Abstract
Flow cytometry has been a fundamental tool of biological discovery for many years. Invasive extraction of cells from a living organism, however, may lead to changes in cell properties and prevents studying cells in their native environment. These problems can be overcome by use of in vivo flow cytometry which provides detection and imaging of circulating normal and abnormal cells directlyin blood or lymph flow. The goal of this mini-review is to provide a brief history, features and challenges of this new generation of flow cytometry methods and instruments. Spectrum of possibilities of in vivo flow cytometry in biological science (e.g., cell metabolism, immune function, or apoptosis) and medical fields (e.g., cancer, infection, cardiovascular disorder) including integrated photoacoustic-photothermal theranostics of circulating abnormal cells are discussed with focus on recent advances of this new platform.
Key terms: flow cytometry; spectral imaging; photoacoustic and photothermal methods; fluorescence; Raman spectroscopy; blood and lymph flows; circulating tumor cells; bacteria, and multicolor nanoparticles; theranostics
Introduction
Flow cytometry (FCM) is a well-established powerful analytical tool that has led to many revolutionary discoveries in cell biology and cellular-molecular disease diagnosis (1–5). In FCM, cells are introduced into a high speed (up to 5–20 m/s) laminar artificial flow, and after they are focused into single file, laser-induced fluorescence and/or forward and sideways scattered light from them are detected using photodetector arrays with spectral filters. This highly accurate technology provides fast (a few million cells in a minute) multiparameter quantification of the biological properties of individual cells at subcellular and molecular levels, including their functional states, morphology, composition, proliferation, and protein expression. Recently an advanced Raman spectroscopy demonstrated the capability for high sensitive detection of individual particles in flow using Surface Plasmon Resonance Scattering (SERS) effects with signal integration times of 10 μsec (6–7). Many methods were developed for positioning cells and particles in a stream including mostly used hydrodynamic cell focusing (2) as well as dielectrophoretic, and acoustic focusing (8–13) and freely cell floating virtual-core flow cytometry (14).
Nevertheless, invasive extraction of cells from a living system may alter cell properties (e.g., morphology or marker expression) and prevents the long-term study of cell metabolism and cell-cell interactions (e.g., aggregation, rolling, or adhesion) in their native complex biological environment.
Other limitations of ex vivo FCM may include 1) low sensitivity for detection of rare abnormal cells (e.g., circulating tumor cells [CTCs]; 2) their time-consuming preparation procedures, taking many hours if not an entire day; and 3) the discontinuity of sampling with limited, discrete time points, making it difficult to detect early-stage disease and delaying effective, well-timed therapy. Indeed, in conventional FCM in the small volume of blood extracted (typically a few milliliters), no less than one abnormal cell (e.g., CTC or bacteria) can be detected. As a result, its current sensitivity level of 1–10 cells/ml is equivalent to 5,000–50,000 cells in the entire volume of blood (~5 L in adult), which is sufficient for the rapid development of a disease to a stage that is barely treatable or is incurable (i.e., metastasis, septic shock, or sickle cell crisis) (15–18). These shortcomings can be solved by development of a FCM instrument that allows in vivo noninvasive, continuous assessment of a significantly larger blood volume than current instruments and potentially a patient’s entire blood volume (15). Detection and quantification of rare circulating cells in vivo is important for the early diagnosis of many diseases (e.g., cancer, infections, cardiovascular dysfunction, inflammation, immune disorders, or diabetes), or for the study of the influence of the different interventions (e.g., drugs, radiation, smoking, alcohol) on individual cells. In particular, to understand the mechanism of the development of metastasis, it is important to monitor tumor cell migration and interaction with other cells. And finally, in vivo study of apoptosis and especially circulating apoptotic cells with advanced molecular imaging is crucial for understanding human metabolic and immune system function.
