Abstract
Objective
To compare the health related quality of life (HRQOL) of children with chronic kidney disease (CKD) to healthy children; to evaluate the association between CKD severity and HRQOL; to identity demographic, socioeconomic and health-status variables associated with impairment in HRQOL in children with mild to moderate CKD.
Patients and Methods
This is a cross-sectional assessment of HRQOL in children aged 2-16 with mild to moderate CKD using the Varni PedsQL™. Overall HRQOL and PedsQL domain means for parents and youth were compared to previously published norms using independent sample t-tests. Study participants were categorized according to kidney disease stage (measured by iohexol based glomerular filtration rate, iGFR) and group differences in HRQOL were evaluated using ANOVA and Cuzick trend tests. The association between hypothesized predictors of HRQOL and PedsQL scores was evaluated with linear and logistic regression analyses.
Results
The study sample was comprised of 402 participants (Mean age =11 yrs, 60% male, 70% Caucasian, 40% anemic, median iGFR=42.5 ml/min/1.73m2, median CKD duration= 7 yrs). Youth with CKD had significantly lower physical, school, emotional and social domain scores than healthy youth (p<.001). IGFR was not associated with HRQOL. Longer disease duration and older age was associated with higher PedsQL scores in the domains of physical, emotional and social functioning (p<.05). Older age was associated with lower school functioning domain scores (p<.05). Maternal education ≥16 years was associated with higher PedsQL scores in the domains of physical, school, and social functioning (p<.05). Short stature was associated with lower scores in the physical functioning domain (p<.05).
Conclusions
Children with mild to moderate CKD, in comparison to healthy children, report poorer overall HRQOL as well as poorer physical, school, emotional and social functioning. Early intervention to improve linear growth and to address school functioning difficulties is recommended.
Keywords: HRQOL, kidney disease, QOL, short-stature
INTRODUCTION
Recently acknowledgment of the importance of health-related quality of life (HRQOL) as a measure of treatment outcomes in children with chronic illnesses has increased and the FDA has recommended the inclusion of HRQOL measurement in clinical trials.1-4 Previously published research illustrates that many children with advanced kidney disease demonstrate significant HRQOL impairments5-11 and many adults with childhood onset kidney disease have significant impairments in educational, social and physical functioning.12-14 Unfortunately, only a few systematic evaluations of the HRQOL of children who are in the early stages of kidney disease have been performed.
As patients are often asymptomatic prior to end-stage renal disease (ESRD) when treatment with dialysis or transplant becomes necessary to sustain life, little epidemiological information is available on the prevalence of children with earlier stages of chronic kidney disease (CKD). From the US Renal Data System, we know the adjusted point prevalence of end stage kidney disease among youth younger than 20 in the US was 82 per million population in 2004-2006.15 The number of children and adolescents who have less severe kidney disease is likely much higher.16
Early identification of children with pre-end stage kidney problems who also have poor quality of life could lead to interventions such as those available to children with more severe educational, physical, emotional and social handicaps. 17 Furthermore, earlier identification of HRQOL problems in children who are in the early stages of kidney disease and intervention may decrease the prevalence of poor educational, occupational and social outcomes in adults with childhood onset of kidney disease.
The National Institutes of Health recently funded a large multi-center prospective observational cohort study of children with mild to moderate kidney disease whose protocol includes yearly assessments of child and parent perceptions of HRQOL.18 In this report we examine the baseline cross-sectional HRQOL data from the Chronic Kidney Disease in Children Cohort Study (CKiD). This analyses provides the first large-scale evaluation of health-related quality of life in youth with pre-end stage kidney disease and serves three related aims: (1) to compare the HRQOL of children with pre-end stage kidney disease to the HRQOL of healthy children, (2) to assess the association between kidney disease severity and HRQOL in children with mild to moderate chronic kidney disease and, (3) to identify demographic, socio-economic and health-status variables associated with significant impairment in physical, school, emotional and social functioning in children with mild to moderate kidney disease. We hypothesized that (1) children with mild to moderate kidney disease will have poorer HRQOL than healthy children, (2) kidney damage severity will be associated in a linear fashion with HRQOL impairment and (3) increased disease duration, socioeconomic status and potentially modifiable health conditions such as growth delay and anemia will be associated with significant impairment in HRQOL.
PATIENTS AND METHODS
Study Design and Participants
This is a cross-sectional assessment of HRQOL of children enrolled in the NIH funded multicenter CKiD study. Human subjects review board approval was obtained at each collaborating site. The inclusion criteria, exclusion criteria and study design have been previously described.18 Briefly, youth were recruited with mild to moderate kidney disease based on their estimated Glomerular Filtration Rate (GFR) from 48 participating pediatric nephrology centers. GFR is the most useful measurement currently available for evaluating the level of kidney function.
Eligible study participants had never been dialyzed or undergone a kidney transplant, had an estimated GFR (eGFR) 19 in the range of 30 to 90 ml/min per 1.73 m2 and were 2-16 years of age. GFR measured from plasma iohexol disappearance curves (iGFR) was determined at the study baseline.20 Normal GFR is approximately 110 - 120 ml/min/1.73m2 after the age of 2 years through adulthood.21 The median GFR of 43 ml/min/1.73m2 of children in the this study represents a loss of more than half of the age appropriate level of normal kidney function, and is associated with an increasing prevalence of complications of chronic kidney disease. Systematic evaluation of differences between enrollees and all eligible subjects was performed at one of the two lead coordinating center sites to assess sample representativeness.
