SYNOPSIS
Rac1, a small GTPase, regulates macrophage matrix metalloproteinase-9 (MMP-9) in an ERK- and SP-1-dependent manner. SP-1 contains a PEST domain that may modulate protein stability. We hypothesize that T578, S586, and/or S587 in the PEST domain are required for SP-1 stability and MMP-9 expression secondary to activation of ERK, a serine/threonine kinase. We determined the effects of Rac1 and ERK on MMP-9 expression driven by SP-1WT and SP-1 mutants, T578A, S586A and S587A. Expression of WT and mutant SP-1 increased MMP-9 promoter activity in alveolar macrophages. However, constitutively active Rac1 suppressed MMP-9 promoter activity in cells expressing SP-1WT, SP-1T578A, and SP-1S587A, but not SP-1S586A. Furthermore, constitutive ERK activation, which was inhibited by Rac1, significantly increased MMP-9 transcription in cells expressing SP-1WT but not SP-1S586A. Because Rac1 activation and ERK inactivation increased degradation of SP-1WT and not SP-1S586A, our results suggest that SP-1 stability mediated at S586 regulates MMP-9 transcription.. In vivo, alveolar macrophages obtained from asbestosis patients had less MMP-9 that was associated with decreased SP-1 expression and ERK activation. These observations demonstrate that S586 in the PEST domain of SP-1 is important for MMP-9 gene expression in alveolar macrophages and highlight the importance of these proteins in pulmonary fibrosis.
Keywords: Rac1, SP-1, ERK, MMP-9, macrophages, pulmonary fibrosis
INTRODUCTION
A hallmark of pulmonary fibrosis is aberrant matrix deposition characterized by an imbalance between the deposition and degradation of extracellular matrix proteins. Under normal conditions, there is a dynamic balance between the two processes that is regulated by a family of zinc-dependent endopeptidases known as matrix metalloproteinases (MMPs) [1]. However, under conditions of excessive remodeling of the extracellular matrix as seen in fibrosis, the precise role of MMPs in the development and maintenance of the pathology is unclear. Although some studies report that MMPs exacerbate fibrosis, others demonstrate their protective role [2–8].
Macrophages are an important cell type in the development of pulmonary fibrosis. Our previous observations suggest that macrophage-derived MMP-9 attenuated asbestos-induced pulmonary fibrosis [9]. In addition, we found MMP-9 production and activity was regulated at the transcriptional level by the small GTPase, Rac1 [9]. Deletion of Rac1 in macrophages significantly enhanced transcription of MMP-9 in vitro and in vivo. Furthermore, mice with a conditional deletion of Rac1 in their macrophages were protected from asbestos-induced pulmonary fibrosis [9, 10].
Specificity protein-1 (SP-1) is a ubiquitous transcription factor expressed in mammalian cells that regulates the transcription of genes involved in multiple cellular processes [11, 12]. SP-1 undergoes several post-transcriptional modifications, such as phosphorylation, ubiquitination, sumoylation, glycosylation, and acetylation, which regulate its transcriptional activity. It is one of many transcription factors that bind to the MMP-9 promoter to induce its transcription [9, 13]. We previously reported that Rac1 inhibits the transcriptional activation of the MMP-9 gene, in part, at its SP-1 binding site [9]. However, the mechanism of SP-1 regulation in pulmonary fibrosis is not known.
In the present study we addressed mechanisms by which Rac1 inhibited SP-1-mediated transcriptional activation of the MMP-9 gene. We identified a PEST domain in murine SP-1 containing one threonine (T578) and two serine (S586 and S587) residues that are potential sites of phosphorylation by serine/threonine kinases. Because the activation of ERK, a serine/threonine kinase, is inhibited by Rac1 [9] and phosphorylation within the PEST domain modulates protein stability [14–16], we hypothesized that Rac1 inhibits MMP-9 promoter activity secondary to suppression of ERK-mediated stabilization of SP-1 at the threonine and/or serine residues within its PEST domain. S→A mutation of the S586 residue, but not the threonine or other serine residues, prevented the modulation of the MMP-9 promoter by Rac1 and ERK. In addition, ERK activation increased the stability and abundance of SP-1, an effect that was mediated at the S586 residue. Furthermore, the importance of these proteins in pulmonary fibrosis was demonstrated ex vivo. MMP-9 expression was significantly less in alveolar macrophages obtained from asbestosis patients compared to normal subjects and was associated with lower levels of SP-1 and active ERK. These observations indicate that Rac1 inhibits MMP-9 gene expression in alveolar macrophages by attenuating the activation of ERK and ERK-mediated stabilization of SP-1 at the S586 residue within its PEST domain.
EXPERIMENTAL
Materials
U0126, anti-β-actin, goat anti-rabbit IgG-HRP, goat anti-mouse IgG-HRP, and cycloheximide were purchased from Sigma (St. Louis, MO). Anti-SP-1 was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-V5 was from Invitrogen (Carlsbad, CA). SimpleChIP enzymatic chromatin IP kit was from Cell Signaling Technology (Beverly, MA) and ChIP grade V5 was from abcam (Cambridge, MA).
Cells
WT and Rac1 null macrophages have been previously described [10]. The mouse alveolar macrophage cell line, MH-S, was obtained from ATCC (Manassas, VA) and were maintained according to the manufacturer’s instructions. All experiments were performed in media containing 0.5% serum.
Mice
Wild-type (WT) and Rac1 null mice have been described [10]. All protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. Chrysotile asbestos (100 µg in 50 µl of normal saline) was administered intra-tracheally to 6- to 10-week old mice after 2– 5 min of anesthesia that was induced by 3% isoflurane delivered via a Fortec vaporizer (Cyprane, Keighley, UK). After 28 days, mice were euthanized with an overdose of isoflurane, and bronchoalveolar lavage (BAL) was performed. BAL cells were used for the determination of total and differential cell counts. BAL fluid was used for the determination of MMP-9 activity.
Human Subjects
The Human Subjects Review Board of the Universityof Iowa Carver College of Medicine approved the protocol for obtaining alveolar macrophages from normal volunteers. Normal volunteers had to meet the following criteria: 1) age between 18 and 55 years; 2) no history of cardiopulmonary disease or other chronic disease; 3) no prescription or nonprescription medication except oral contraceptives; 4) no recent or current evidence of infection; and 5) lifetime nonsmoker. Alveolar macrophages were also obtained from patients with asbestosis. Patients with asbestosis met the following criteria: 1) FEV1 and DLCO at least 50% predicted; 2) current nonsmoker; 3) no recent or current evidence of infection; and 4) evidence of restrictive physiology on pulmonary function tests and interstitial fibrosis on chest computed tomography. Fiberoptic bronchoscopy with bronchoalveolar lavage was performed after subjects received intramuscular atropine, 0.6 mg, and local anesthesia. Three subsegments of the lung were lavaged with five 20-ml aliquots of normal saline, and the first aliquot in each was discarded. The percentage of alveolar macrophages was determined by Wright-Giemsa stain and varied from 90 to 98%.