FCM is the general term for quantitative detection, identification and enumeration of individual cells in flow (2,5,18). Most significant contribution in development of in vivo FCM for single cell analysis directly in the blood and lymph flow was made since 2004 by Vladimir Zharov’s group in the University of Arkansas for Medical Sciences (15, 19–42) and Charles Lin’s team in Wellman Center for Photomedicine at Massachusetts General Hospital (43–57) using photothermal [PT]-based (e.g., thermal lens and photoacoustic [PA]) and fluorescent methods, respectively. The advances in in vivo FCM were also made by other researchers and research groups (52, 58–79), including Irene Georgakoudi (Tufts University), Theodore Norris (University of Michigan), Xunbin Wei (Fudan University), Eric Tkaczyk (University of Tartu), and Anja Hauser (Deutsches Rheumaforschungszentrum). These advances were made mostly in the fluorescent-based in vivo FCM (58, 61–64,80), confocal fluorescence (59,60,62) backscattering (65,66) microscopy, multi-photon laser scanning microscopy (63,67–78), and coherent anti-Stokes Raman scattering (CARS) (79) techniques. Gold nanostructures were proposed to be used as scattering contrast agents in vivo FCM (80–81).
The adaptation of FCM principles from in vitro technology with cells flowing in well-controlled single file, to an in vivo approach using the blood vessels as natural tubes with native cell flows faces many challenges to in vivo. Cell velocity in blood, and especially in lymph flow, is much slower (from 0.5–5 mm/s in blood microvessels to 0.1–0.5 m/s in large blood vessels of animals and humans) than the maximum speed of analysis achieved in FCM in vitro (see above), which from this perspective makes FCM’s technical platform easy to adapt to an in vivo setting. However, in vivo studies may be limited by 1) poor optical conditions (compared to an almost ideal situation in vitro), such as absorption, scattering and autofluorescent background from the vessel wall, surrounding tissues (e.g., skin layers, connective tissue, muscles, or fat), or from blood cells; 2) multiple-file cell flow in vessel cross-sections; 3) difficulties of noninvasively accessing deep vessels; 4) problems with the use of a transillumination (forward) or sideways optical schemes, which is more convenient for achieving high spatial resolution; and 5) instability of blood-and, especially, lymph-flow parameters (e.g., fluctuation of cell velocity and the positions of cells in vessel cross-sections) compared to the well-controlled flow parameters of in vitro FCM. These limitations required some precautions in the choices of a detection system and proper animal models as a first step toward transitioning this technique to human applications. This can be done taking into account circulation properties and features. Significant contribution in developing optical methods for in vivo imaging of blood and lymph flow, measuring flow velocity, and study of circulation at different conditions has been made by Valery Tuchin’s group in Saratov State University (SSU) starting since 1994 (82–99). It included the speckle techniques for cell velocity measurements in mostly used rat mesentery as animal model and vessels of eye conjunctiva in humans. In particular, complex dynamics of cell flows in lymph and blood vessels was studied, including the reactivity of lymphatic microvessels on topical application of a number of biologically important substances different in their nature, mechanisms, and action intensity, such as staphylococcal exotoxin (α-hemolysin), N-nitro-L-arginin (L-NNA), dimethyl sufoxide (DMSO), glucose, glycerol, etc. It was shown that nitric oxide (NO) plays an important role in the lymphatic microvessel regulation (94). One of the possible mechanisms of low-intensity laser therapy fundamentally based on NO signaling and regulating the drainage function of lymph microvessels was also proposed (96). The natural property of lymph vessel and valve function were studied in the detail (87, 94). The key principle investigator in this direction in Tuchin’s group was Ekaterina Galanzha, who the further developed in Vladimir Zharov’s group a video-imaging flow cytometry to make it faster and be applicable to blood flows, cell deformability quantification in situ, and cell-cell and cell-vessel wall interactions (100–112). A high-speed (up to 40,000 fps), high-resolution (up to 300 nm) continuous in vivo optical imaging technique (106) allows one to monitor and identify red blood cells (RBCs), white blood cells (WBCs), and platelets in the blood flow of rat mesenteric microvessels and other animal models without conventional labeling. It was demonstrated that the frame rate up to 10,000 fps at the high optical resolution could be sufficient for full estimation of individual cell behavior and cellular biomechanical properties in vivo in microvascular net. Cell focusing in vivo in native lymph flow was demonstrated using lymph valve as a natural nozzle (26).
A few other groups and researchers, including Stephen Morgan (University of Nottingham), Vyacheslav Kalchenko (Weizmann Institute of Science), and Gerard L. Coté (Texas A&M University) made a brilliant input in the problem of the label-free imaging of blood flow and measurement of cell velocity and oxygenation (16,17, 111–112, 113–119), which are partly summarized in (110) and presented in this issue (17).