The initial administration of the PedsQL survey completed by the primary caregiver and study participant at enrollment was analyzed. The sample comprised 402 families who completed the PedsQL22 at enrollment and for whom iGFR and duration of kidney disease was available as of June 2008. Children 8 years of age and older filled out the PedsQL child form, while parents/caregivers of all eligible children completed the PedsQL parent proxy form.
Measurement of HRQOL
HRQOL was evaluated using the 23-item Pediatric Inventory of Quality of Life Core Scales (PedsQL™, Version 4.0). The PedsQL is a generic HRQOL instrument that assesses physical, emotional, social and school functioning in children and adolescents. It has been shown to be an appropriate assessment tool for healthy children,22-26 children with kidney disease 10;11;27 as well as children with a variety of other chronic medical conditions.28-31 The PedsQL directions instruct respondents to review a list of problem statements and rate the degree to which each has been experienced over the past month. PedsQL domain scores are derived by summing the problem frequency within each domain and then linearly transforming the resulting sum into a standardized score.32 The overall HRQOL score is calculated by summing the problem frequency endorsements from the entire survey and dividing by the number of items answered.32 Overall HRQOL and domain scores range from 0 to 100 with higher scores reflecting better functioning and better HRQOL.
Data Analyses
The outcomes of interest were the PedsQL overall score and domain scores. Chronbach alphas were calculated for each PedsQL domain to assess how well individual items measure the latent construct of the quality of life (QOL) domains in our study cohort.
Independent sample t-tests evaluated mean differences between the study cohort and published PedsQL population sample norms.33 Based on a priori assumptions about putative risk factors for adverse HRQOL outcomes, potential differences in HRQOL scores between children with comorbid conditions, lower SES, or with reported prematurity at birth (gestational age <36 weeks) and the other participants were assessed using T-tests.
To assess the study hypothesis that poorer kidney function (lower GFR) is associated with poorer HRQOL, youth were categorized into kidney disease severity groups based on their iGFR after study enrollment (≥ 90 ml/min/1.73m2 =Stage 1, 60-89 ml/min/1.73m2=Stage 2, 30-59 ml/min/1.73m2=Stage 3, 15-29 ml/min/1.73m2= Stage 4, <15 ml/min/1.73m2=Stage 5)34 and then group differences in PedsQL domain scores were evaluated using ANOVAs and a Cuzick test (a non-parametric trend test and an extension of the rank sum test for more than two groups used to assess for monotonic trends).
A staged approach was used to identify demographic, socioeconomic and health status variables associated with significant impairment in HRQOL. First, the association of variables shown previously to be related to QOL was evaluated using parametric and non-parametric tests of association (T-tests, Chi-Squares and Wilcoxon rank). Separate analyses were conducted for the parent proxy and child PedsQL scores given the possibility that different predictors would emerge.
Next, linear regression analysis was used to evaluate the relationship between the PedsQL domain scores and variables found to be associated in the univariate analyses or supported by clinical practice observations. Three variables were used to represent socioeconomic status in the models: maternal education, parental marital status and maternal income. Due to the high correlation between the socio-economic status variables, they were assessed in separate models. The model that included maternal education is presented in this report as maternal education is considered an excellent indicator of SES and has consistently been linked to health-enhancing activities.35;36
Thirdly, an alternate set of analyses evaluated predictors of the poorest performing segment of this cohort. For these analyses, the study sample was dichotomized based on the PedsQL score, with a cut-off score for ‘at-risk’ status set at one standard deviation below the population mean.24;33 Using the same predictors from the linear regression models, logistic regression analyses were used to evaluate the effect demographic, socio-economic and health-status on the odds of having ‘at risk’ (i.e. significantly impaired) quality of life scores.
Data were analyzed using SAS version 9.1 and STATA/SE version 9.0.
RESULTS
Participants
The characteristics of the 402 study participants are provided in Table 1. The cohort had a mean age of 11±4 years, was 60% male and 70% Caucasian. Mean CKD duration was 7.4 ± 5 years with a median iGFR of 42.5 ml/min/1.73m2. The predominant cause of CKD was congenital or hereditary kidney disease.
Table 1.
