Abstract
Steel corrosion resulting from the penetration of chloride ions or carbon dioxide is a major cause of degradation for reinforced concrete structures,. The objective of the present investigation was to develop a low-cost sensor for steel corrosion, which is based on a very simple physical principle. The flat end of a cut optical fiber is coated with an iron thin film using the ion sputtering technique. Light is then sent into a fiber embedded in concrete and the reflected signal is monitored. Initially, most of the light is reflected by the iron layer. When corrosion occurs to remove the iron layer, a significant portion of the light power will leave the fiber at its exposed end, and the reflected power is greatly reduced. Monitoring of the reflected signal is hence an effective way to assess if the concrete environment at the location of the fiber tip may induce steel corrosion or not. In this paper, first the principle of the corrosion sensor and its fabrication are described. The sensing principle is then verified by experimental results. Sensor packaging for practical installation will be presented and the performance of the packaged sensors is assessed by additional experiments.
Keywords: Steel corrosion, iron thin film, ion sputtering technique, optical fiber
1. Introduction
Reinforced concrete structures make up a large portion of the physical infrastructure of the world, and their durability is an issue of great concern. Steel corrosion is a major culprit for the degradation of concrete members. In order to perform proper maintenance which can extend the lifetime of concrete structures, techniques for steel corrosion detection need to be developed. Within the alkaline environment of concrete, steel is in a passivated state, with a negligible corrosion rate, but when carbon dioxide and chlorides penetrate through the concrete cover to reach the steel, depassivation occurs and the corrosion rate becomes significant. One approach to assess the corrosion of steel is hence to measure the depth of carbonation or the chloride profile in the concrete structure. The conventional approach is to perform chemical analysis on samples cored from the concrete structure. A detailed description can be found in [1]. For carbonation, phenol-phthalate solution is often applied to the sample to identify the depth where color change occurs. However, since the pH at which color change occurs is not the same as the pH below which steel corrosion may occur, some subjective judgment is required for interpreting the results. For chloride penetration, the cored sample is further sliced into thin sections, and each section is ground into powder for chemical analysis. While this can provide an accurate chloride profile in the structure, a major limitation should be pointed out. The chloride concentration does not have a direct correlation with steel corrosion [2]. The critical chloride concentration beyond which steel is depassivated depends also on the pH of the concrete, and this exact relation is not known. Indeed, different researchers [3-4] have come up with different relations between the critical chloride concentration and pH. As another major drawback, taking cores from a large concrete structure at critical locations is often difficult and costly. As a result, the coring operation can only be occasionally performed.
Embeddable corrosion sensors have been developed and installed in concrete structures. The most notable example is the ‘ladder’ sensor developed in Germany [5]. Parallel steel rods are fixed across two parallel rails to form a small ladder. The ladder is then placed inside a concrete structure in such a way that the rods are at different depths from the surface. By comparing the potential of each rod with a reference electrode, one can assess if corrosion has occurred at the level of a particular rod. The penetration of the corrosion front is then monitored. Note that a major advantage of this technique is the actual assessment of corrosion activity, rather than measuring a variable (such as chloride concentration) for indirect inference of whether corrosion has occurred. A disadvantage of the technique is the relatively high cost associated with the sensing system, and the fact that the ‘ladder’ sensor can only be installed in new structures. Recently, a cylindrical sensor, based on the same principle has been developed [6]. The cost is still relatively high. Also, when a hole is cored in an existing concrete structure for installing the cylinder, the prevention of preferential chloride penetration along the sensor/concrete interface is an important issue that needs to be further studied.
Optical fiber sensors for chloride detection have also been developed [7]. A particular chloride sensitive solution or indicator paper is brought into contact with concrete. Broad-band light is then sent from one optical fiber through the solution (or paper) to another fiber. In the presence of chlorides, formation of precipitates in the solution or color change of the paper leads to changes in the intensity or spectrum of the transmitted light. For this sensor, an ingenious installation technique, which allows the sensor to be changed over the life span of the structure, has been developed. A limitation of the sensor is that it cannot be applied to existing concrete structures. Also, it is quite large in size, so if a large number of sensors are to be employed to monitor chloride profile at various locations, the installation becomes very inconvenient. Another fiber optic sensor was developed in [8]. The cladding of the optical fiber is made with a special polymer that is sensitive to the environment. Changes in chloride concentration will lead to a change in the properties of the fiber cladding. The corresponding change in the evanescent wave can be monitored through optical spectroscopy. While this is certainly a powerful technique, the principle is rather complicated, and the results may need to be interpreted by a specialist. Also, both optical techniques suffer the limitation that the measurand is the chloride concentration, which does not have a one-to-one relation with corrosion activity [2].
If there is penetration of carbon dioxide or other acids into concrete, steel corrosion starts when the pH drops below 11.5. In [9], a fiber optic pH sensor is developed to monitor pH values between 9 and 12, which covers the critical pH value below which corrosion will occur. The sensor is based on the absorption intensity ratio between two different wavelengths at a pH sensitive membrane. In [10], a different fiber optic pH sensor is reported. An optical fiber is placed inside a polymer matrix with covalently coupled dye. When the pH values vary between 11 and 12.5, the color change of the dye is monitored with a micro-fabricated electro-optical sensing system. Using a different type of dye, a humidity sensor based on the same principle has also been developed [10]. While the measurement of pH and humidity is useful in identifying corrosion caused by loss of alkalinity in the concrete environment, chloride-induced corrosion cannot be revealed.