Basic principles and detection methods
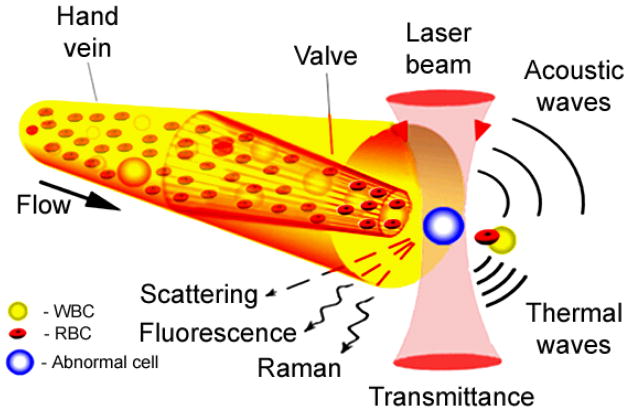
In FCM techniques individual cells of interest in blood or lymph flows are imaged with a sufficient rate and resolution by a CCD or CMOS camera using conventional microscopic lamp illumination and/or irradiated with one or several laser beams. Laser-associated physical effects in individual cells such as absorption, fluorescence, elastic and inelastic (Raman) scattering, PT and PA phenomena (Fig. 1) are detected with corresponding optical or non-optical detectors depending on the method used. Opto-physical time-dependent phenomena are generated in a cell via interaction with pulsed or intensity modulated optical radiation at different wavelengths (multicolor FCM).
Fig. 1.
Principle of in vivo flow cytometry integrating multimodal detection of individual circulating cells directly in the bloodstream or lymph flow.
Label-free video-imaging
The principle of this in vivo FCM method is illustrated in Fig. 1 and demonstrated in Fig. 2. A video-recording using a high-resolution and high-speed CCD or CMOS cameras in transmittance or reflectance mode allows one to get pretty good cell images in lympth or blood flows. As an example, high-speed transmittance digital microscopy (TDM) images of cells in the area close to valve of the lymph vessel of rat mesentery demonstrating the natural cell flow hydrodynamic focusing are shown in Fig. 2. A vein (or lymphatic) valve works as a nozzle, it is like a natural hydrodynamic cell used in conventional in vitro FCM allowing for cell flowing one by one.
Fig. 2.
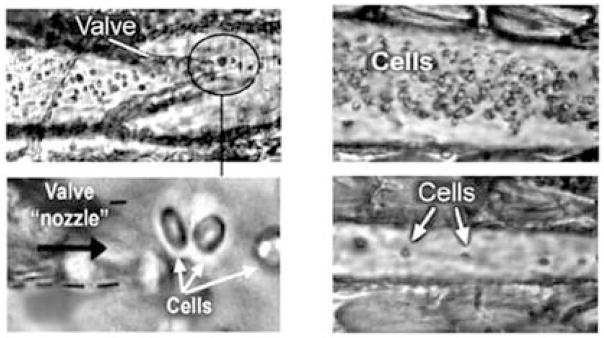
High-speed transmittance digital microscopy (TDM) images of cells in the space within the valve area (left) and immediate after a valve (right) of the lymph vessel of rat mesentery at ×10 (top) and ×100 (bottom) magnifications demonstrating the principle of cell flow hydrodynamic focusing. TDM images of cells in the central part of a lymphangion in diastole (top, right) and systole (bottom, right) phases are also shown [from (26)].
Potential and already realized applications of a high-speed TDM include (19,35,100–112): fundamental study of cell–cell interactions in native flow; estimation of the proportion of fast-moving WBCs traveling with RBCs in axial flow to slow-moving (rolling) WBCs, which may have diagnostic value for the study of some pathologic conditions; identification of rare abnormal cells (e.g., cancerous or sickled cells) on the basis of their different deformability in flow; imaging of platelets during thrombus formation and interaction with metastatic cancer cells; study of the impact of drugsorradiation on individual cells; estimation of blood viscosity in a high-velocity flow; study of cell aggregation dynamics and adhesion to endothelial cells; estimation of velocity profiles in fast flow; and imaging and detection of individual cell dynamics in afferent lymph flow with cell velocity up to 7 mm/s.
As mentioned, an exposure of the vessel by a laser light induces a number of physical phenomena each of which give rise to a unique cytometry techniques such as light scattering, light transmittance, fluorescence, Raman, PT, or PA methods (Fig. 1), where TDM serves also as a navigator instrumentation for laser beams and finding the region of interest of the vessel net for cytometry in situ using relatively thin transparent tissue.