Plasmids and Transfections
The entire coding sequence of murine SP-1 was directionally cloned into pcDNA3.1D/V5.His (Invitrogen, Carlsbad, CA). S→A mutations of SP-1 at T578, S586, and S587 was accomplished using QuikChange Lightning Multi Site Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA). The MMP-9 promoter reporter vectors have been previously described [9]. Constitutively active Rac1 (Q61L) in pUSEamp plasmid was from Millipore. Constitutively active pCMV-MEK1 and dominant negative pCMV-HA-ERK2 (K/A) plasmids (generous gifts from Dr. Roger Davis, University of Massachusettes, Worcester, MA) have been described previously [17]. Cells were transfected with Effectene Transfection Reagent or X-Treme Gene 9 Transfection Reagent, according to manufacturer’s instructions. To correct for transfection efficiency cells were co-transfected with phL-TK Renilla luciferase vector (Promega, Madison, WI). Firefly and Renilla luciferase activities were assayed in cell lysates using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI).
Quantitative RT-PCR
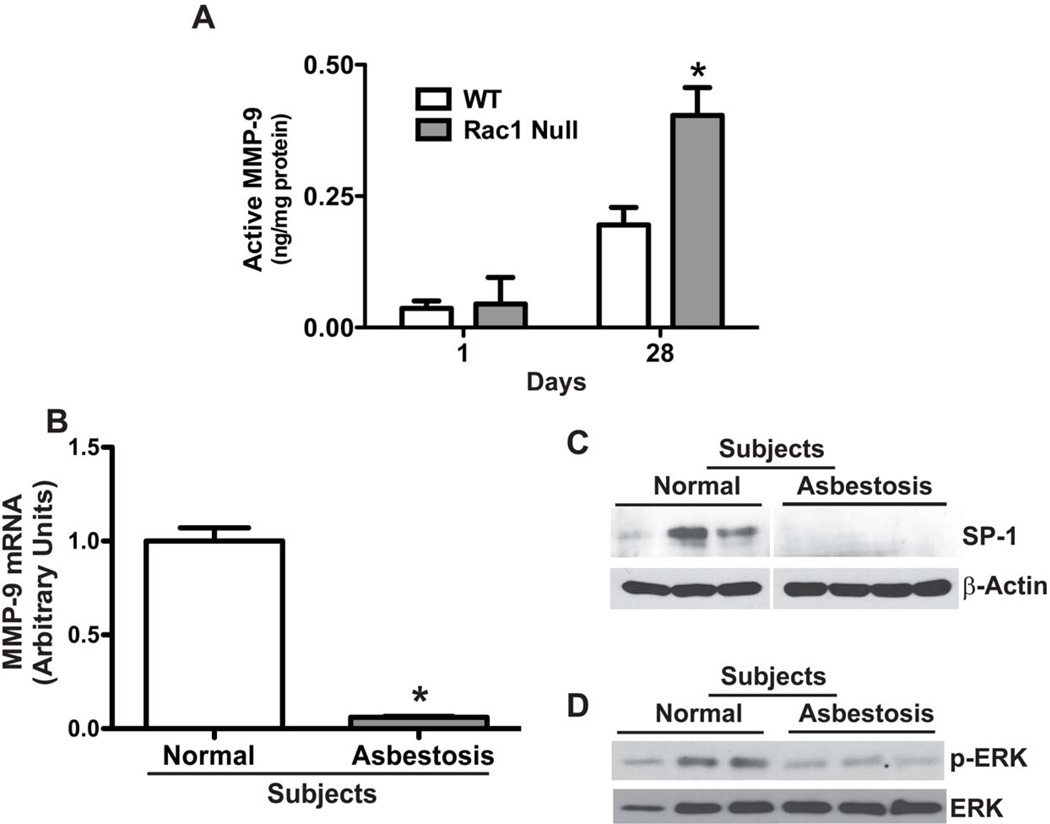
Total RNA from BAL cells obtained from normal subjects and asbestosis patients was isolated by TRIzol, DNase treated, and reverse transcribed using the reverse transcriptase kit Iscript (Bio-Rad Laboratories, Hercules, CA). MMP-9 and hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA transcripts were determined by quantitative real-time PCR using SYBR Green (Bio-Rad Laboratories) and respective primers on an IQ5 real-time PCR machine (Bio-Rad Laboratories). The following primers were used: 5′-GGCAAGGGCGTCGTGGTTCC-3’ (forward) and 5′-TCCGTGGTGCAGGCGGAGTA-3′ (reverse) for human MMP-9 and 5’-AGCCCTGGCGTCGTGATTAGTGA-3’ (forward) and 5’-TGTCCCCTGTTGACTGGTCATTACA-3’ (reverse) for human HPRT. Data were calculated by the cycle threshold (ΔΔCT) method. MMP-9 mRNA was normalized to HPRT and expressed as arbitrary units.
Chromatin Immunoprecipitation (ChIP) Assay
MH-S cells were transfected with pcDNA3.1 (Empty) or pcDNA3.1V5.His vectors expressing SP-1WT, SP-1T578A, SP-1S586A, SP-1S587A. After an overnight incubation, nuclei were isolated and subjected to chromatin digestion using the SimpleChIP™ enzymatic chromatin IP kit as previously described [9]. The resulting cross-linked chromatin preparations were used for input controls (2% of total) or for immunoprecipitation using anti-V5 (abcam), anti-Histone 3 (Cell Signaling) as a positive control or normal rabbit IgG antibody (Cell Signaling) as a negative control. Chromatin/immune complexes were eluted, and the chromatin was subjected to reversal of cross-links followed by DNA purification as described in the protocol. DNA was analyzed by quantitative real-time PCR on an IQ5 real-time PCR machine (Bio-Rad Laboratories) using Sybr Green (Bio-Rad Laboratories) and the following primers: 5′-GCT CCC ACA TGT GTG TGT C-3′ and 5′-CCT AGC TCC AGC AGG CTG for the murine MMP-9 SP-1 binding site (76-bp product) and RPL30 primers (Cell Signaling) for murine ribosomal protein gene locus (159-bp product). PCR products were resolved on a 2% agarose gel. Results were calculated as arbitrary units relative to Empty vector transfected cells (control).
Electrophoretic Mobility Shift Assays (EMSA)
Nuclear proteins were extracted as previously described from MH-S cells transfected with either empty pcDNA3.1, WT SP-1 or mutant SP-1 vectors [18]. Consensus SP-1 (5’-ATT CGA TCG GGG CGG GGC GAG C-3’) oligonucleotide was labeled with [γ-32P]ATP (NEN Life Science Products) and allowed to bind to 12.5 µg of nuclear proteins as previously described [18]. Protein-DNA complexes were separated on a 5% non-reducing polyacrylamide gel which was dried and exposed to X-ray film.
