Abstract
A novel needle-type biosensor based on carbon nanotubes is reported. The biosensor was prepared by packing a mixture of multi-wall carbon nanotubes (MWCNTs), graphite powder and glucose oxidase (Gox) freeze-dried powder into a glass capillary of 0.5 mm inner diameter. The resulting amperometric biosensor was characterized electrochemically using amperometry in the presence of hydrogen peroxide and in the presence of glucose. The glucose biosensor sensitivity was influenced by the glucose oxidase concentration within the MWCNTs mixture. The optimized glucose needle-type biosensor displayed better sensitivity and stability, and a detected range of up to 20 mM. Based on its favorable stability, the needle biosensor was first time used in real-time monitoring system as a kind of online glucose detector. The decay of current response is less than 10% after 24-hour continuous observation.
Keywords: Multiwall carbon nanotubes, needle-type biosensor, glucose
1. Introduction
Diabetes mellitus has become the third leading cause of death and disability in the world. The Diabetes Control and Complications Trial (DCCT) showed that intense therapy and keeping blood glucose levels as close to normal as possible can reduce the risk of development of some microvascular complications, such as the retinopathy and nephropathy, so proper control of blood glucose has become more and more important in the field of clinical diabetes therapy. Nowadays, to detect the blood glucose most blood samples are collected from vena and the capillary vessel of the fingertip. Because these blood samples are used in off-line detection, many hyper- and hypoglycemia points may be missed and it's difficult to know the actual fluctuations in blood glucose during 24 hours. Recently, numerous works have focused on micro-type biosensors [1-3] which could be imbedded in the human body and used in a continuous glucose monitoring system. Electrochemical methods have gradually become considered as the most effective method for this purpose.
Carbon nanotubes (CNTs), a new type of carbon material, have been identified as excellent electrode materials because of their unique electronic properties. Previous works had proven [6-12] that CNT based sensors had improved sensitivity and wider linear detection range than the traditional carbon material based sensors.
Wang et al. [4] were the first to use CNT as an electric material to fabricate needle-like microsensors. A Gox and CNT mixture was packed within polyimide tubing and coated with a Nafion film at the end of the sensor. The current response of the sensor was then evaluated. Yun et al. [5] developed another new method to construct micro-biosensors based on CNTs. A bundle of nanotubes was welded onto the tip of a tungsten needle under a microscope, the resulting needle was encased in glass and a polymer coating leaving only the tip of the needle exposed. The glucose oxidase was physically attached on the needle tip. This biosensor detected glucose and the amperometric response showed a high sensitivity with a low detection limit.
Because Multiwall Carbon Nanotubes (MWCNTs) are cheaper and easier to be treated by acid than single-wall CNTs (SWCNTs) without losing their excellent electroconductivity, in this paper they were used as the electrode material to fabricate a micro needle-type glucose biosensor. What we really interested in is whether such a kind of biosensor could be used in an online glucose monitoring system and how its long-time stability would be. After optimization of various technical parameters, it is found that this new needle-type glucose biosensor is useful for continuous glucose monitoring and has good potential applications in the field of real-time monitoring.