In this paper, we will present a new fiber optic based technique for detecting the onset of steel corrosion under a certain environment, with special application on concrete structures. The physical principle is first explained. The sensor will be tested in sodium chloride solution and embedded in cement mortar with sodium chloride to illustrate the feasibility of corrosion sensing. Then, sensor packaging for practical application will be presented. Additional tests were conducted on the packaged sensor to study its performance. First, sensors are installed inside holes drilled in hardened cement mortar blocks with different sodium chloride content to verify the capability to detect corrosion activity in existing structures. Second, sensors are glued to embedded steel reinforcements under corrosive environment and the sensor output will be compared to corrosion current measurement. Third, packaged sensors are embedded in cement mortar blocks with the sensing tip at different distances from the surface. With these blocks placed inside a salt water bath, the feasibility to monitor the penetration of chloride into a concrete member, in a way similar to the corrosion “ladder” sensor [5], can be assessed.
2. Physical Principle
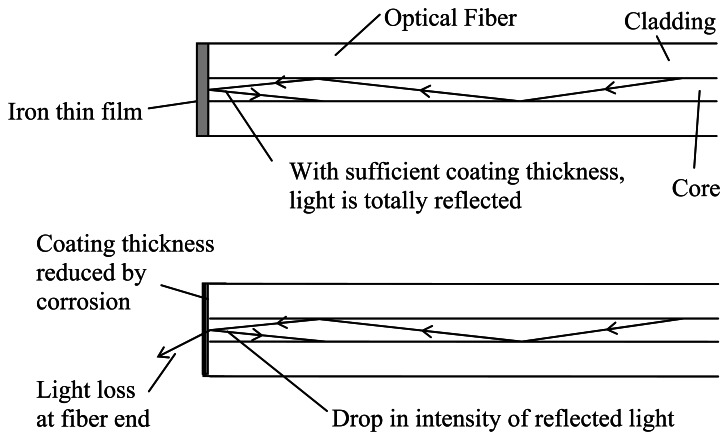
The novel corrosion detection method is based on the simple principle of light reflection illustrated in Figure 1. An optical fiber is stripped and cut with a cleaver to produce a flat surface at its end that is perpendicular to the fiber axis. Using the ion sputtering technique, an iron thin film (thickness around one hundred to several hundred nanometers) is deposited on the cleaved fiber end. Light is then sent through the optical fiber and the reflected light intensity is monitored as a function of time. Initially, the iron film at the end of the fiber acts like a mirror and all or most of the light is reflected (Figure 1a). As corrosion occurs, the film thickness decreases with time. Consequently, the reflected signal also drops (Figure 1b). Ultimately, after the film is completely removed, the reflected light intensity will return to the level for the cleaved glass surface.
Figure 1.
Illustration of the corrosion sensing principle.
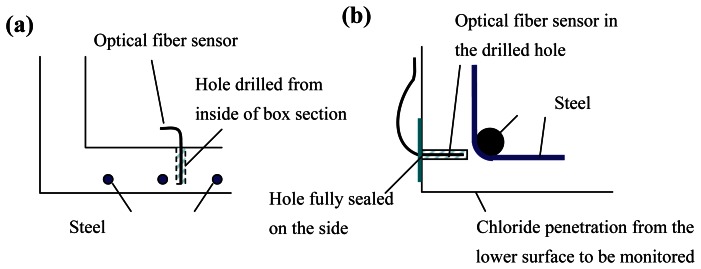
Compared to other corrosion sensors, the ‘reflection’ sensor possesses a number of advantages. Since the principle is very simple, interpretation of results is easy and direct. It is applicable to corrosion induced by either chloride penetration or carbonation. The technique does not require the use of special optical fibers, so low-cost single mode telecommunication fibers can be employed. In the sputtering process, a large number of fibers can be placed in the sputtering chamber and coated at the same time. Mass production of the sensor at low cost should therefore be possible. Moreover, with the small size of the sensor, it can be retrofitted to existing structures. Two plausible ways to install the sensor are illustrated in Figure 2.
Figure 2.
Plausible ways for retrofitting the sensor on an existing structure with (a) boxed section, (b) solid section.
For a bridge with boxed section, a hole of required depth can be drilled from the inside of the box for placement of the sensor (Figure 2a). To monitor chloride or carbon dioxide penetration from a surface of a solid section, the hole can be drilled on a perpendicular surface and sealed (Figure 2b). With these approaches, the drilled hole will not act as an easy diffusion path that will affect the monitoring results. Since the monitoring involves light intensity measurement, to avoid variations due to fluctuations in the light source, and changes in connector efficiency when fibers are disconnected and re-connected, the loss in reflected light power is best measured with an optical time domain reflectometer (OTDR). This method measures the relative power of light reflected at points along the fiber, and that at the fiber end. Since it is a relative measurement, results will not be affected by the variations mentioned above. From our experience, single mode optical fiber is superior to multimode fiber in terms of measurement stability. This is because the intensity of reflected light at the film depends on the incident angle of the ray, which varies for different modes. For a multimode fiber, any small deformation along the fiber may lead to mode redistribution that causes fluctuation of the sensor response. Our tests are conducted in 850nm wavelength, as this is the operating wavelength of our OTDR. The cost of 850nm single mode optical fiber and its accessories is much higher than those of 1550nm single mode optical fiber (which is the common type for communication applications). As the operating wavelength is not an essential parameter of the sensor, an OTDR system and single mode optical fiber at 1550nm should be employed in practical applications. However, as light absorption by the film is wavelength dependent, the sensor should be calibrated at the wavelength at its operation. Also, the spatial resolution of the sensor does not affect the sensor performance as long as the fiber end is sufficiently far away from the bulkhead of the OTDR. Hence, a portable OTDR with low cost (around US$6000) can be used for the measurement.