Descriptive Statistics for Demographic Characteristics and Analytical Variables of 402 Study Participants
| Characteristic | Value |
|---|---|
| Age, years (M±SD) | 11 ± 4 |
| Male (%,N) | 60% (242) |
| Race (%,N)a | |
| Caucasian | 70% (281) |
| African American | 15% (61) |
| Asian | 2% (9) |
| Other | 12% (50) |
| Hispanic Ethnicity (%)a | 14% (57) |
| Maternal Education (M±SD) a | 13 ± 3 |
| High School or less / ≤12 yrs (%,N) | 42% (168) |
| Post High School / 13-15 yrs (%,N) | 28% (112) |
| 4 yrs College / ≥ 16 yrs (%,N) | 28% (112) |
| Height for age, < 5th %ilea,b | 23% (86) |
| Anemia (%,N)c | 40 % (160) |
| Primary CKD diagnosis (%,N) | |
| Glomerular | 22% (87) |
| Genitourinary,cystic,hereditary | 77% (311) |
| Other or missing | 1% (4) |
| CKD Duration (year M±SD) | 7 ± 5 |
| % of life with CKDd (M±SD) | 71% ± 34% |
| Iohexol-based GFR, ml/min per 1.73m2 (Median,IQR) | 43 (32,53) |
M = mean SD = standard deviation IRQ=Interquartile range
Missing data: n=1 missing race; n=6 missing Hispanic ethnicity; n=10 missing maternal education; n=24 missing height %ile; n=6 missing anemia
Percentiles based on CDC growth charts for age and gender
Anemia defined as hemoglobin <5th % of normal (g/dl) for age and gender or if reported taking an erythropoiesis-stimulating agent
CKD duration/age * 100 = % of life with CKD
A systematic evaluation of the differences between study participants and non-enrollees at one of our larger participating sites located in an east coast urban environment showed that of 64 eligible youth, 20 were enrolled. Enrollees had a similar mean age, mean eGFR and gender distribution compared to youth not enrolled. However, although 25% of eligible youth had underlying glomerular disease and 75% had underlying structural urologic disease, 15% of those enrolled had glomerular disease, and 85% had urologic disease. Although 42% of eligible youth were African American, 30% of those enrolled subjects were African American.
To evaluate whether the subgroup of more susceptible children with additional health co-morbidities or prematurity at birth was influencing the observed overall and domain HRQOL means of our sample, we compared mean scores of children with health comorbidities or prematurity to the mean scores of children with only a diagnosis of kidney disease using T-tests. Statistical differences were found in the physical, emotional and social domains of the parent report data and in the social domain of the child report data with those reporting a comorbidity having lower mean scores. However, when those individuals with comorbidities were removed, the mean scores of the remaining youth with kidney disease without health comorbidities or prematurity were still statistically lower than the normative mean scores in all domains. No differences were found between those participants reporting premature birth and those reporting term birth in overall HRQOL or any of the PedsQL domains. Therefore the analyses reported in this manuscript include the entire cohort.
Missing data
Eighteen parent proxy PedsQL reports were missing school functioning domain scores. Youth with missing school functioning domain scores were not attending school. The majority of the missing data were in the 2-4 year old group.
Internal consistency reliability
Chronbach’s alphas were estimated for each PedsQL domain for parents and children separately to assess internal consistency. In addition, separate Chronbach alphas were calculated for the parent assessment of toddlers and parent assessment of school-age youth with CKD. The standardized alpha’s based on parent proxy of overall HRQOL for the toddler and school-age group were 0.91 and 0.89 respectively. The standardized alpha based on child report of overall HRQOL was 0.92. The lowest standardized alpha (0.67) was observed in the parent proxy of school functioning for the toddler group.
Comparison of CKD group to Norm group
Table 2 displays the means and standard deviations of the PedsQL overall and domain scores for the kidney disease group and the published normative sample.33 We found statistically significant differences between the kidney disease group and the normative group means (p< .001) on all domains of HRQOL with the kidney disease group having consistently lower scores. The largest between-group differences were observed in the school functioning domain.
Table 2.
PedsQL Mean Score Comparisons of CKD and Normative (Varni et al 2003) Data
| CKD data | Varni et al data: Ambulatory Pediatrics (2003), 3:329-341 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Mean | SD | N | Mean | SD | |
| Physical Functioninga | ||||||
| Parent Proxy | 402 | 76.91 | 22.41 | 10,050 | 83.26 | 19.98 |
| Childc | 283 | 79.42 | 16.58 | 5,962 | 86.86 | 13.88 |
| Emotional Functioninga | ||||||
| Parent Proxyb | 401 | 73.26 | 18.55 | 10,044 | 80.28 | 16.99 |
| Childbc | 282 | 73.92 | 18.15 | 5,961 | 78.21 | 18.64 |
| Social Functioninga | ||||||
| Parent Proxyb | 401 | 76.80 | 21.89 | 10,036 | 82.15 | 20.08 |
| Childc | 283 | 79.24 | 19.50 | 5,948 | 84.04 | 17.43 |
| School Functioninga | ||||||
| Parent Proxyb | 384 | 65.00 | 21.34 | 8,466 | 76.91 | 20.16 |
| Childc | 283 | 64.14 | 18.20 | 5,908 | 79.92 | 16.93 |
| Overall HRQOLa | ||||||
| Parent Proxy | 402 | 73.72 | 17.42 | 10,070 | 81.34 | 15.92 |
| Childc | 283 | 74.78 | 14.26 | 5,972 | 82.87 | 13.16 |
CKD Means < Varni Means, p<0.001
Missing data: n=1 Parent Emotional & Social Functioning; n=18 Parent School Functioning; n=1 Child Emotional Functioning
Only children ≥ 8 years complete the child version of the PedsQL; n=283
The association between GFR and HRQOL
Analyses evaluating the existence of an association between kidney disease severity, as assessed by iGFR measurement, and PedsQL scores indicated that HRQOL is not associated with level of GFR within the range of GFR of the population studied in this report. Table 3 shows the median PedsQL score for each PedsQL domain by GFR category corresponding to KDOQI CKD stage. 34 Analysis of variance evaluating median scores did not reflect any differences in HRQOL between GFR categories. Non-parametric trend tests did not reveal any significant trends in HRQOL by KDOQI stage of CKD.