Immunoblot Analysis
Nuclei were isolated as described [18]. Nuclear extracts, cytosol, and whole cell lysates were separated by SDS-PAGE and used for immunoblot analyses as described [19].
Measurement of MMP-9 Activity
Total and pro-MMP-9 were determined in BAL fluid isolated from asbestos-exposed mice using mouse quantikine ELISA kits (R&D Systems, Minneapolis, MN). Active MMP-9 was calculated as the difference between total and pro-MMP-9.
Statistical Analyses
Statistical comparisons were performed using either an unpaired, one-tailed t test or one-way analysis of variance followed by Tukey's t test. Values in the figures are expressed as means ± S.E., and p < 0.05 was considered to be significant.
RESULTS
Rac1 regulates SP-1-mediated MMP-9 gene expression at S586
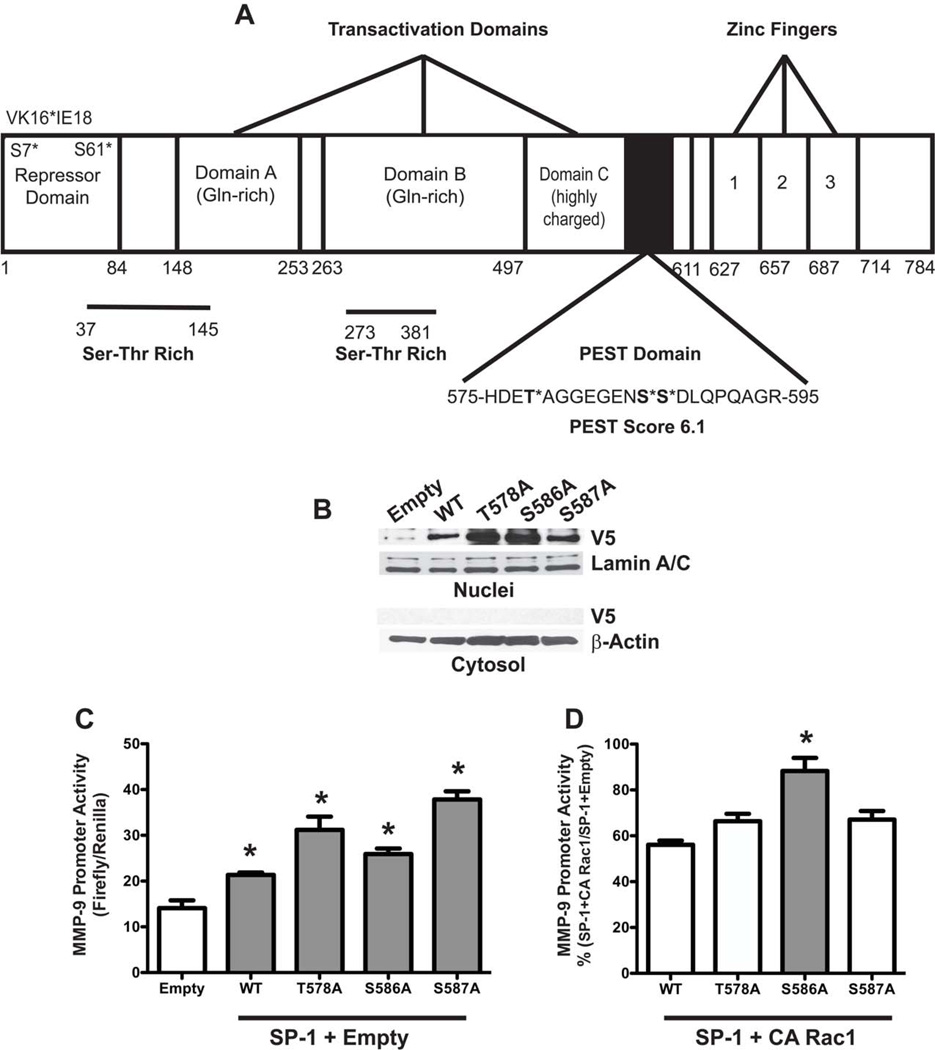
Previous observations show that Rac1 modulates MMP-9 expression and activity in macrophages, in part, by decreasing SP-1 transcriptional activity and nuclear abundance [9]. The mechanism by which this occurs is not known. Stability of proteins is often determined by a PEST domain, a region rich in amino acids proline (P), glutamic acid (E), serine (S), and threonine (T). A PEST domain score greater than 5 typically targets proteins for degradation [20]. We identified a PEST domain (575–595) in murine SP-1 having a score of 6.1 that contains three potential sites of phosphorylation by serine/threonine kinases, T578, S586, and S587 (Figure 1A). ERK-mediated phosphorylation of amino acid residues within the PEST domain is known to stabilize proteins [14, 15]. We hypothesized that Rac1 inhibits MMP-9 transcription by increasing SP-1 degradation and thereby decreasing its transcriptional activity. To address this, we generated T→A and S→A mutations of SP-1 at T578, S586, and S587 residues and transfected alveolar macrophages with vectors expressing these mutants. SP-1WT and the three SP-1 mutants were predominantly expressed in the nucleus with non-detectable levels observed in the cytoplasm (Figure 1B). To examine the effects of the PEST domain of SP-1 on MMP-9 promoter activity, macrophages were transfected with SP-1WT or the SP-1 PEST domain mutants together with a reporter plasmid driven by the MMP-9 promoter. Cells expressing the SP-1WT and the SP-1 mutants significantly enhanced MMP-9 promoter activity compared to cells expressing the empty vector (Figure 1C). Because recombinant SP-1 expression increased MMP-9 expression, we next evaluated the effect of Rac1. Macrophages were transfected with SP-1WT or the SP-1 PEST domain mutants, a constitutively active (CA) Rac1 vector, and the MMP-9 promoter reporter vector. Cells transfected with CA Rac1 significantly decreased SP-1-mediated MMP-9-luciferase in cells expressing SP-1WT (Figure 1D). A similar effect was observed with SP-1T578A and SP-1S587A mutants. In contrast, MMP-9 promoter activity was unchanged by CA Rac1 in cells expressing SP-1S586A suggesting that the S586 residue is important for MMP-9 gene expression in macrophages.
Figure 1. Rac1 regulates SP-1 driven MMP-9 gene expression at S586 in the PEST domain.