3. Sensor Fabrication and Verification of the Detection Principle
To form an iron thin film with controlled thickness on the cleaved end of the optical fiber, the sputtering deposition technique was employed. The fibers were placed inside a vacuum chamber, with the cleaved ends facing a pure iron target. The sputtering process involves the bombardment of target with energetic ions to induce the ejection of vapor particles. Inside the vacuum, the vapor will deposit as a thin film on the surface of the fiber. The film thickness depends on the distance of the fiber from the target as well as the deposition time. Before the coating of fibers, a calibration experiment was performed using a glass plate with a strip of thin tape bonded to its surface. The plate was placed at a distance to the target that was equal to the fiber/target distance in the actual sputtering process. Sputtering deposition was performed for different periods of time. After the deposition process, the tape was removed and the thickness of coated film was obtained from the surface profile along the coated plate, measured with a profileometer. With the calibration experiment, the relation between film thickness and deposition time was found to be linear and the slope was 2.92nm/min.
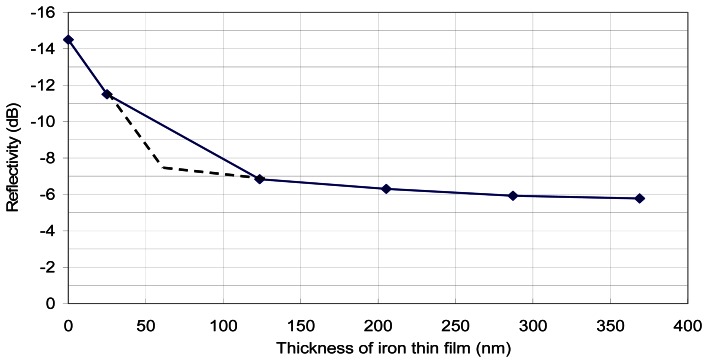
Based on the aforementioned sensing principle, a bundle of cleaved fiber (Nufern 780-HP) was put into the sputtering chamber facing the pure iron target. With different deposition times, fibers with different coating thicknesses were prepared. In this study, the definition of reflectivity is 10log(Proportion of light reflected). The reflectivity of cleaved fiber end without any coating is calculated theoretically to be -14.6dB (the refractive index of the core of the optical fiber is 1.458). For fibers with various thicknesses of iron coated at its tips, the reflectivity was experimentally determined by an OTDR (Opto-Electronics, OFM-20). The results (Figure 3) indicate significant difference (about 8dB) in reflectivity between the coated and uncoated fiber. Also, the reflectivity seems to approach an asymptotic value (about -6dB) for film thickness greater than 300nm. There was no reflectivity data for film thickness in the range of 25nm to 100nm. The dotted lines represent a hypothetical bilinear relationship. There is a sharp change in the sensitivity of the sensor at about 60nm film thickness.
Figure 3.
Relationship between the reflectivity at the coated fiber end and the film thickness.
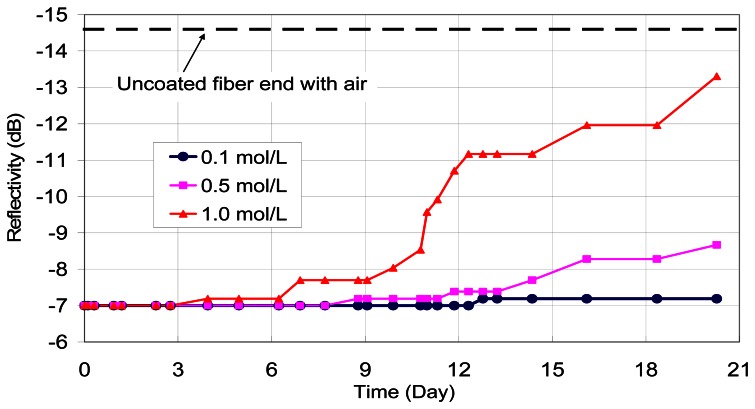
The first verification experiment was performed using 100 nm iron film on their tips. The coated end of optical fibers were dipped into calcium hydroxide solutions (pH=13 to simulate the concrete environment) with 0.1, 0.5 and 1.0 mol/L of sodium chloride, respectively. The reflected signal from the fiber end was monitored daily with OTDR. The variations of reflectivity (in dB) with time (in days) under different chloride concentrations are shown in Figure 4. It should be pointed out that an optical fiber sensor has also been placed in alkaline solution without any sodium chloride added, and no change in optical power was observed over two months. Based on the test results, two observations can be made. First, the presence of a corrosive agent (chloride ions) leads to a drop in reflected optical power with time, which can be attributed to the gradual removal of the iron film on the fiber end. Second, the power drop which represents the total corrosion of the film increases with increasing chloride concentration. These findings demonstrate the feasibility of the proposed sensing concept.
Figure 4.
Effect of chloride concentration on corrosion sensor output.
From the test results, the measured loss does not increase smoothly with time, but may show a sudden jump followed by a period of little or no increase. This is most obvious for the case with 1.0 mol/L of sodium chloride. Actually, what is monitored is the film thickness at the core of the optical fiber with a small area of only 64 μm2. In other words, we are measuring the corrosion activity within a small zone, rather than the overall corrosion over a reasonably large region (such as in macrocell current measurements). The results are therefore very sensitive to the local chloride concentration and moisture content, which can vary with time. In particular, once reaction occurs, chlorides are used up and diffusion of additional chlorides to the region takes time. This explains the large reduction in corrosion rate (indicated by constant signal loss versus time) after a rapid increase in corrosion activities.