Table 3.
CKD Parent Proxy and Child PedsQL Overall and Domain Median Scores and Interquartile range by iGFR Stage
| iGFR STAGE | |||
|---|---|---|---|
| Stage 2 (60-89 ml/min/1.73m2) | Stage 3 (30-59 ml/min/1.73m2) | Stage 4 (15-29 ml/min/1.73m2) | |
|
| |||
| Parent N=71 | N=254 | N=77 | |
| Child N=49 | N=182 | N=52 | |
| Physical Functioning | |||
| Parent Proxy | 88 (75,94) | 84 (63,94) | 81 (69,94) |
| Child | 88 (78,94) | 81 (69,91) | 81 (66,94) |
| Emotional Functioning | |||
| Parent Proxy | 65 (55,85) | 75 (60,90) | 75 (60,85) |
| Child | 75 (60,88) | 75 (60,90) | 75 (60,90) |
| Social Functioning | |||
| Parent Proxy | 80 (60,95) | 80 (60,95) | 80 (60,100) |
| Child | 85 (70,95) | 85 (70,95) | 78 (60,95) |
| School Functioning | |||
| Parent Proxy | 65 (50,83) | 65 (50,83) | 65 (45,80) |
| Child | 70 (55,75) | 65 (55,75) | 65 (50,75) |
| Overall HRQOL | |||
| Parent Proxy | 78 (65,87) | 77 (62,88) | 76 (64,88) |
| Child | 80 (67,85) | 76 (67,85) | 76 (60,85) |
No significant group differences identified with ANOVA or Cusick non-parametric trend tests
Predictors of HRQOL
Linear regression analyses were performed to estimate the association between hypothesized predictors of QOL and the PedsQL scores. Based on univariate analyses and a priori hypotheses, seven variables were assessed in a multivariate model: iGFR, percent of life with CKD, anemia, gender, age at study entry, height percentile (age and gender adjusted) and, level of maternal education. The adjusted results from the youth self-report and parental assessment of child HRQOL are displayed in Table 4.
Table 4.
Adjusted Linear Regression Results for Predictors of Child and Parent Proxy PedsQL Overall and Domain Scores
| RISK FACTOR | OVERALL QOL | PHYSICAL QOL | SCHOOL QOL | EMOTIONAL QOL | SOCIAL QOL | |
|---|---|---|---|---|---|---|
| β | β | β | β | β | ||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| Female | CHILD | 0.2 (-3.4,3.9) | -0.4 (-4.6,3.8) | 2.0 (-2.7,6.8) | -3.4 (-8.0,1.3) | 2.9 (-2.1,7.8) |
| PARENT PROXY | 2.6 (-1.1,6.2) | 1.8 (-2.8,6.4) | 5.7c (0.9,10.4) | 0.4 (-3.7,4.5) | 3.5 (-1.2,8.2) | |
| Maternal Education(13-15yrs) | CHILD | -0.2 (-4.4,4.0) | 2.5 (-2.4,7.3) | -1.4 (-6.9,4.0) | -1.9 (-7.3,3.4) | -1.6 (-7.2,4.1) |
| PARENT PROXY | 2.4 (-1.7,6.5) | 4.3 (-0.8,9.4) | 5.6c (0.2,10.9) | -3.2 (-7.7,1.4) | 3.5 (-1.8,8.8) | |
| Maternal Education (≥16yrs) | CHILD | 5.4c (1.4,9.3) | 4.6 (-0.1,9.1) | 8.0c (2.9,13.2) | 3.4 (-1.7,8.4) | 5.9c (0.6,11.2) |
| PARENT PROXY | 4.7c (0.5,8.8) | 5.5c (0.3,10.7) | 7.3c (2.0,12.7) | 1.8 (-2.9,6.4) | 4.7 (-0.6,10.1) | |
| Age per 2 years | CHILD | 2.1b (0.9,3.3) | 1.7c (0.3,3.1) | 1.1 (-0.5,2.7) | 2.6c (1.0,4.1) | 3.2b (1.5,4.8) |
| PARENT PROXY | -0.7 (-1.6,0.1) | -0.7 (-1.7,0.4) | -1.4c (-2.5,-0.2) | -0.1 (-1.0,0.9) | -0.4 (-1.5,0.7) | |
| iGFR per 10ml decline | CHILD | -0.1 (-1.3,1.1) | -0.2 (-1.6,1.1) | -0.9 (-2.4,0.7) | 0.7 (-0.9,2.2) | 0.0 (-1.6,1.6) |
| PARENT PROXY | -0.1 (-1.4,1.1) | -0.7 (-2.2,0.9) | -0.3 (-1.9,1.3) | 0.6 (-0.8,1.9) | 0.3 (-1.3,1.9) | |
| % of life with CKD per 10% | CHILD | 0.4 (-0.1,0.9) | 0.8c (0.2,1.4) | 0.2 (-0.5,0.9) | 0.4 (-0.3,1.0) | 0.0 (-0.7,0.7) |
| PARENT PROXY | 0.6c (0.1,1.2) | 0.9c (0.2,1.5) | 0.2 (-0.5,0.9) | 0.7c (0.1,1.3) | 0.6 (-0.2,1.3) | |
| Anemia | CHILD | 0.1 (-3.7,3.8) | 0.5 (-3.8,4.8) | -0.9 (-5.8,4.0) | 1.5 (-3.3,6.2) | -0.9 (-6.0,4.1) |
| PARENT PROXY | -1.4 (-5.3,2.5) | -0.7 (-5.5,4.1) | -2.9 (-7.9,2.1) | 0.5 (-3.8,4.8) | -3.9 (-8.9,1.0) | |
| <= 5% Height %ile | CHILD | -2.7 (-6.8,1.4) | -4.0 (-8.8,0.7) | -1.6 (-7.0,3.8) | -0.2 (-5.4,5.0) | -4.2 (-9.7,1.4) |
| PARENT PROXY | -4.0 (-8.1,0.2) | -5.7c (-10.9,-0.6) | -1.7 (-7.1,3.6) | -1.3 (-5.9,3.4) | -5.0 (-10.3,0.4) |
Significant, p<0.0001
Significant, p<0.001
Significant, p<0.05
Youth self-report of HRQOL
Youth whose mothers had ≥ 16 years of education reported overall HRQOL scores 5.4 points on average higher than those whose mothers had no more than a high school education (p<.05, 95% CI: 1.4, 9.3). The estimated increases resulting from maternal education were significant (p<.05) in the domains of school and social functioning (8.0 and 5.9 points, respectively).