2. Results and Discussion
2.1. Influence of percentage of GOX to the current response in the needle-type biosensor
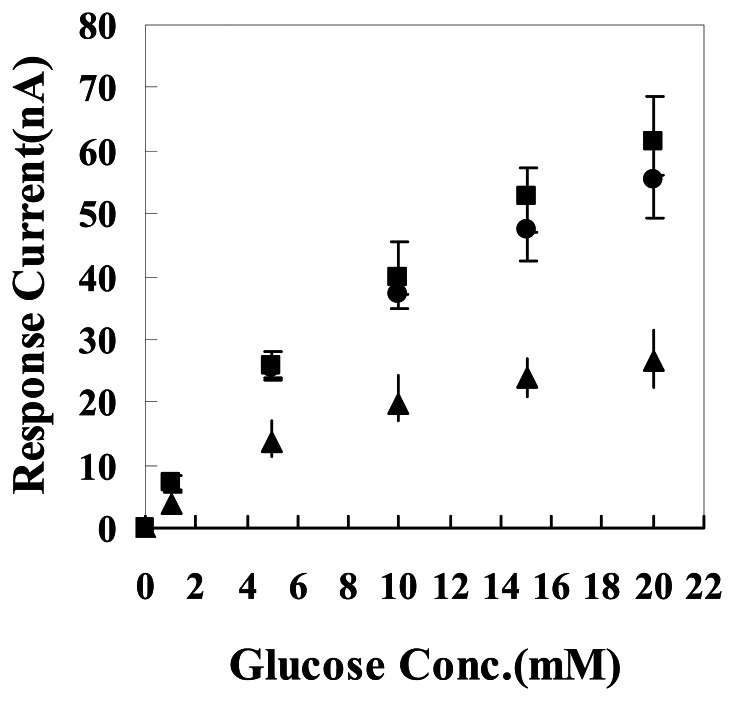
In our previous work (data not shown), current responses to hydrogen peroxide of the needle electrodes fabricated with different conducting materials, such as a pure graphite powder sealed electrode, a mixture of MWCNTs and graphite powder sealed electrode, a pure MWCNTs sealed electrode, were studied. It was found that the needle-type electrode showed the highest response to H2O2 when the ratio of graphite and carbon nanotubes up to 1:2 (w/w). This optimized mixture of MWCNTs/graphite powder, together with different percentages of Gox powder, were packed into the glass capillary to construct the glucose needle-type biosensors. The current responses of this biosensor to the presence of different glucose concentrations were then studied (see Figure 1). As indicated from this figure, the current response of biosensor increased with the percentage of Gox. At certain range of Gox, a larger amount of Gox in the biosensor correspond to a higher current response. When the composition ratio of MWCNTs mixture/Gox is increased from 5:3 to 5:5, the intensity of current response was not increased so obviously. This indicates that when the percentage of Gox is increased to a certain level, the corresponding increase of current response to the percentage of Gox become small. The reason is that the decrease of percentage of MWCNTs results in an increase of the amount of Gox in the whole electrical material, therefore the electrical conductivity and hydrogen peroxide catalysis of the biosensor will be decreased. This decrease can counteract the effect of Gox in the biosensor, thus the biosensor can't display a sustained increase in current response along with the increase of Gox amount.
Figure 1.
Influence of Gox contents upon the amperometric glucose response. ▲-MWCNTs mixture/Gox needle biosensor at 5:1 composition ratio, ●- MWCNTs mixture/Gox needle biosensor at 5:3 composition ratio, ■- MWCNTs mixture/Gox needle biosensor at 5:5 composition ratio.