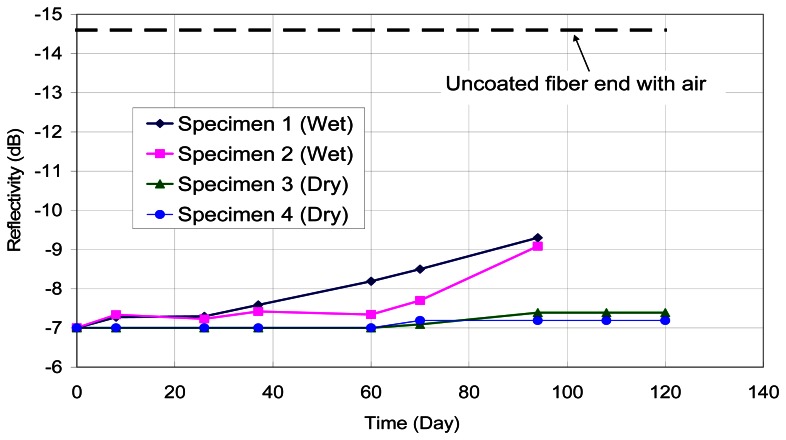
A second set of experiments was performed to investigate the corrosion sensing capability inside an actual concrete environment. The coated optical fibers were cast inside small blocks of cement mortar with water:cement:sand ratio of 0.5:1:2. Sodium chloride was added at 5% of the cement weight. After curing for 7 days, two specimens were placed inside a warm water bath at 30°C. Another two specimens were taken out of the curing room and exposed to the dry laboratory environment. For each specimen, the reflectivity of the end of the embedded optical fiber sensor was monitored as a function of time. The results are shown in Figure 5 for both the wet and dry specimens. When kept wet, corrosion (which is indicated by the drop of reflectivity) started much earlier and occurs to a much larger extent. For the specimen in the laboratory environment, which is relatively dry but not completely dry, corrosion started to occur after two months, and did not reach a high level. The findings indicate that the sensor can detect chloride ions inside the concrete environment, and it can distinguish between the seriousness of corrosion activities under different environments.
Figure 5.
Output from sensor embedded in concrete under different environments.
4. Packaging of the Sensor
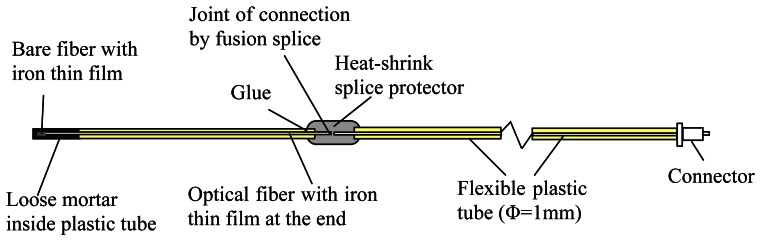
The sensor employed in the above experiments is simply a bare fiber with an iron coating. The installation of sensor into concrete has to be carried out with great caution to avoid fiber breakage. While this is achievable in the laboratory, field application of the sensor requires proper packaging to make it robust against the concrete casting process. An approach to package the sensor is illustrated in Figure 6. To fit the fiber bundle into the sputtering chamber, the length of each coated optical fiber is about 200 mm. The coated fiber is embedded in a 1 mm diameter hollow flexible plastic tube and fixed inside by glue. The coated end inside the plastic tube is then well protected (from mechanical actions) during the concrete casting process.
Figure 6.
Schematic diagram of sensor packaging.
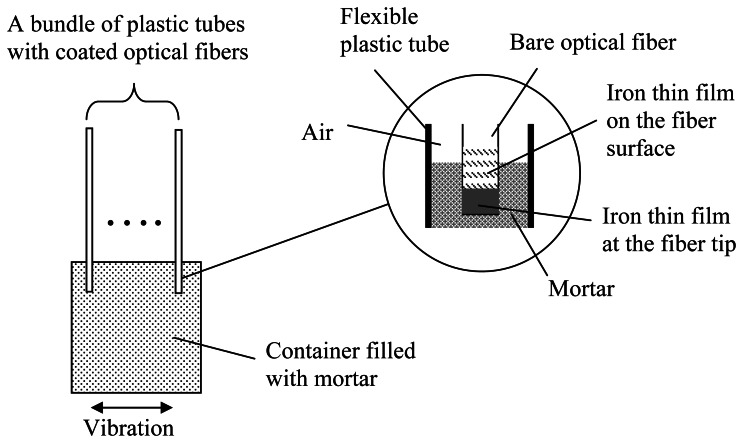
To enable proper transfer of corrosive agents from the concrete to the tip of the fiber, mortar is added into the plastic tube (see Figure 6 and inset of Figure 7). A proper mortar mix can be made with fine sand (diameter less than 0.2 mm) and high water/cement ratio (w/c=0.6). A high water/cement ratio is necessary to ensure the easy transportation of corrosive agents to the end of the optical fiber. To get the mortar into the fine plastic tube, a bundle of tubes with optical fiber (about ten per batch) is inserted into mortar with the tube end just under the mortar surface (Figure 7).
Figure 7.
Fiber sensor packaged inside a mortar block.
For proper filling, the mortar container is shaken on a vibration table to facilitate the penetration of mortar into the plastic tube. The filled plastic tube is then taken out and placed horizontally until the mortar is hardened. After mortar filling, the fiber end should be surrounded completely by mortar (see the inset of Figure 7), and this has been verified by cutting some of the plastic tubes to reveal the mortar and fiber inside. While the optical fiber is surrounded by mortar (which is alkaline), it would not be attacked as the glass surface is covered with iron thin film during the sputtering deposition process. The length of the coated optical fiber, which is only 200 mm, is certainly not long enough in practical applications. It is therefore connected to an extension cable by fusion splicing and the joint is protected by a heat-shrink splice protector (Figure 6). The whole fiber (coated as well as fiber extension) is protected by flexible plastic tube and no bare fiber is exposed. For use in harsh environment of the construction site, the extended fiber can be further protected by an armored loose tube optical fiber cable.
5. Experiments on Packaged Sensors
5.1. Experimental set-up and procedure
In Section 3, the sensors were tested on their own to show that they can respond to chlorides in the environment. In real applications, however, we need to ensure that the packaged sensor is effective in providing information on steel corrosion inside a concrete structure. In the following, the experimental set-ups and procedure for three separate tests on the packaged sensor in Section 4 will be described in detail.