(A) Schematic of murine SP-1. Threonine and serine residues in the PEST domain are shown in bold. (B) Macrophages were transfected with Empty vector or vectors containing SP-1WT, SP-1T578A, SP-1S586A, or SP-1S587A. (C) Macrophages were co-transfected with MMP-9 luciferase and empty, SP-1WT, SP-1, T578A, SP-1S586A, or SP-1S587A vectors with an empty vector. Twenty four hours after transfection, cells were lysed and luciferase activity determined. Results show Mean±SEM of firefly luciferase normalized to Renilla. n=3, *p < 0.05. (D) Macrophages were co-transfected with MMP-9 luciferase and SP-1WT, SP-1T578A, SP-1S586A, or SP-1S587A vectors with a constitutively active (CA) Rac1 vector. Results are expressed as % (SP-1+CA Rac1/SP-1+Empty) MMP-9 promoter activity. n=3, *p < 0.019 S586A compared to all other constructs.
Recombinant SP-1 binds to the endogenous MMP-9 promoter
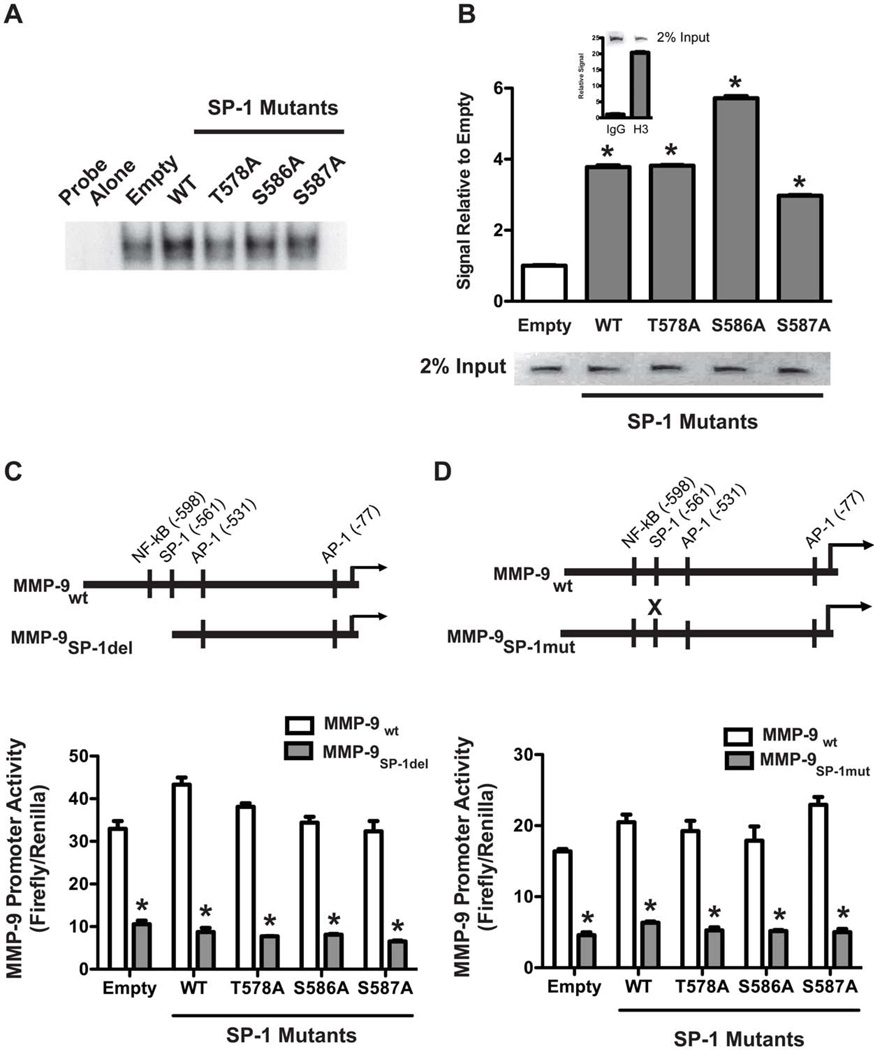
To investigate whether the difference in MMP-9 promoter activity was due to an alteration in DNA binding, we determined binding of WT and mutant SP-1 to a consensus SP-1 oligonucleotide. Compared to cells transfected with empty pcDNA3.1, all of the SP-1 vectors had an increase in binding to the SP-1 oligonucleotide compared to cells expressing the empty vector, although there was a slight reduction in the binding of the T578A mutant compared to the other vectors (Figure 2A).
Figure 2. WT and mutant SP-1 bind to SP-1 sites on the MMP-9 promoter.
(A). MH-S cells were transfected with pcDNA3.1.V5.His vectors expressing SP-1WT, SP-T578A, SP-1S586A or SP-1S587A. Control cells were transfected with pcDNA3.1 (empty). Twenty four hours later, nuclei were isolated and subjected to chromatin immunoprecipitation with anti-V5, anti-histone 3 (H3) antibodies or normal rabbit IgG as described in Methods. Immunoprecipitated purified DNA was amplified by real-time PCR using Sybr Green and primers directed against SP-1 binding sites on the MMP-9 promoter or histone binding RPL30 gene. Results show Mean±SEM of signal relative to control and gel separation of PCR products (inset). N=3. (B) MH-S cells were transfected as in A. Nuclear proteins were subjected to EMSA using 32P-labeled consensus SP-1 oligonucleotide as described in Methods. A representative autoradiogram from one out of two representative experiments are shown. (C&D). MH-S cells were transfected with empty, WT or mutant SP-1 vectors together with WT MMP-9 promoter (#2)-, truncated MMP-9 promoter (#4)-(C) or mutated MMP-9 promoter-(D) driven luciferase vectors. Schematic of the MMP-9 promoters is shown (inset). Twenty four hours after transfection, luciferase activity was determined. Results show Mean±SEM of firefly luciferase normalized to Renilla. n=3, *p < 0.05.
To more specifically determine the DNA binding ability of the SP-1 vectors, we evaluated binding to the endogenous MMP-9 promoter. Cells were transfected with and empty vector or with the SP-1WT, SP-1T578A, SP-1S586A, or SP-1S587A vectors. Nuclei were isolated from these cells and analysed by ChIP assay using anti-V5 antibody to pull down chromatin bound to V5-tagged SP-1. Subsequent real-time PCR analysis of purified chromatin DNA with primers specific for the SP-1 binding site on the MMP-9 promoter revealed that SP-1WT and the mutant SP-1 vectors bound with greater affinity to the MMP-9 promoter relative to cells expressing the empty vector (Figure 2B).
To determine the specificity of SP-1-driven MMP-9 promoter activity, MMP-9 promoter constructs with the SP-1 binding site either deleted or mutated were evaluated. Macrophages were transfected with the SP-1 vectors together with the MMP-9 promoter reporter constructs. WT and mutant SP-1 increased WT MMP-9 promoter activity compared to cells expressing the empty vector (Figure 2C–D). In contrast, luciferase activity driven by the truncated MMP-9 promoter with its SP-1 site deleted (MMP-9SP-1del) was markedly diminished in all cells expressing either empty, WT, or SP-1 mutants (Figure 2C). Similar results were obtained with the MMP-9 promoter with its SP-1 binding site mutated (MMP-9SP-1mut). Promoter activity observed with this vector was dramatically less than WT MMP-9 promoter in all cells regardless of whether they were transfected with WT or SP-1 mutants. Taken together, these results establish the specificity of recombinant SP-1-driven MMP-9 gene expression.