Every additional two years of youth age was associated with higher overall HRQOL score by an average of 2.1 points (p<.001, 95% CI: 0.9, 3.3). In addition, significantly higher scores were associated with increased age by 2 years in the physical (p<.05), emotional (p<.05) and social (p<.001) domain scores (1.7, 2.6 and 3.2, respectively).
Percent of life with CKD was also positively associated with PedsQL physical domain scores, with a increase in the physical functioning score of 0.8 for every 10% increase in percent of life with CKD (p<.05, 95% CI: 0.2, 1.4). In other words, youth who had spent a higher proportion of their life with diagnosed CKD had slightly higher HRQOL scores in the physical functioning domain.
Parent evaluation of youth HRQOL
When parents were asked to assess their child’s HRQOL, maternal education remained a significant predictor of higher overall HRQOL, physical and school domain scores. Our results indicate that mothers with ≥16 years of education had children with overall QOL scores that were on average 4.7 points higher than children whose mothers had less than a high school education (p<.05, 95% CI: 0.5, 8.8). Additionally, the physical and school domain scores were 5.5 (p<.05, 95% CI: 0.3, 10.7) and 7.3 (p<.05, 95% CI: 2.0 12.7) points higher on average, respectively, comparing the same two maternal education groups.
In the parent-proxy assessment of HRQOL, youth age was negatively associated with the school component of HRQOL, subtracting on average 1.4 points per every two years of increased age (p<.05, 95% CI: -2.5, -0.2).
Mirroring youth HRQOL assessment, percent of life with CKD (per 10%) was significantly associated with several aspects of QOL according to parent perception. Longer disease duration was associated with higher HRQOL scores of 0.6 (p<.05, 95%CI: 0.1, 1.2), 0.9 (p<.05, 95%CI: 0.2, 1.5) and 0.7 (p<.05, 95% CI: 0.1 1.3) for overall, physical and emotional domains of QOL, respectively.
Short Stature (being less than the 5th percentile for height) was associated with lower overall HRQOL as well as lower physical, school, emotional and social domain scores with a statistically significant result in the physical domain (p<.05, -5.7, 95% CI: -10.9, -0.6) and lower point estimates (although not statistically significant) for overall HRQOL and social functioning (-4.0 and -5.0, respectively).
Female gender (in comparison to male gender) was associated with higher school functioning scores on average by 5.7 points (p<.05, 95% CI: 0.9, 10.4). Anemia was associated with lower point estimates for scores in the social functioning domain (p>.05, -3.9, 95% CI: -8.9, 1.0) and similarly lower overall, physical and school scores, although these were not statistically significant.
Assessment of ‘At Risk’ participants
Logistic regression analyses examined the effect of the predictors on a dichotomous outcome comparing those whose HRQOL score was greater than or equal to 1 standard deviation below the normative sample mean to those above that threshold. Figure 1 illustrates the prevalence of children in this cohort who experienced significant HRQOL problems as measured by Peds QL scores falling more than 1 SD below the normative sample mean. For both youth and parent proxy data, inferences from the logistic regressions were similar to the linear regressions for all factors including the strongest predictors of adverse effects on HRQOL (lower maternal education, lower age and shorter height).
Figure 1.