5.1.1. Sensors in hardened cement blocks
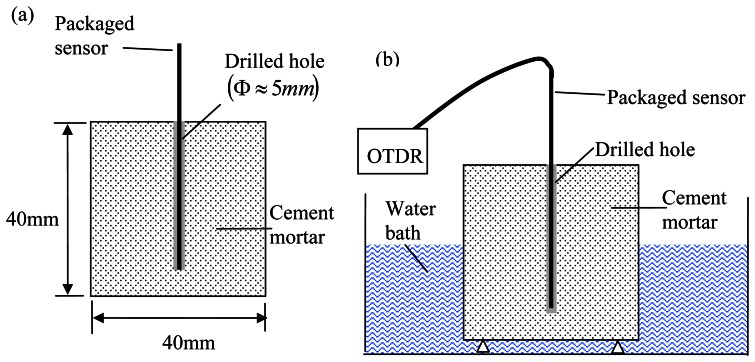
The possibility to post-install the proposed sensor in existing structures is one of its major advantages over other available sensors. To verify such capability of the sensor, cement mortar cubes (40 mm × 40 mm × 40 mm) were first cast with 0.4%, 1% and 2% of sodium chloride by the cement weight (see Figure 8a).
Figure 8.
Experimental set-up of the test of sensors in drilled hole of a hardened cement mortar block.
The mix proportion of the cement mortar was water:cement:sand ratio=0.6:1:2. After curing the specimens for 14 days, a 5 mm diameter hole was drilled into each specimen to reach a depth that was 5 mm from the bottom. The hole was cleaned by water and a packaged sensor was inserted into the hole. In the tests, the coated film on the fiber tip was about 200 nm in thickness, and the tube diameter was about 1 mm. The hole was then filled by cement mortar with the same mix proportional as the specimen (but without sodium chloride). In practice, the cement mortar for filling the hole should be at least as porous as the parent material, so the water/cement ratio should be the same or higher. Also, a hole size of 5 mm is appropriate, as a smaller hole will be difficult to be filled. After the sensor was installed and the hole was filled, the specimens were put into a water bath to keep the moisture inside the specimens (Figure 8b). As shown in the figure, the bottom of the specimen and part of the vertical surfaces were exposed to water during the test.
5.1.2. Modified ASTM G109 test
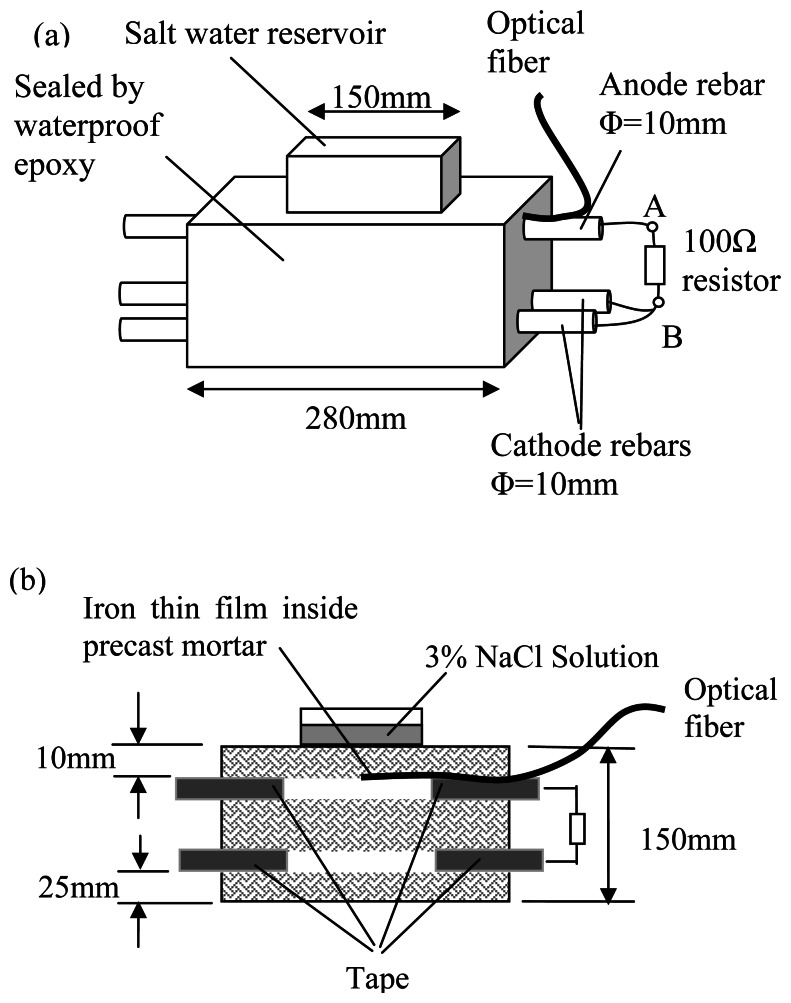
The objective of this experiment is to compare the sensor response to a different approach of corrosion detection, to see if consistent results can be obtained. The chosen approach of comparison is the measurement of corrosion current, which is a standard method described in ASTM G109 [11]. The employed experimental set-up is shown in Figure 9 with all the relevant dimensions.
Figure 9.
Set-up to compare optical sensor output with corrosion current of steel.
A salt water reservoir with 3% sodium chloride solution was placed on top of a concrete prism, to provide a source for chloride ions that would penetrate into the concrete specimen. A steel reinforcement, 10 mm in diameter, was placed near the top surface as an anode with a cover of 10 mm. Two additional steel rods were also placed close to the bottom of the member as cathodes with a cover of 25 mm. All the steel reinforcement was covered by tape, except for the portion under the salt water reservoir. All the vertical surfaces of the concrete prism were sealed by epoxy to ensure a one-dimensional diffusion path. At the top of the steel reinforcement facing the upper surface, a packaged optical corrosion sensor was attached. The thickness of iron film on the fiber was about 200 nm. The specimens were cured for 14 days before the salt water reservoir was installed. During the test, the reflectivity of the sensor was measured at different times. Also, the potential difference over a 100 Ω resistor (AB in Figure 9a) between the anode and cathode was measured to calculate the macrocell current. Since the bars at the bottom of the specimen should not corrode, an increase in macrocell current indicated the onset of corrosion at the upper bar. The threshold of corrosion macrocell current is about 10 μA [1]. The corrosion initiation times obtained from the drop of reflectivity of the sensor and the rise of macrocell current can be compared to see if the two methods give rise of consistent results.