ERK inhibition by Rac1 modulates SP-1-mediated MMP-9 gene expression
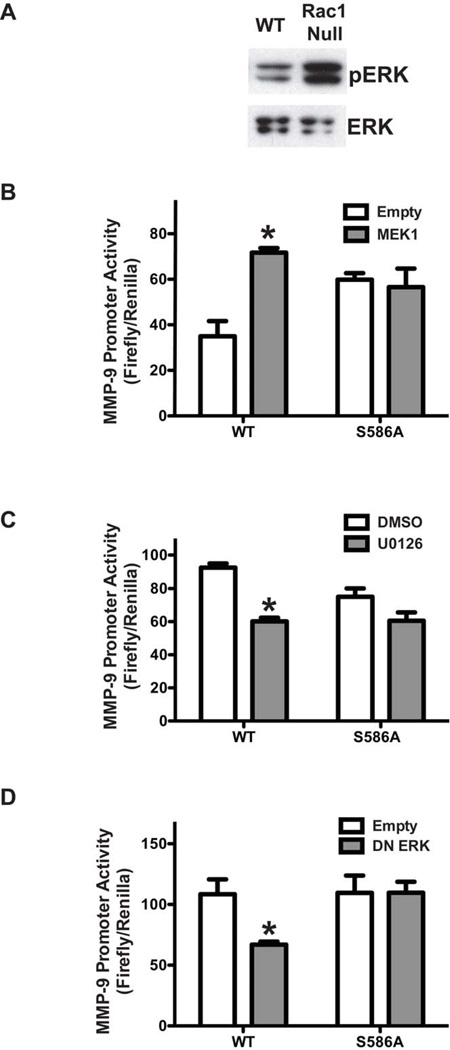
To address whether Rac1 inhibited MMP-9 transcription secondary to ERK inactivation, we first determined the effect of Rac1 on ERK activation. WT and Rac1 null macrophages were assayed for levels of activated p-ERK and found that p-ERK was significantly lower in WT cells compared to Rac1 null macrophages (Figure 3A). Because ERK is known regulate SP-1 transcriptional activity [9, 21–23], we asked if ERK could regulate SP-1-driven MMP-9 promoter activity. Macrophages were co-transfected with a MMP-9 promoter reporter and either SP-1WT or SP-1S586A in combination with either an empty vector or the constitutively active MEK1, which is the upstream kinase that activates ERK. Compared to cells transfected with the empty vector alone, MEK1 significantly increased MMP-9 promoter activity in cells expressing SP-1WT but not SP-1S586A mutant (Figure 3B). To verify the role of ERK, cells were co-transfected with the MMP-9 promoter vector together with either SP-1WT or SP-1S586A. Twenty-four hours later cells were incubated with the MEK1 inhibitor, U0126 for 7 hours. U0126 significantly reduced MMP-9 transcription in cells expressing SP-1WT but not the SP-1S586A mutant (Figure 3C). Similarly, macrophages transfected with a dominant-negative ERK (pCMV-HA-ERK2 (K/A)) vector decreased MMP-9 promoter activity in cells over expressing SP-1WT but not in cells transfected with SP-1S586A mutant (Figure 3D). Taken together, these results strongly suggest that Rac1 regulates MMP-9 gene expression by inhibition of ERK, which modulates SP-1 at S586.
Figure 3. ERK regulates SP-1-driven MMP-9 gene expression at S586.
(A) Whole cell lysates were obtained from WT and Rac1 null macrophages. (B) Macrophages were cotransfected with MMP-9-luciferase and either empty or constitutively active MEK1 in combination with either SP-1WT or SP-1S586A. n=3, *p < 0.05. (C) Macrophages were transfected with MMP-9-luciferase and twenty four hours later, cells were incubated with U0126 (10µM) or 0.1% DMSO for 6 hrs. Results show Mean±SEM of firefly luciferase normalized to Renilla. n=8, *p<0.05. (D) Rac1 null macrophages were co-transfected with MMP-9-luciferase and dominant negative (DN)-ERK and SP-1WT or SP-1S586A vectors. Results show Mean±SEM of firefly luciferase normalized to Renilla. n=3, *p < 0.05.
ERK increases the stability of SP-1 at S586
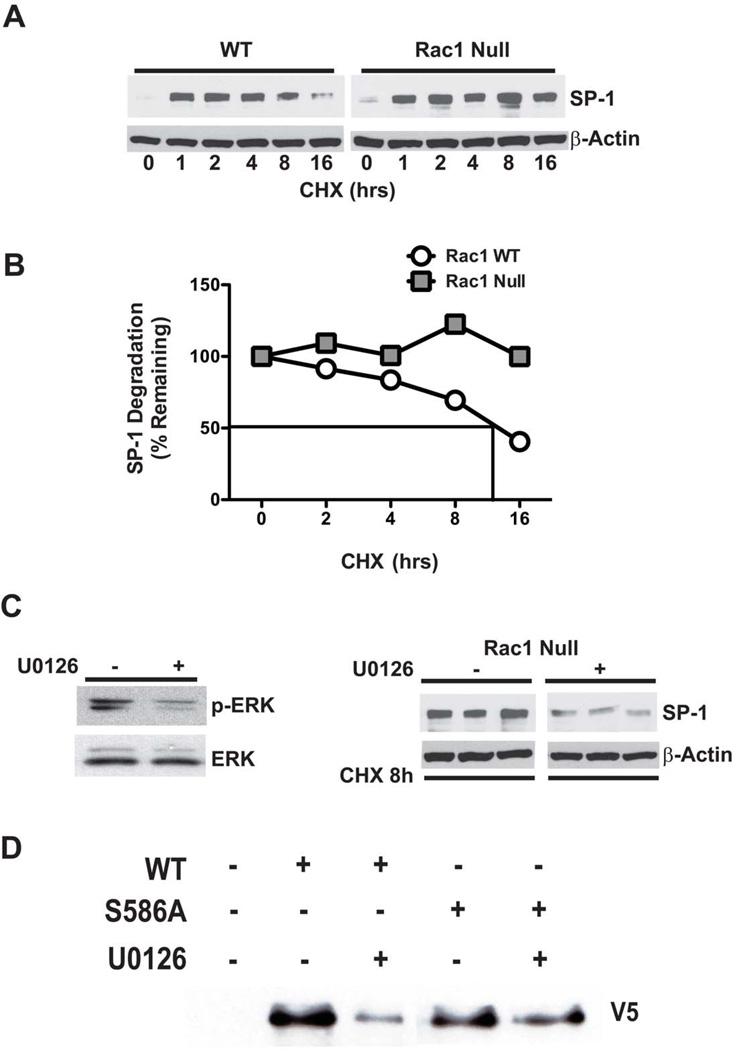
ERK phosphorylation of serine and/or threonine residues within the PEST domain is linked to increased stability of proteins [14, 15]. To address whether SP-1 stability can be regulated by ERK, we first determined the rate of SP-1 degradation in WT and Rac1 null macrophages incubated with cycloheximide, a general inhibitor of protein translation, for up to 16 hours. The abundance of SP-1 was determined in cells by immunoblot analysis. SP-1 degradation in WT cells decreased in a time-dependent manner in the presence of cycloheximide, whereas there was no evidence of degradation of SP-1 in Rac1 null macrophages (Figure 4A). The t1/2 of native SP-1 in WT cells was about 12 hours, whereas the t1/2 of native SP-1 in Rac1 null cells was greater than 16 hours (Figure 4B). To determine whether the rate of SP-1 degradation was due to ERK inactivation, we inhibited ERK in Rac1 null cells using U0126 and measured SP-1 degradation in the presence of cycloheximide. ERK inhibition significantly decreased the abundance of SP-1 in the absence of protein synthesis in Rac1 null cells suggesting that ERK activation increases SP-1 stability (Figure 4C). To confirm that ERK acted on SP-1 S586, cells were transfected with either SP-1WT or SP-1S586A. Whole cell lysates were subjected to affinity purification, and the expression of the V5 tag following U0126 exposure was determined. ERK inactivation by U0126 significantly decreased the expression of SP-1WT but not the expression of SP-1S586A (Figure 4D).