2.2. Biosensor Stability
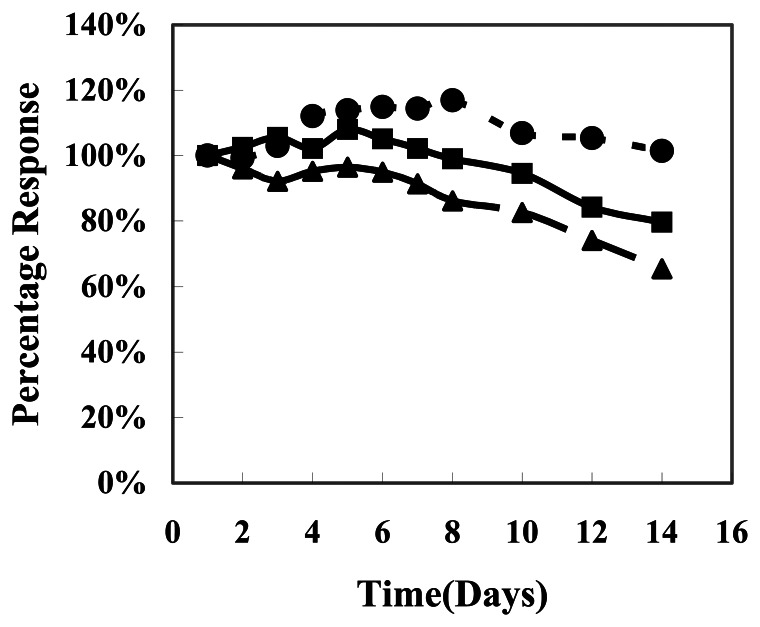
In order to study the stability and reproductivity of the needle-type biosensors, three biosensors with different ratios of MWCNTs mixture and Gox were tested in the presence of 20 mM glucose solution for 14 days (Figure 2). The biosensors were removed from a 4°C refrigerator and put in the reaction cell to perform the experiment each day, and then dried and stored at 4 °C again after each experiment was completed. During the continuous observation, the MWCNTS mixture/Gox biosensor with a composition ratio of 5:3 showed the best reproductivity and stability. After 14 days observation, three biosensors all showed a decay trend; the current response of the biosensor with a ratio of 5:3 had the smallest decay and the current response of the biosensor with a ratio of 5:1 decreased deeply (the current response of the 14th day was only 65 % to the result of 1st day). Due to the continuous observation, the biosensors had to pass through a dry to wet to dry again cycle. This cyclic procedure had a negative influence to the activity of enzyme, and normally the activity of enzyme will decrease and hence the current response of biosensor; but in our investigation, these decays were not so obvious over a long period. This can be explained by the integration of enzyme protein with MWCNTs: the acid treated MWCNTs have a lot of hydrophilic carboxyl groups, which are easily combined with enzyme protein (Gox) in the moist environment and form stable enzyme-MWCNTs complexes [13]. These complexes are more stable and result in a long-term stability of the Gox compared with the free Gox. Therefore this kind of biosensor showed a better current response and stability.
Figure 2.
Storage stability of needle-type biosensor. ▲- MWCNTs mixture/Gox needle-type biosensor (5:1 composition ratio), ●-MWCNTs mixture/Gox needle-type biosensor (5:3 composition ratio), ■- MWCNTs mixture/Gox needle-type biosensor (5:5 composition ratio). The data points represent biosensor's current response to 20 mM glucose, pH 7.0 PBS, measured for 100 second at 0.60 V on a given day. The response was normalized with respect to that on day 1.
2.3. 24-hour online monitoring
To test whether or not this needle-type biosensor based on carbon nanotubes can be used in a Continuous Glucose Monitoring system, we put the biosensor with a composition ratio of 5:3 (MWCNTs mixture/Gox) in a micro reaction cell containing glucose solution and observed its current responses continually.
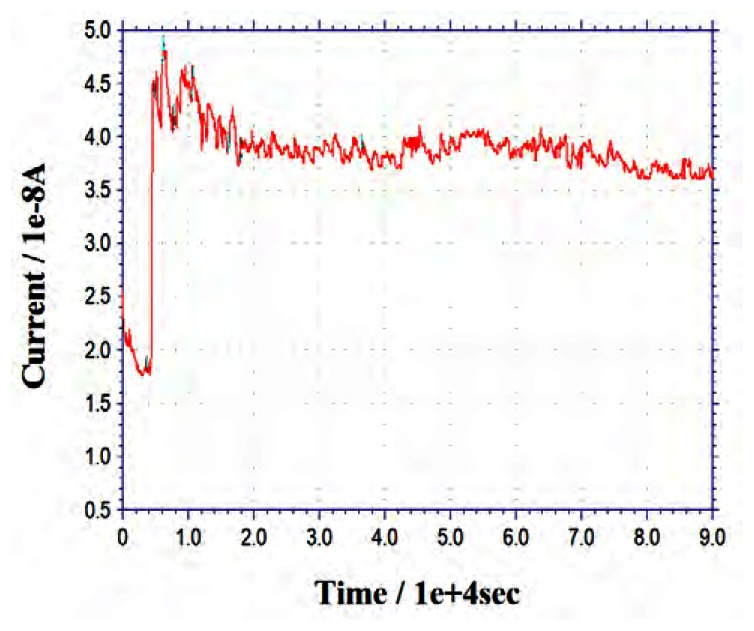
First, the needle biosensor was equilibrated in the PBS (pH 7.0) for 3600 s (1-hour). Then the current response of the biosensor was measured in PBS for another 3600 s (1-hour) (at the applied potential of +0.6V vs. SCE). Later, we pumped glucose solution into the micro reaction cell continually to maintain a constant glucose concentration of 5 mM, and then recorded the current response for 23 hours. The total observation time is 24 hours (86400 s) and the results are shown in Figure 3. As shown in this figure, when the glucose concentration changed from 0 to 5 mM, the current response of the needle biosensor rapidly jumped from 