It should be pointed out that our experimental set-up was identical to the one recommended by ASTM G109 except that the cover of the anode rebar was reduced from 25 mm to 10 mm, so that the corrosion process could be accelerated. As both diffusion and absorption occurs in the first 10-20 mm of concrete from the surface during wet/dry cycle [12,13], a test with small concrete cover is not suitable for the comparison of material parameters from one mix to another (as the transport process is governed by more than one parameter). However, since the objective of this experiment is simply to compare the corrosion initiation time indicated by the sensor and the macrocell current, the use of non-standard set-up is acceptable.
5.1.3. Diffusion test
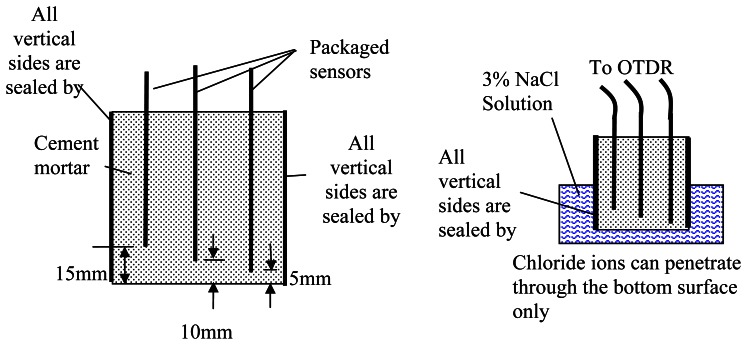
For long term monitoring, it is necessary to know the penetration front of corrosion activity. The present experiment aims at verifying the potential of the proposed corrosion sensor to monitor the penetration of corrosive agents, such as chloride ions, carbon dioxide and sulphur dioxide. In this test, three packaged sensors were embedded into a cement mortar cube (40mm× 40mm× 40mm) with the tip at different distances (5mm, 10mm and 15mm) to the surface (Figure 10). In this experiment, the thickness of the coated iron thin film on the fiber was about 400nm. During the casting of mortar to make the blocks, toothpicks were used to hold the fiber in place. After the blocks were cast and cured, epoxy was applied on all vertical sides so the corrosive agents and water can only penetrate from the bottom. The specimen was then put into a salt water bath with 3% sodium chloride. The reflectivity of the three fibers was then measured as a function of time. Note that the concentration may change due to evaporation and diffusion. Although the corrosion initiation time may not be sensitive to the chloride concentration of the source as long as it is larger than 1% [14], the salt water bath was replaced by a new solution every two weeks to maintain a constant solution during the test.
Figure 10.
Set-up of diffusion test.
In practice, we are interested to knowing if corrosion has started in the reinforcing steel inside a concrete structure. Several optical sensors can be placed inside the concrete member with their tips at a smaller distance from the surface than the steel bar. Monitoring of corrosion activities indicated by the sensors can provide sufficient warning to the corrosion of steel reinforcement, and proper maintenance action can be taken.
5.2. Experimental results and discussion
In this section, the experimental results of the tests in Section 5.1 are presented to demonstrate the viability of the proposed optical corrosion sensor. The output from the optical sensor is converted to a reflectivity value. When the coated iron film has not been completely removed, the fiber end is isolated from the environment and the reflectivity versus film thickness relationship should follow the calibration curve in Figure 3. Once the whole thin film is removed, the reflectivity depends on the surrounding environment, which can give rise to two different situations. In the first situation, the fiber end is in contact with air, and the reflectivity is -14.6 dB. In the second situation, the fiber end is surrounded by water (when the pores inside concrete are saturated), and the theoretical reflectivity reaches a much higher value of -26.8 dB.
5.2.1. Sensors in hardened cement mortar blocks
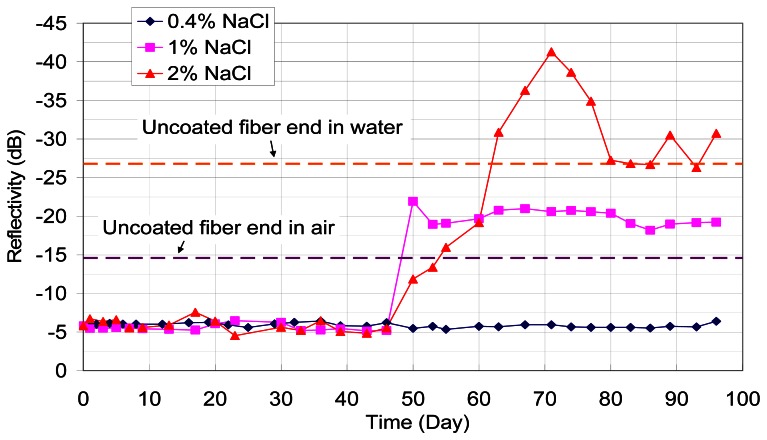
Packaged sensors were inserted into holes in cement mortar blocks with different concentrations of sodium chloride. The results are shown in Figure 11. The reflectivity of the sensor embedded into 1% and 2% sodium chloride was reduced after about 40 days of embedment, while the reflectivity remained at the same level for the 0.4% case. The threshold of chloride concentration for steel corrosion is about 0.4% for structural steel [1]. For the sensor, corrosion of pure iron at its end was measured. At 0.4% sodium chloride, there was nearly no change in reflectivity in a period of three months. For the other two cases (with 1% and 2% sodium chloride, which are both above the threshold), significant drop in reflectivity was found to occur at about the same time (after 46 days). In these tests, the sensors were not directly cast into the mortar blocks, but inserted into a drilled hole which was later filled with mortar (without chloride). The results indicate that corrosion activities in a concrete member with corrosive agents can be detected by a post-installed sensor. The potential for corrosion monitoring in existing structures is hence demonstrated.