Figure 4. ERK activation increases stability of the SP-1 protein.
(A) WT and Rac1 null macrophages were incubated with 10µM of cycloheximide (CHX) for the indicated times. (B) Densitometric analysis of representative experiments of SP-1 decay in WT and Rac1 null macrophages. (C) Rac1 null cells were incubated for 18 hrs with 0.1% DMSO or U0126 (10µM) and then treated for 8 hrs with CHX (10µM). Immunoblot analysis for p-ERK and SP-1 was performed. (D) Macrophages were transfected with an empty, SP-1WT, or SP-1S586A. Twenty-four hours later cells were cultured in the presence or absence of U0126 for 8 hrs. Immunoblot analysis was performed after affinity purification.
Alveolar macrophages from asbestosis patients have less MMP-9, SP-1, and active ERK
Because Rac1 null mice express high levels of MMP-9 in their alveolar macrophages and do not develop asbestos-induced pulmonary fibrosis [9], we determined MMP-9 activity in BAL fluid from WT and Rac1 null mice after exposure to chrysotile asbestos. Although there was no significant difference in MMP-9 activity one day after exposure, there was a significant increase in activity in BAL from Rac1 null mice at 28 days post-exposure (Figure 5A). Our previous observations demonstrate that macrophages constitute the majority of cells when fibrosis becomes evident [10]. Therefore these data suggest that increased macrophage-derived MMP-9 expression and activity in Rac1 null mice contribute, in part, to protection from development of pulmonary fibrosis.
Figure 5. Decreased MMP-9 and SP-1 expression and ERK activation in asbestosis patients.
(A) WT and Rac1 null mice were intratracheally administered 100 µg chrysotile asbestos. Animals were euthanized at one or 28 days after exposure, and BAL was obtained. Active MMP-9 was determined by measuring the difference between total and pro-MMP-9 which were determined. N=4 (WT and Rac1 null); *p < 0.041. (B) Total RNA was isolated from alveolar macrophages obtained from normal subjects (n=3) or patients with asbestosis (n=3). MMP-9 mRNA expression was measured by real-time PCR. * p < 0.001. (C–D) Alveolar macrophages from asbestosis patients (n=3) and normal subjects (n=3) were lysed and subjected to immunoblot analysis for the determination of SP-1 and phosphorylated ERK.
To further address the biological significance of MMP-9, we determined MMP-9 expression in alveolar macrophages isolated from asbestosis patients. Compared to normal subjects, MMP-9 mRNA was about 16-fold less in alveolar macrophages obtained from asbestosis patients (Figure 5B). To determine if our in vitro data was recapitulated in patients, we investigated whether patients with asbestosis have lower levels of SP-1 expression and decreased ERK activation in alveolar macrophages. The expression of the SP-1 (Figure 5C) and phosphorylated ERK (Figure 5D) were significantly decreased in alveolar macrophages from asbestosis patients compared to normal subjects. In aggregate, these results demonstrate that Rac1, SP-1, and ERK are important in the regulation of MMP-9 expression in alveolar macrophages.
DISCUSSION
Asbestosis, which is the most debilitating asbestos-related lung disease, is a prototypical form of pulmonary fibrosis. Despite tight regulatory controls to limit exposure, more than 1.3 million workers are exposed to hazardous levels annually [24, 25]. A distinguishing feature of pulmonary fibrosis is aberrant matrix deposition that results from alteration in the balance between matrix deposition and degradation [26]. MMPs have been implicated in the development of fibrosis, but their precise role has not been determined [2–8]. Several studies suggest that MMPs increase pulmonary fibrosis, whereas other studies suggest that inhibition or deletion of MMPs may alleviate the development of fibrosis [3–5, 7, 27]. We previously showed that Rac1 is required for the development of pulmonary fibrosis in mice exposed to asbestos [9]. Because the production of the macrophage-derived MMP-9 is inhibited by Rac1, these results suggest that the effects of Rac1 on fibrosis development are mediated, in part, by an inhibition of MMP-9 production. In the current study we demonstrated that MMP-9 expression is dependent on post-translational regulation of SP-1. Rac1 inhibited MMP-9 gene transcription by inhibiting ERK activation and increasing SP-1 degradation. We report a novel finding that ERK activation increases SP-1 stability at S586 and, thereby, increases MMP-9 promoter activity. These results establish, for the first time, the molecular mechanism by which Rac1 modulates the matrix remodeling enzyme MMP-9 via ERK and SP-1 and suggest that this mechanism could account for the fibrotic phenotype observed in asbestosis (Figure 6).