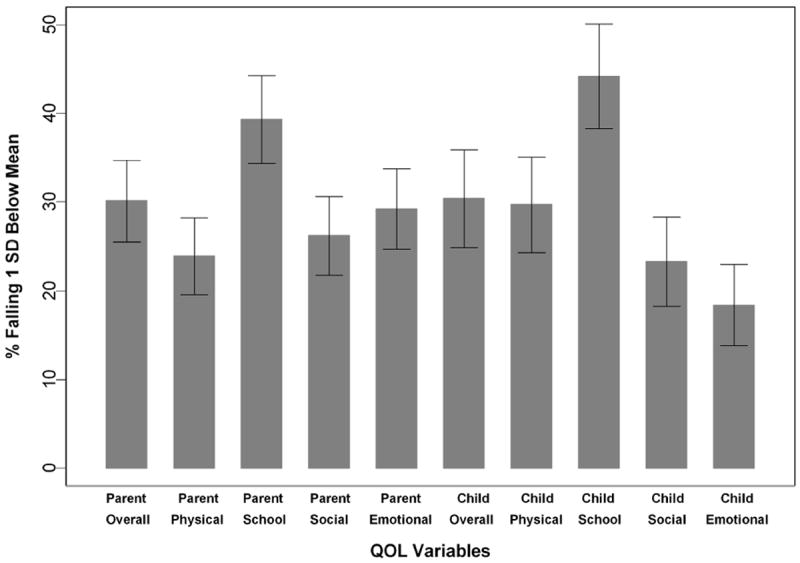
Percent of CKD group with poor quality of life (>1SD below published normative sample mean) and standard error (based on 2 SDs)
DISCUSSION
The analyses described in this report had three related aims: (1) to compare the HRQOL of children with pre-end stage renal disease to the HRQOL of healthy children, (2) to assess the association between kidney disease severity and HRQOL in children diagnosed with mild to moderate CKD and, (3) to identify demographic, socio-economic and health-status variables associated with significant impairment in physical, school, emotional and social functioning in children with mild to moderate kidney disease.
Our results provide strong evidence that the HRQOL of children with mild to moderate chronic kidney disease is poorer than that of healthy children. On average, youth with CKD and their parents, report that overall HRQOL as well as physical, social, emotional and school functioning is poorer than healthy youth. The most marked differences from norms were in school functioning. This may be due to the complexities of the children’s medical care and the frequency of medical visits interfering with school attendance, or possibly impairments in attention or cognitive functioning associated with deteriorating kidney function. The negative impact on HRQOL scores in the physical and social domains may be due to growth impairment and pubertal delay associated with childhood onset CKD. These possibilities can be further assessed in the longitudinal analysis of CKiD data, as more follow-up study visits and QOL assessments are obtained in these children.
While it is well established that children with end stage kidney disease have significant impairments in HRQOL, 10;11 to our knowledge, this is the first large scale study to demonstrate that HRQOL is significantly impacted early on in the course of chronic kidney disease. Figure 1 illustrates the magnitude of HRQOL problems and shows that a sizable faction of youth with pre-end stage kidney disease has significant impairment in physical, school, emotional and social functioning.
We did not find support for our primary study hypothesis that the severity of kidney damage, measured by GFR or KDOQI stage of CKD, was associated with HRQOL impairment. It is possible that the use of cross sectional data obscured our ability to detect a relationship between kidney disease severity and HRQOL. Longitudinal analysis of HRQOL as GFR declines is needed and is an ongoing aim of the CKiD cohort study.
Contrary to our expectations, youth who had CKD for a longer period of time (i.e. greater % of life with CKD) were observed by their parents to have better physical and emotional functioning than youth who had CKD for a shorter period of time. Similarly, according to youth, greater percent of life with CKD was associated with better physical functioning and older age was found to be associated with better physical, emotional and social functioning. These findings are quite interesting and may suggest that with the passage of time, children and their families accommodate to having kidney disease such that their perception of the negative impact of CKD on physical, emotional and social functioning decreases over time. This phenomenon has been observed in other chronic illness groups and has been labeled “response shift”. 37;38 It has been suggested that response shift may result from a change in an individual’s internal standards, personal values or conceptualization of perceived HRQOL and may involve behavioral, cognitive and affective mechanisms or processes necessary for a person to accommodate to a changed health state.39 Alternatively, given the cross sectional nature of the data, this finding could be a function of survivor bias (i.e. youth participants enrolled in CKiD who have CKD for a long time may be better off in some ways). Longitudinal analysis of HRQOL in CKiD will allow us to distinguish between these possibilities.
Youth with mild to moderate kidney disease and their parents report that short stature is associated with a negative impact on overall QOL and increases the odds of significant impairment in the area of physical functioning. These results may foreshadow poor long-term social outcomes that have been observed by a number of research groups 13;40 and highlight the importance of early interventions to improve linear growth and normalize height in children with CKD. 41;42
Advanced maternal education was associated with higher HRQOL scores in both parent proxy and child assessments. This study replicates other published accounts of the positive association between maternal education and HRQOL43-45 and more globally between education and health.36 Furthermore, as maternal education was used as an indicator of SES in this study, these results replicate previous findings that SES impacts HRQOL.23;46
There are a few issues that limit the generalizability and inferential value of these results. First, this is a cross-sectional analysis and it is important for these findings to be replicated in longitudinal analyses. Secondly, it is possible that our results may not generalize to all children with CKD as the sample enrolled in the CKiD study may be different than the general population of youth with kidney disease in terms of racial distribution and cause of kidney failure. Specifically, white children and children who have had kidney disease longer, with underlying urologic disease were more likely to participate in our study. This limitation, however, should not change the internal validity of our study in examining factors that are associated with lower health related quality of life in childhood CKD.