Figure 11.
Results of tests of drilled hole of hardened cement mortar block.
If one takes a reflectivity of -14.6 dB to indicate the complete corrosion of the iron film on the fiber, the corrosion rate appears to be higher for the case of 1% sodium chloride. The results indicate that corrosion initiation and corrosive rate may not be sensitive to the chloride concentration, as long as it is sufficiently high. After the reflectivity goes below -14.6 dB, large fluctuations can be observed for the 2% sodium chloride case. A plausible explanation is the presence of cement hydration products right around the fiber tip that affects the reflectivity. This phenomenon may be similar to the effect of soil particles on in-situ spectroscopic measurements reported in the literatures [15, 16].
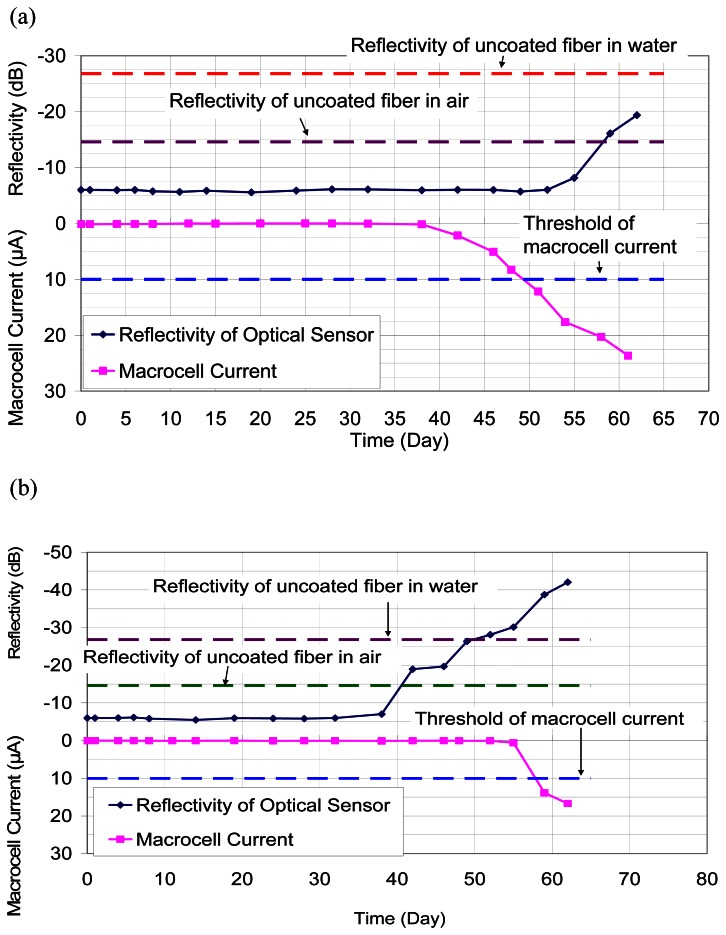
5.2.2. Modified ASTM G109 Test
By the tests of drilled hole of hardened cement mortar block, the packaged sensors are shown to be responsive to chloride ions. Here, the corrosion initiation time obtained from the sensors is compared to the macrocell current measurement by the standard ASTM G109 test. The results are shown in Figure 12. For the first specimen (Figure 12a), the macrocell current indicated the start of steel corrosion (when the macrocell current reaches 10 μA) at about the 50th day while the decrease of reflectivity was at about the 55th day. The difference is about 5 days. The time of corrosion initiation from the optical sensor and the macrocell current was very close. For the second specimen (Figure 12b), the reflectivity of the optical sensor showed significant drop at about the 42nd day while the macrocell current indicated that the steel corrosion started at about the 59th day. The difference is about 17 days. It should be pointed out that the difference in initiation time, which is significant for the experiment, may not be significant in practical applications. In practice, the initiation time of steel corrosion is over several years. After initiation, corrosion progresses at very slow rate. Even if the corrosion is detected a few months after it starts, there is little consequence.
Figure 12.
Results of standard ASTM G109 tests. (a) 1st specimen, (b) 2nd specimen.
5.2.3. Diffusion test
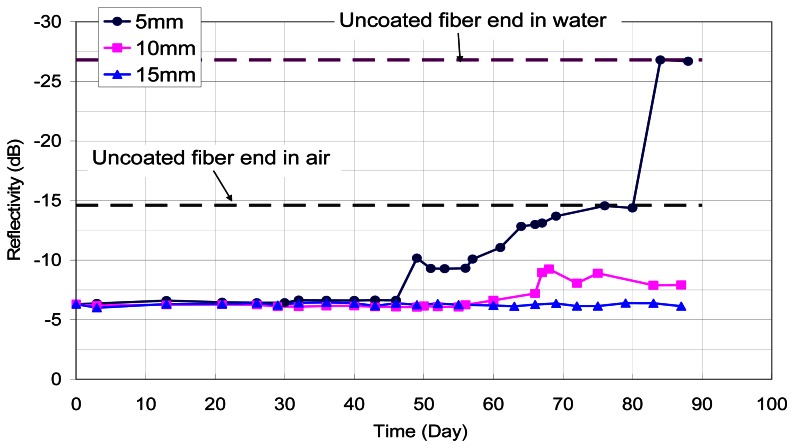
In this test, the penetration front of chloride ion is monitored by measuring the output of sensors at different distances to the specimen surface. The results are shown in Figure 13. For the sensor at 5 mm from the surface, the reflectivity started to drop at the 47th day, while the iron film was completely removed at the 77th day (when the reflectivity dropped to -14.6dB). The sensor at 10mm from the surface showed a significant drop of reflectivity at about the 67th day, while the reflectivity of the sensor at 15 mm from the surface remained constant up to about 90 days. It should be noted that the coated film thickness in this experiment is 400nm while that in the previous experiment is around 200 nm. Therefore, it is not surprising that the corrosion initiation time in this test is higher than that in the previous one, even through the distance of the fiber from the surface is smaller.