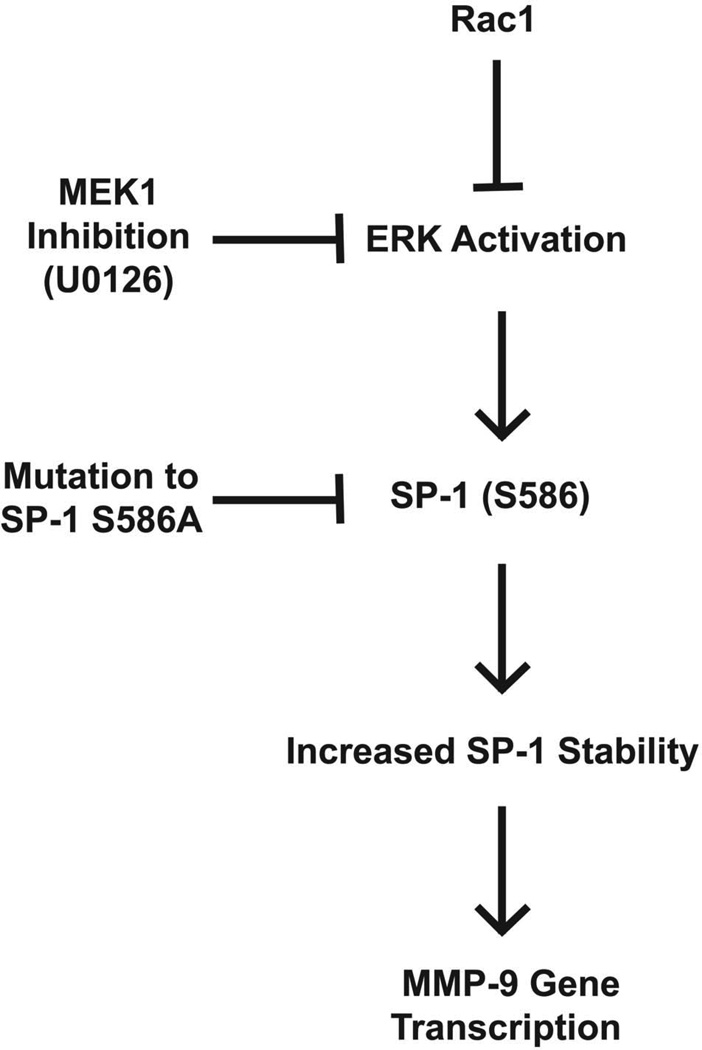
Figure 6. Schematic of the effects of Rac1, SP-1, and ERK on MMP-9 expression.
Rac1 decreases SP-1 protein stability by inhibition of ERK. ERK modulates SP-1 degradation via S586 in the PEST domain. SP-1 is critical for MMP-9 expression in alveolar macrophages. This provides a mechanism by which Rac1 modulates macrophage-derived MMP-9 expression and activity.
SP-1 is a ubiquitously expressed transcription factor that belongs to the C2H2-type zinc-finger protein family. It binds to GC-rich motifs on the promoters of various genes to either enhance or repress transcription. It is known to be highly regulated by post-translational modifications such as phosphorylation, ubiquitination, sumoylation, acetylation, and O-glycosylation that regulate its transcriptional activity [23, 28–31]. Phosphorylation modulates the transcriptional activity of SP-1 by regulating its stability [30, 32]. Proteins having a region rich in amino acids proline (P), glutamine (E) or asparagine (D), serine (S), and threonine (T) are often targeted to be rapidly degraded [20]. We identified a PEST domain in murine SP-1 between amino acids 575 and 595 containing one threonine (T578) and two serine (S586 and S587) residues that are potential sites of phosphorylation by serine/threonine kinases. Phosphorylation within the PEST domain of serine/threonine residues stabilizes proteins as observed with ERK phosphorylation of serine residues in A170, MKP-7, and MCL1 [14–16]. Although multiple kinases phosphorylate SP-1 at its many serine and threonine residues to either increase or decrease its transcriptional activity, none have been shown to phosphorylate SP-1 on residues within its PEST domain [12]. Because our previous results demonstrate the involvement of ERK in Rac1-mediated suppression of MMP-9 gene expression [9], we questioned whether ERK altered SP-1 stability and the transcriptional activation of SP-1 by modulating the PEST domain. To our knowledge, our observations are the first to suggest that ERK regulates SP-1 stability within the PEST domain and consequentially, its transcriptional activation of the MMP-9 promoter.
SP-1 has been shown to be phosphorylated by MAP kinases only on threonine residues [23, 32]. JNK phosphorylates SP-1 on threonine residues, T278 and T739, during mitosis and protects SP-1 from ubiquination and degradation, which results in increased transcriptional activation of the 12(S)-lipoxygenase gene [32]. ERK phosphorylates SP-1 on T453 and T739, which is associated with increased VEGF transcription or decreased FGF-mediated PDGFR alpha gene expression [21, 23]. Our results demonstrate that the S586 residue within the PEST domain is another site of ERK action on SP-1. Furthermore, while other serine residues have been shown to mediate SP-1 degradation [30, 32], the involvement of the S586 residue in SP-1 protein stability has not been reported. Previous reports link SP-1 degradation to post-translational modifications such as sumoylation and/or ubiquitination or phosphorylation [30, 32]. Phosphorylation of serine 7 results in SP-1 ubiquitination and degradation [30], whereas phosphorylation of serine 59 regulates sumoylation and ubiquitination of SP-1 [32]. Given that PEST domains target proteins for ubiquitination and subsequent degradation, it is likely that phosphorylation of S586 regulates proteasomal targeting [20].
Our observations do not rule out the role of other regions in the SP-1 protein that modulate MMP-9 transcription. The phosphorylation of SP-1 at serine 7 results in ubiquitination and subsequent degradation [33]. We found that MMP-9 promoter activity increased with a mutation of serine 7 to alanine (data not shown). In addition, mutation of lysine 16 to arginine also enhanced MMP-9 promoter activity (data not shown). This is consistent with the previous report that sumoylation at this residue represses SP-1 transcriptional activity {}. However, our observations indicate that constitutively active Rac1 did not alter the MMP-9 expression in cells expressing these SP-1 mutants (data not shown). Interactions between residues on the SP-1 protein have been shown to control protein activity [33, 34]. Interestingly, mutations of T578, S586, and S587 increased basal MMP-9 gene transcription. It is likely that these residues either modulate one another’s effect and/or cooperate with other regions in the protein to regulate the overall functional activity of SP-1.
A novel finding of this study is that asbestosis patients were found to have less MMP-9 expression in alveolar macrophages compared to normal subjects. Moreover, the modulation of MMP-9 by Rac1 is supported by our recent data showing that alveolar macrophages from asbestosis patients have increased Rac1 activity compared to normal subjects [35]. This, together with our observation that asbestos-exposed Rac1 null mice have greater MMP-9 expression and activity supports the notion that macrophage-derived MMP-9 is important in matrix remodeling. In aggregate, this study reveals that SP-1 (S586), Rac1, and ERK are potential biomarkers for pulmonary fibrosis.
ACKNOWLEDGEMENTS
FUNDING
This work was supported by National Institutes of Health grants ES015981, ES014871, and a Merit Review from the Department of Veterans Affairs.
Footnotes
Author contributions: Conception and design, ABC and SM; acquisition of data, SM, AJR, and ABC; analysis and interpretation of data, SM, AJR, and ABC; writing and critical review of manuscript, SM and ABC; final approval of version to be published, ABC.