CONCLUSION
This study presents strong evidence that many children with mild to moderate kidney disease suffer HRQOL disturbances. School functioning problems are particularly prominent and severe. While previously published studies have described impaired quality of life in children with end-stage renal disease and in adults who had childhood onset of kidney disease, 6;8;9;11-14;27 this is the first study to describe quality of life impairments in children with early kidney disease. Additional data analyses need to be undertaken to assess the extent to which at-risk children are receiving optimal school-based supplementary services aimed at preventing poor long-term occupational and social outcomes. In addition, the effect of growth, development, time from diagnosis and GFR decline on these quality of life disturbances will be more fully evaluated in subsequent analyses of longitudinal follow-up in the CKiD cohort.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, Ph.D.), and data coordinating center (Principal Investigator) at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.). The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, UO1-DK-66116). The CKID website is located at http://www.statepi.jhsph.edu/ckid. We also especially want to acknowledge and thank the youth with kidney problems who are participating in this study and their families.
Abbreviations
- HRQOL
Health Related Quality of Life
- CKD
Chronic kidney Disease
- ESRD
End Stage Renal Disease
- CKiD
Chronic Kidney Disease in Children Prospective Cohort Study
- iGFR
iohexol based glomerular filtration rate
References
- 1.Eiser C, Jenney M. Measuring quality of life. Arch Dis Child. 2007;92:348–350. doi: 10.1136/adc.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeCivita M, Regier D, Alamgir AH, Anis AH, FitzGerald MJ, Marra CA. Evaluating health-related quality-of-life studies in paediatric populations. Pharmacoeconomics. 2005;23:659–685. doi: 10.2165/00019053-200523070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein S, Gerson AC, Goldman CW, Furth S. Quality of life of children with Chronic Kidney Disease. Semin Nephrol. 2006;26:114–117. doi: 10.1016/j.semnephrol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.FDA. Guidance for industry: Patient reported outcome measures: Use in medical product development to support labeling claims. Rockville, MD: Center for Drug Evaluation and Research, Food and Drug Administration; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fadrowski JJ, Cole SR, Hwang W, Gerson AC, Furth SL. Prospective study of health related quality of life in adolescents with chronic kidney disease. Pediatr Nephrol. 2006;22 doi: 10.1007/s00467-0050212203. [DOI] [PubMed] [Google Scholar]
- 6.Fukunishi I, Honda M. School adjustment of children with end-stage renal disease. Pediatr Nephrol. 1995;9:553–557. doi: 10.1007/BF00860928. [DOI] [PubMed] [Google Scholar]
- 7.Manificat S, Dazord A, Cochat P, Morin D, Plainguet F, Debray D. Quality of life of children and adolescents after kidney or liver transplantation: Child, parents and caregiver’s point of view. Pediatr Transplant. 2003;7:228–235. doi: 10.1034/j.1399-3046.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 8.Ehrich JHH, Rizzoni G, Broyer M, et al. Rehabilitation of young adults during renal replacement therapy in Europe. 2. Schooling, employment, and social situation. Nephrol Dial Transplant. 1992;7:579–586. doi: 10.1093/ndt/7.7.579. [DOI] [PubMed] [Google Scholar]
- 9.Roscoe JM, Smith LF, Williams EA, et al. Medical and social outcome in adolescents with end-stage renal failure. Kidney Int. 1991;40:948–953. doi: 10.1038/ki.1991.299. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW. Health related quality of life in pediatric patients with ESRD. Pediatr Nephrol. 2006;21:846–850. doi: 10.1007/s00467-006-0081-y. [DOI] [PubMed] [Google Scholar]
- 11.McKenna AM, Keating LE, Vigneaux A, Stevens S, Williams A, Geary DF. Quality of life in children with chronic kidney disease--patient and caregiver assessments. Nephrol Dial Tranplant. 2006;21:1899–1905. doi: 10.1093/ndt/gfl091. [DOI] [PubMed] [Google Scholar]
- 12.Morton MJS, Reynolds JM, Garralda ME, Postlethwalte RJ, Goh D. Psychiatric adjustment in end-stage renal disease: a follow up study of former paediatric patients. J Psychosom Res. 1994;38:296–303. doi: 10.1016/0022-3999(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz J, Reicwald-Klugger E, Oh J, Turzer M, Mehls O, Schafer F. Psychosocial rehabilitation and satisfaction with life in adults with childhood-onset of end-stage renal disease. Pediatr Nephrol. 2005;20:1288–1294. doi: 10.1007/s00467-005-1952-3. [DOI] [PubMed] [Google Scholar]
- 14.Rosenkranz J, Bonzel K-E, Bulla M, et al. Psychosocial adaptation of children and adolescents with chronic renal failure. Pediatr Nephrol. 1992;6:459–463. doi: 10.1007/BF00874014. [DOI] [PubMed] [Google Scholar]
- 15.U.S.Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. [Google Scholar]
- 16.Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatric Nephrology. 2007;22:1989–2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong FD. Neurodevelopment and chronic illness: Mechanisms of disease and treatment. MRDDRR. 2006;12:168–173. doi: 10.1002/mrdd.20114. [DOI] [PubMed] [Google Scholar]
- 18.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods in the chronic kidney disease in children (CKiD) prospective cohort study. CJASN. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 20.Schwartz GJ, Furth S, Cole SR, Warady BA, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 21.Brodehl J, Gellissen K, Wbber HP. Postnatal development of tubular phosphate reabsorption. Clin Nephrol. 1982;17:163–171. [PubMed] [Google Scholar]