18 nA to 45 nA. It was found the baseline of the biosensor decreased very slowly during the monitoring period, so after eliminating this baseline influence, the decay of the current response was less than 10% during the 24 hour online monitoring. This indicates that the needle biosensor has acceptable long-term stability. This result makes it possible to employ such kinds of CNT biosensor in the continuous glucose monitoring system field. In addition, the baseline problem could be solved by using two identical microbiosensors together, one is used as a glucose sensor and the other is used as a baseline reference sensor. Software changes in future use should result in another more efficient method.
Figure 3.
24-hour current response of the needle-type biosensor. The needle-type MWCNTs mixtue/Gox biosensor used in this experiment has 5:3 composition ratio of MWCNTs mixtue and Gox.
2.4. Conclusions
A attractive amperometric biosensor for Continuous Glucose Monitoring has been presented, based on the MWCNTs/graphite/Gox packed needle-type electrode. The glucose biosensor sensitivity was strongly influenced by the glucose oxidase concentration within the MWCNTs mixture. After optimization, the biosensor displayed good sensitivity and stability, and a detected range of up to 20 mM. We also assessed the use of needle-type biosensor in a real-time glucose monitoring system. Our preliminary results indicate that the new needle-type biosensor is promising and useful in this field (current decay is lower than 10% during 24 hour observation). In addition, because all the experiments were processed in vitro, the system could not provide all the significative results we are interested in, so animal experiments will be considered for future work.
3. Experimental
3.1. Instrument and reagent
A CHI800 Electrochemical Workstation (American CH Instrument Company) was used., Glucose oxidase (Gox, 100U/mg) was obtained from Sangon Bio Inc. All other reagents were of the best quality available commercially. Deionized water was used throughout. Glass capillaries (inner diameter is 0.5mm) were purchased from American Drummond Scientific Company. Multi-Wall Carbon Nanotubes (MWCNTs) were provided by the College of Materials Sciences and Chemical Engineering, Zhejiang University. The MWCNTs had an average length of 20 μm and a mean diameter of 15 nm. The MWCNTs were treated with mixed acid (H2SO4-HNO3 = 3:1, v/v), and ultrasonically agitated for 8 hours. The acid treated MWCNTs were filtered and then washed with deionized water until neutral, and finally dried in an oven.
3.2. Methods
3.2.1. Construction of the carbon nanotubes based glucose needle biosensor
The acid treated MWCNTs, graphite powder and Gox were homogeneously mixed in a certain ratio. The mixture were compactly pressed into the cavity (0.5-mm i.d., 2-mm depth) at the end of a glass capillary, with electrical contact to its inner end made with a 0.1 mm diameter copper wire [4]. Then the end of capillary which contains the MWCNTs was soaked in paraffin oil for 20 minutes, and dried in the oven at 50°C for 10 minutes. Finally, the end surface of this electrode was polished to a smooth surface with weighing paper.