PT and PA methods
These methods are based on nonradiative relaxation of absorbed laser energy into heat and accompanying acoustic effects (Fig. 1,3). Among different PT-based methods (120, 121), time-resolved two beam (pump-probe) PT thermal lens detection and imaging methods with one (121) and multiplex (122) channel schematics was mostly used in in vivo FCM. In PA FCM, cells in blood or lymph flow are irradiated with a focused laser beam and laser-induced PA waves (referred to as PA signals) are detected with an ultrasound transducer attached to the skin (Fig. 3). These methods offer the highest absorption sensitivity at the single-cell level, 10−2–10−4 cm−1. This level of sensitivity enables imaging of cells noninvasively (i.e., with a short-term temperature elevation ≤0.5°C), with molecular specificity based on label-free intrinsic absorption spectroscopic contrast (e.g., from hemoglobin, melanin, or cytochromes) or on low-toxicity, functionalized strongly absorbing nanoparticles (NPs) (Fig.3) PA technique demonstrates an advantage in assessing of deep vessels in vivo (up to 1–3 cm) (123) while PT methods have higher sensitivity in the study of relatively transparent thin structures like animal ear or mesentery. Recent technical advances include use a high pulse repetition rate nanosecond lasers (up to 0.5 MHz) such as diode laser at 905 nm (29) and fiber-based Yab laser at 1064 nm (34,40), two-color PA FCM (15, 26,29, 41), integration of in vivo magnetic enrichment and multiplex PA detection (15), minimally invasive fiber delivery of laser radiation to vessels (29,30), and potential to use contrast agent with tunable ultrasharp PT and PA spectral resonances and holes (up to a few nm width) for multicolor detection (124).
Fig.3.
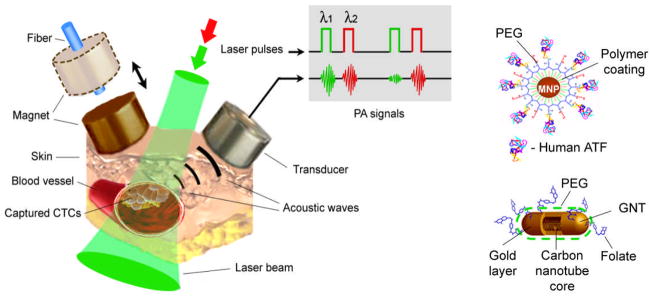
In vivo magnetic enrichment and multiplex two – color photoacoustic (PA) detection of circulating tumor cells (CTCs) molecularly targeted by cocktail of golden carbon nanotubes (GNTs) and magnetic nanoparticles (MNP) [from (15)].
Fluorescent methods
Fluorescence detection schematics were used in in vivo FCM in different modifications (e.g., confocal or two-photon) employing standard fluorescent labels as in conventional FCM in vitro (1). In the confocal scheme, fluorescent signals from the cell populations of interest were recorded as the cells passed through a slit of laser (e.g., He-Ne) light focused across 20–50-μm mouse ear blood vessels (43,44). Emitted fluorescence was collected by microscope objectives, and directed through a dichroic splitter and mirrors to photomultiplier tubes. Compared to single-photon fluorescence microscopy, multiphoton fluorescent technique (68,76) can increase the depth of light penetration in microvessels located deeper in tissue (few hundred μm) and reduce out-of-focus photodamage. However, this technique is typically using focused circle laser beams (compared to classic linear beam shape) that may lead to missing cells flowing in relatively large vessels outside irradiated zone. Recent advances include use of two color schematics (49,71) and fiber-based deliverylaser light to deep vessels (75).
Raman spectroscopy
Zharov’s team introduced in vivo Raman flow cytometry in which scattering signals from Raman active vibrational states were detected using a high sensitive optical technique with fast spectral acquisition down to few milliseconds (28). This technique can use either intrinsic contrast agents such as lipids with CH2 state or exogenous labels such as carbon nanotubes and as proposed in (6,7 33) surface-enhanced Raman scattering (SERS) agents including gold nanoparticle coated with Raman active dyes.