Figure 13.
Results of diffusion tests.
The test results, which show earlier initiation of corrosion at a smaller distance from the source of chlorides indicates the feasibility of corrosion front monitoring with the packaged sensors. However, the current results are mostly qualitative in nature. Further investigations are required to relate the corrosion initiation time to the distance from the surface, so theoretical extrapolation can be performed with measured results to predict the future penetration rate of the corrosion front. Also, experiments should be performed to study the response of the sensor under other corrosive environments, such as concrete with penetrated carbon dioxide or sulphur dioxide.
6. Conclusions
In this paper, we have presented a new fiber optic sensor for the detection of steel corrosion in concrete structures. The physical principle is based on the corrosion of an iron thin film at the end of an optical fiber, which will reduce the reflectivity from the fiber end. The sensing concept is verified by experiments. Practical sensor packaging method is proposed for embedding the sensor into existing and new concrete structures. Experimental results indicate that the sensor can detect the presence of chloride ions and the change in sensor output is dependent on the chloride concentration or moisture condition of the surrounding environment. The corrosion initiation time determine from the drop in sensor reflectivity is consistent with that obtained from standard macrocell current measurement. By placing sensors at different distances from a concrete surface exposed to chloride ions, the potential to monitor the penetration of chloride front inside a concrete member is demonstrated. While further work is certainly necessary to fully understand the correlation between the change in reflectivity and the corrosion process, the available experimental results do illustrate the feasibility and potential of the proposed sensing technique for practical applications.
Acknowledgments
Financial support of the work by the Hong Kong Research Grant Council, under CERG Project 616405, is gratefully acknowledged.
References
- 1.Broomfield J.P. Corrosion of Steel in Concrete. E & FN SPON; London: 1997. [Google Scholar]
- 2.Bentur A., Diamond S., Berke N.S. Steel Corrosion in Concrete. E & FN SPON; London: 1997. [Google Scholar]
- 3.Hausmann D.A. Steel Corrosion in Concrete. Materials Protection. 1967;6(11):19–22. [Google Scholar]
- 4.Diamond S. Chloride Concentrations in Concrete Pore Solutions Resulting from Calcium and Sodium Chloride Admixtures. Cement and Concrete Aggregates. 1986;8(2):97–102. [Google Scholar]
- 5.Schiessl P., Raupach M. Monitoring System for the Corrosion Risk for Steel in Concrete. Concr. Int. 1992;14(7):52–55. [Google Scholar]
- 6.Raupach M. Proceedings of 15th International Corrosion Congress. Frontiers in Corrosion Science and Technology; Granada: 2002. Corrosion Behaviour of the Reinforcement under On-Site-Conditions. Art.096. [Google Scholar]
- 7.Huston D.R., Fuhr P.L. Distributed and Chemical Fiber Optic Sensing and Installation in Bridges. Ansari F., editor. Fiber Optic Sensors for Construction Materials and Bridges. 1998:79–88. [Google Scholar]
- 8.Ghandehari M. Proceedings of the 15th ASCE Conference on Engineering Mechanics. New York: 2001. Ingress Monitoring in Concrete Structures. CD-ROM. [Google Scholar]
- 9.Habel W.R., Hofmann D. Fibre Optic Sensors for long-term SHM in Civil Engineering and geotechnique. ISHMII-3 Conference; Vancouver Canada. Nov. 2007. Art. 191. [Google Scholar]
- 10.Melhorn K., Flachsbarth J., Kowalsky W., Johannes H.H. Proceeding of the 6th IWSHM Conference. Stanford CA: 2007. Novel Sensors for long-term monitoring of pH and humidity in concrete; pp. 387–394. [Google Scholar]
- 11.ASTM International. West Conshohocken, PA: 2007. ASTM Standard G109. Standard Test Method for Determining Effects of Chemical Admixtures on Corrosion of Embedded Steel Reinforcement in Concrete Exposed to Chloride Environments. www.astm.org. [Google Scholar]
- 12.Weyers R.E., Prowell B.D., Sprinkel M.M. Concrete Bridge Protection, Repair, and Rehabilitation Relative to Reinforcement Corrosion: A Methods Application Manual. 1993 SHRP-S-360. [Google Scholar]
- 13.Tuutti K. Durability of Concrete on Saline Environment. Uppsala: 1996. Chloride induced corrosion in marine concrete structures; pp. 81–93. [Google Scholar]
- 14.Zhang J.Y., Lounis Z. Sensitivity analysis of simplified diffusion-based corrosion initiation model of concrete structures exposed to chlorides. Cem. Concr. Res. 2006;36:1312–1323. [Google Scholar]
- 15.Sinfield J.V., Germaine J.T., Hemond H.F. Journal of Geotechnical and Geoenvironmental Engineering. 12. Vol. 125. ASCE; 1999. Effects of soils on laser induced fluorescence of BTX contaminated pore waters; pp. 1072–1077. [Google Scholar]
- 16.Bogrekci I., Lee W.S. Design of a portable Raman sensor for phosphorus sensing in soils. ASAE Meeting Presentation; ASAE Annual International Meeting; Tampa, Florida. 17 - 20 July; 2005. p. 051040. [Google Scholar]