3.2.2. Measurements
In order to examine the response character of the MWCNTs-based biosensor to glucose, experiments were carried out in an aqueous solution buffered at pH 7.0 with 0.1 M phosphate in a conventional three-electrode cell. The needle biosensor served as the working electrode. A platinum wire and a saturated calomel electrode (SCE) were used as counter and reference electrode, respectively. A peristaltic pump was used to refresh the solution and maintain solution equilibrium in the cell. The response current (under 0.6V static potential referred to SCE) was marked with the change value between the steady-state current and background current. All experiments were performed at room temperature.
Acknowledgments
This work was financially supported by the Science and Research Plan of Zhejiang, P.R. China (Grant 2004C33008).
References and Notes
- 1.Matsumoto T., Ohashi A., Ito N. Development of a micro-planar AgAgCl quasi-reference electrode with long-term stability for an amperometric glucose sensor. Analytica Chimica Acta. 2002;462:253–260. [Google Scholar]
- 2.Ahmed S., Dack C., Farace G., Rigby G., Vadgama P. Tissue implanted glucose needle electrodes: early sensor stabilisation and achievement of tissue-blood correlation during the run in period. Analytica Chimica Acta. 2005;537:153–162. [Google Scholar]
- 3.Wang J., Zhang X. J., Prakash M. Glucose microsensors based on carbon paste enzyme electrodes modified with cupric hexacyanoferrate. Analytica Chimica Acta. 1999;395:11–16. [Google Scholar]
- 4.Wang J., Musameh M. Enzyme-dispersed carbon-nanotube electrodes: a needle microsensor for monitoring glucose. Analyst. 2003;128:1382–1385. doi: 10.1039/b309928h. [DOI] [PubMed] [Google Scholar]
- 5.Yun Y., Bange A., Shanov V. N., Heineman W., Halsall H. B., Dong Z., Jazieh A., Tu Y., Wong D., Pixley S., Behbehani M., Schulz M. J. A Carbon Nanotube Needle Biosensor. Journal of Nanoscience and Nanotechnology. 2007;7:2293–2300. doi: 10.1166/jnn.2007.408. [DOI] [PubMed] [Google Scholar]
- 6.Gooding J. J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochimica Acta. 2005;50:3049–3060. [Google Scholar]
- 7.Wang S.G., Zhang Q., Wang R. L., Yoon S.F. A novel multi-walled carbon nanotube-based biosensor for glucose detection. Biochemical and Biophysical Research Communications. 2003;311:572–576. doi: 10.1016/j.bbrc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z. A., Chen X., Qu X. H., Jia J. B., Dong S. J. Single-wall carbon nanotube-based voltammetric sensor and biosensor. Biosensors and Bioelectronics. 2004;20:579–584. doi: 10.1016/j.bios.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Rubianes M. D., Rivas G. A. Carbon nanotubes paste electrode. Electrochemistry Communications. 2003;5:689–694. [Google Scholar]
- 10.Tang H., Chen J. H., Yao S. Z., Nie L. H., Deng G. H., Kuang Y. F. Amperometric glucose biosensor based on adsorption of glucose oxidase at platinum nanoparticle-modified carbon nanotube electrode. Analytical Biochemistry. 2004;331:89–97. doi: 10.1016/j.ab.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Musameh M., Wang J., Merkoci A., Lin Y. H. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochemistry Communications. 2002;4:743–746. [Google Scholar]
- 12.Gavalas V. G., Law S. A., Ball J. C., Andrews R., Bachas L. G. Carbon nanotube aqueous sol-gel composites: enzyme-friendly platforms for the development of stable biosensors. Analytical Biochemistry. 2004;329:247–252. doi: 10.1016/j.ab.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Guan W. J., Li Y., Chen Y. Q., Zhang X. B., Hu G. Q. Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes. Biosensors and Bioelectronics. 2005;21:508–512. doi: 10.1016/j.bios.2004.10.030. [DOI] [PubMed] [Google Scholar]