An integrated Raman-based cytometry was developed with PT and PA two-beam detection of Raman-induced thermal and acoustic signals in biological samples with Raman-active vibrational modes (33) that can be applied also for in vivo FCM. Coherent anti-Stokes Raman scattering (CARS) demonstrated promises for detection of tumor cells with high lipid content (79).
Detection of the scattered light
In conventional in vitro FCM, detection of forwardly and orthogonally scattered light allows for discrimination between lymphocytes, monocytes, and granulocytes (1). Light scattering spectroscopy has identified differences in the wavelength dependence of backscattered light between normal and cancer cells due to cellular transformations in early stages of disease development. Recently the backscattering confocal systems allows for characterizing differentially leukemic and normal WBCs and RBCs were developed (65,66). This approach has good perspectives for in vivo light scattering FCM.
To reduce the interference from light scattering background from surrounding tissue and normal cells, gold NPs with strong light scattering due to plasmonic resonance was proposed as contrast agents for in vivo FCM (80,81). Further development of in vivo scattering FCM may include detection of enhanced scattering effects from laser-induced nano- and microbubbles around overheated absorbing contrast agents as demonstrated in vivo for non-moving cells (125).
Cell flows
Most studies were performed on superficial ~50 μm blood microvessels in mouse ear. PA FCM with ability to assess deep vessels demonstrated noninvasive detection of CTCs in ~300 μm skin vessels and 0.9 mm aorta at depth around 2–3 mm using focused cylindrical ultrasound transducer (29). Zharov’s team extended application of in vivo FCM on lymph flow using natural cell focusing after lymphatic valves (19, 22, 26) and recently on bones (36) and plants (37, 126).
Labeling in vivo
The great advantage of in vivo FCM is the possibility for label-free detection using intrinsic contrast agents, such as hemoglobin, melanin, or cytochromes, in video-imaging and PT/PA methods (19,20, 23) or lipids in Raman-based techniques (79), and intrinsic scattering (1, 65,66) or autofluorescence properties of cells. Nevertheless, cells can be labeled directly in the bloodstream by intravenous injection of florescent dyes (43, 46, 57) of functionalized NPs (Fig.3) (15). Depending on cell and contrast agent types labeling procedure on mice models takes from 10–20 min (15) to approximately one hour (57).
Animal models and potential for humans
To fully exploit the capabilities of FCM, it is extremely important to choose the proper animal model. The acute preparation is widely used and usually requires a surgical procedure on the anesthetized animal while placing it on a specially heated platform (22, 96, 109). Such preparations are usually required for studying the microcirculation of internal organs, tumor development, angiogenesis, inflammation, thrombosis, and the progression of infection. FCM utilizes acute preparations in rodent models to expose the vessels of mesentery, skin flap, bone, brain, liver, kidney, lungs, and cremaster muscle (109,118). Less invasive are models of eye conjunctiva (99), iris (119) and retina (59,60) which are directly applicable in humans. A widely spread and intensively developing human noninvasive model is the nailfold model, which is on use in in vivo capillaroscopy for many years (16, 17).
Many studies in vivo FCM were performed in thin (250–300 μm), relatively transparent (i.e., also suitable for PT FCM (in transillumination mode) mice ear with well-distinguished blood microvessels. The ear blood vessels under examination are located 30–100 μm deep and have diameters in the range of 30–50 μm and blood velocities of 1–5 mm/sec (23,43). Also many experiments were performed with the rat mesentery which has an almost ideal vascular and surrounding tissue structure to proof concept of in vivo FCM because it consists of very thin (7–15 μm) transparent connective tissue with a single layer of blood and lymph microvessels (109).
These animal models were successfully used in various promising applications (see below) at fluorescent in vivo FCM. However, translation of this technology to in vivo situation in human faces many challenges such as toxicity of fluorescent probes, broad emission spectra in near-infrared (NIR) window of tissue transparency (i.e., multicolor problem), strong scattering and autofluorescence background, high intensity of CW laser used (10–100 W/cm2) compared to laser safety standard (0.2 – 0.5 W/cm2) (127), and assessment only superficial (≤ 0.3 mm) microvessels with a slow flow rate. For example, in 50-μm blood vessel with flow velocity of 5 mm/s, approximately two days are required to continuously assess total ~2-ml blood mouse volume.