None of the authors have a conflict of interest.
REFERENCES
- 1.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera S, Gaxiola M, Arreola JL, Ramirez R, Jara P, D'Armiento J, Richards T, Selman M, Pardo A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int J Biochem Cell Biol. 2007;39:2324–2338. doi: 10.1016/j.biocel.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Jiang HD, Guan HS. MS80, a novel sulfated oligosaccharide, inhibits pulmonary fibrosis by targeting TGF-β1 both in vitro and in vivo. Acta Pharmacol Sin. 2009;30:973–979. doi: 10.1038/aps.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. Transforming growth factor (TGF)-β1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 5.Karakiulakis G, Papakonstantinou E, Aletras AJ, Tamm M, Roth M. Cell type-specific effect of hypoxia and platelet-derived growth factor-BB on extracellular matrix turnover and its consequences for lung remodeling. J Biol Chem. 2007;282:908–915. doi: 10.1074/jbc.M602178200. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. Faseb J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 7.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 8.Tan RJ, Lee JS, Manni ML, Fattman CL, Tobolewski JM, Zheng M, Kolls JK, Martin TR, Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol. 2006;34:226–232. doi: 10.1165/rcmb.2005-0212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated Mitochondrial H2O2, Generation Regulates MMP-9 Gene Expression in Macrophages via Inhibition of SP-1 and AP-1. J Biol Chem. 2010;285:25062–25073. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, Metwali N, Meyerholz DK, Wang Y, Glogauer M, Thorne PS, Carter AB. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L846–L855. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruzalegui FH, Cano E, Treisman R. ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene. 1999;18:7948–7957. doi: 10.1038/sj.onc.1203362. [DOI] [PubMed] [Google Scholar]
- 12.Cheeran MC, Hu S, Gekker G, Lokensgard JR. Decreased cytomegalovirus expression following proinflammatory cytokine treatment of primary human astrocytes. J Immunol. 2000;164:926–933. doi: 10.4049/jimmunol.164.2.926. [DOI] [PubMed] [Google Scholar]
- 13.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 14.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 15.Katagiri C, Masuda K, Urano T, Yamashita K, Araki Y, Kikuchi K, Shima H. Phosphorylation of Ser-446 determines stability of MKP-7. J Biol Chem. 2005;280:14716–14722. doi: 10.1074/jbc.M500200200. [DOI] [PubMed] [Google Scholar]
- 16.Yanagawa T, Yuki K, Yoshida H, Bannai S, Ishii T. Phosphorylation of A170 stress protein by casein kinase II-like activity in macrophages. Biochem Biophys Res Commun. 1997;241:157–163. doi: 10.1006/bbrc.1997.7783. [DOI] [PubMed] [Google Scholar]
- 17.Wartmann M, Davis RJ. The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J Biol Chem. 1994;269:6695–6701. [PubMed] [Google Scholar]
- 18.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 19.Carter AB, Hunninghake GW. A constitutive active MEK-->ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J Biol Chem. 2000;275:27858–27864. doi: 10.1074/jbc.M003599200. [DOI] [PubMed] [Google Scholar]
- 20.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 21.Bonello MR, Khachigian LM. Fibroblast growth factor-2 represses platelet-derived growth factor receptor-alpha (PDGFR-α) transcription via ERK1/2-dependent Sp1 phosphorylation and an atypical cis-acting element in the proximal PDGFR-α promoter. J Biol Chem. 2004;279:2377–2382. doi: 10.1074/jbc.M308254200. [DOI] [PubMed] [Google Scholar]
- 22.Hsu MC, Chang HC, Hung WC. HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J Biol Chem. 2006;281:4718–4725. doi: 10.1074/jbc.M510937200. [DOI] [PubMed] [Google Scholar]
- 23.Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two SP1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–20639. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- 24.Attfield MD, Wood JM, Antao VC, Pinheiro GA. Changing patterns of pneumoconiosis mortality--United States, 1968–2000. MMWR Morb Mortal Wkly Rep. 2004;53:627–632. [PubMed] [Google Scholar]
- 25.Guidotti TL, Miller A, Christiani D, Wagner G, Balmes J, Harber P, Brodkin CA, Rom W, Hillerdal G, Harbut M, Green FHY. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 26.Cook DN, Brass DM, Schwartz DA. A matrix for new ideas in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:122–124. doi: 10.1165/ajrcmb.27.2.f245. [DOI] [PubMed] [Google Scholar]
- 27.Tan RJ, Fattman CL, Niehouse LM, Tobolewski JM, Hanford LE, Li Q, Monzon FA, Parks WC, Oury TD. Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice. Am J Respir Cell Mol Biol. 2006;35:289–297. doi: 10.1165/rcmb.2005-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung JJ, Wang YT, Chang WC. SP1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol Cell Biol. 2006;26:1770–1785. doi: 10.1128/MCB.26.5.1770-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumdar G, Harrington A, Hungerford J, Martinez-Hernandez A, Gerling IC, Raghow R, Solomon S. Insulin dynamically regulates calmodulin gene expression by sequential o-glycosylation and phosphorylation of SP1 and its subcellular compartmentalization in liver cells. J Biol Chem. 2006;281:3642–3650. doi: 10.1074/jbc.M511223200. [DOI] [PubMed] [Google Scholar]
- 30.Spengler ML, Guo LW, Brattain MG. Phosphorylation mediates SP1 coupled activities of proteolytic processing, desumoylation and degradation. Cell Cycle. 2008;7:623–630. doi: 10.4161/cc.7.5.5402. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to SP1 activation domain inhibits its transcriptional capability. Proc Nat Acad Sci USA. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu TC, Wang Z, Feng X, Chuang P, Fang W, Chen Y, Neves S, Maayan A, Xiong H, Liu Y, Iyengar R, Klotman PE, He JC. Retinoic acid utilizes CREB and USF1 in a transcriptional feed-forward loop in order to stimulate MKP1 expression in human immunodeficiency virus-infected podocytes. Mol Cell Biol. 2008;28:5785–5794. doi: 10.1128/MCB.00245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spengler ML, Brattain MG. Sumoylation inhibits cleavage of SP1 N-terminal negative regulatory domain and inhibits Sp1-dependent transcription. J Biol Chem. 2006;281:5567–5574. doi: 10.1074/jbc.M600035200. [DOI] [PubMed] [Google Scholar]
- 34.Wang YT, Yang WB, Chang WC, Hung JJ. Interplay of Posttranslational Modifications in SP1 Mediates SP1 Stability during Cell Cycle Progression. J Mol Biol. 2011;414:1–14. doi: 10.1016/j.jmb.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, Sieren JC, Spitz DR, Carter AB. Mitochondrial Rac1 import and electron transfer from cytochrome c is required for pulmonary fibrosis. J Biol Chem. 2011 doi: 10.1074/jbc.M111.308387. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]