- 22.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Burwinkle T, Seid M. The PedsQL 4.0 as a school population health measure: Feasibility, reliability, and validity. Quality of Life Research. 2006;15:203–215. doi: 10.1007/s11136-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 24.Seid M, Varni JW, Segall D, Kurtin PS. Health-related quality of life as a predictor of pediatric healthcare costs: A two year prospective cohort analysis. Health and Quality of Life Outcomes. 2004;2 doi: 10.1186/1477-7525-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limbers CA, Newman DA, Varni JW. Factorial invariance of child self-report across healthy and chronic health condition groups: A confirmatory factor analysis utilizing the PedsQL 4.0 generic core scales. J Ped Psych. 2008;33:630–639. doi: 10.1093/jpepsy/jsm131. [DOI] [PubMed] [Google Scholar]
- 26.Limbers CA, Newman DA, Varni JW. Factorial invariance of child self-report across socioeconomic status groups: a multigroup confirmatory factor analysis utilizing the PedsQL 4.0 Generic Core Scales. J Behav Med. 2008;31:401–411. doi: 10.1007/s10865-008-9166-3. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein SL, Graham N, Warady BA, et al. Measuring health-related quality of life in children with esrd: Performance of the generic and ESRD-specific instrument of the Pediatric quality of life inventory. AJKD. 2008;51:285–297. doi: 10.1053/j.ajkd.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Sawyer MG, Reynolds KE, Couper JJ, et al. Health-related quality of life of children and adolescents with chronic illness-a two year prospective study. Quality of Life Res. 2004;13:1309–1319. doi: 10.1023/B:QURE.0000037489.41344.b2. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Seid M, Knight TS, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: Reliability, validity, and responsiveness of the pediatric quality of life inventory generic core scales and rheumatology module. Arthritis & Rheumatism. 2002;46:714–725. doi: 10.1002/art.10095. [DOI] [PubMed] [Google Scholar]
- 30.Panepinto JA, Pajewksi NM, Foerster LM, Hoffmann RG. The performance of the PedsQL Generic Core Scales in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30:666–673. doi: 10.1097/MPH.0b013e31817e4a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PdsQL 4.0 Generic Core Scales. Health and Quality of Life Outcomes. 2007;5 doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varni J. Scaling and scoring of the Pediatric Quality of Life Inventory (PedsQL) Lyon: Mapi Research Trust; 2005. [Google Scholar]
- 33.Varni JW, Burwinkle TM, Rapoll MA, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation’s kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics. 2003;111:1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 35.Duncan GJ, Daly MC, McDonough P, Williams DR. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92:1151–1157. doi: 10.2105/ajph.92.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross C, Wu C. The links between education and health. Am Soc Review. 1995;60:719–745. [Google Scholar]
- 37.Saigal S, Tyson J. Measurement of quality of life of survivors of neonatual intensive care: critique and implications. Semin Perinatol. 2008;32:59–66. doi: 10.1053/j.semperi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Parsons SK, Barlow SE, Levy SL, Supran SE, Kaplan SH. Health-related quality of life in pediatric bone marrow transplant survivors: according to whom? Int J Cancer Suppl. 1999;12:46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 39.Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 40.Broyer M, LeBihan C, Charbit M, et al. Long-term social outcome of children after kidney transplantation. Transplantation. 2004;77:1033–1037. doi: 10.1097/01.tp.0000120947.75697.8b. [DOI] [PubMed] [Google Scholar]
- 41.Sheppard L, Eiser C, Davies HA, et al. The effects of growth hormone treatment on health-related quality of life in children. Horm Res. 2006;65:243–249. doi: 10.1159/000092455. [DOI] [PubMed] [Google Scholar]
- 42.Stabler B, Siegel PT, Clopper RR, Stoppani CE, Compton PG, Underwood LE. Behavior change after growth hormone treatment in children with short stature. J Pediatr. 1998;133:366–373. doi: 10.1016/s0022-3476(98)70271-9. [DOI] [PubMed] [Google Scholar]
- 43.Van Dellon OM, Stronks K, Bindels PJ, Ory FG, Bruil J, Van Alderen WM Peace Study Group. Health-related quality of life in children with asthma from different ethnic origins. J Asthma. 2007;44:125–131. doi: 10.1080/02770900601182459. [DOI] [PubMed] [Google Scholar]
- 44.Hassan K, Loar R, Anderson BJ, Heptulla RA. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J Pediatr. 2006;149:426–531. doi: 10.1016/j.jpeds.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Moorthy LN, Harrison MJ, Peterson M, Onel KB, Lehman TJ. Relationship of quality of life and physical function measures with disease activity in children with systemic lupus erythematosus. Lupus. 2005;14:280–287. doi: 10.1191/0961203305lu2075oa. [DOI] [PubMed] [Google Scholar]
- 46.Von Rueden U, Gosch A, Rajmil L, Bisegger C, Ravens-Sieberer U. Socioeconomic determinants of health-related quality of life in childhood and adolescence: Results from a European study. J of Epi Comm Health. 2006;60:130–135. doi: 10.1136/jech.2005.039792. [DOI] [PMC free article] [PubMed] [Google Scholar]