PT and, especially PA, methods are almost free of these limitations. For example, optical-resolution photoacoustic microscopy (OR-PAM) working in reflection-mode is described (128). It allows for in vivo, noninvasive, label-free, three-dimensional (3D) microvascular imaging down to single capillaries and quantification of total hemoglobin concentration and hemoglobin oxygenation saturation. Several successful pilot trials on humans demonstrated a clinical significance and safety of PA technique including: 1) continuous monitoring of blood oxygenation in the internal jugular vein (10–20 mm in diameter, 5–10 mm deep) in the presence of strong light scattering and attenuation in the 15–20 mm layer of overlying tissue (129); 2) detection and imaging of breast tumor at a depth of 3 cm (130); and 3) monitoring of blood hemoglobin in the wrist area overlying the radial artery at a depth of 3 mm (131). PA technology is safe for human subjects as it requires a laser energy fluence of only 5–20 mJ/cm2 which is well within the laser safety standard (35–100 mJ/cm2) in NIR range (e.g., 700–1100 nm) (127). The high sensitivity of nonlinear PT/PA technique based on synergistic amplification of PT/PA signals associated with high localized (within single cells) laser-induced nanobubbles around overheated multilayer (e.g., with ethanol) plasmonic NPs, and especially their clusters allows further reduction the threshold of laser pulse fluence to 1–5 mJ/cm2 (32,36, 132–136). First clinical prototype of fiber-based PA FCM is shown in Fig. 4 (29,41).
Fig.4.
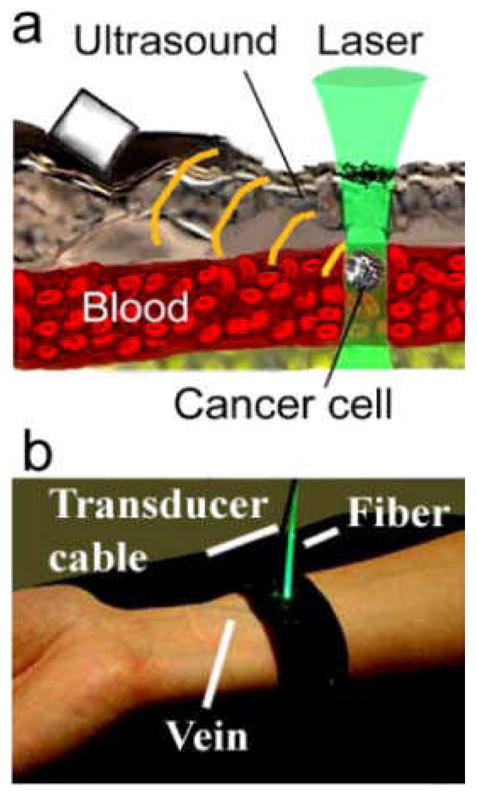
(a) Schematic of in vivo PA flow cytometry (FCM). (b) Clinical prototype of personal in vivo PA FCM (from [29,41]).
Applications
The status and potential applications of different modification of in vivo FCM are discussed in previous (5,22,25–27) and presented in this issue’s papers. Most studies were performed on 50–300 μm blood microvessels with cell flow rate in the range of 104 – 106 cells/s (i.e., comparable or higher than in conventional in vitro FCM with typical cell rate of 104 –105 cell/s) with focus on detection of relatively rare cells with linear laser beam shape overlapping vessel diameter. Briefly, the capacity of clinically relevant in vivo PA FCM technologies was demonstrated by the real-time detection in blood and lymph flows of circulating individual normal cells (e.g., erythrocytes and leukocytes) in different functional states (e.g., normal, apoptotic, or necrotic), tumor cells (melanoma, breast, squamous), bacteria (e.g., E. coli and S. aureus), NPs (e.g., gold nanorods, carbon nanotubes, magnetic and golden carbon nanotubes, and dyes (e.g., Lymphazurin, Evans Blue, and Indocyanine Green) (15, 19–42). Compared to other promising approaches PT FCM and PA FCM offer the following advantages: 1) use of low-toxicity NPs approved for a pilot study in humans; 2) high-resolution (300 nm) PT and optical imaging (in vivo image FCM) of flowing single cells of interest at velocities up to 2 m/s (23,34,40); 3) PT and PA measurements of the velocity of individual flowing cells (19, 29,42) and of cell flows using optical speckle measurements (96, 97, 99); 4) theranostic of circulating abnormal cells (e.g., CTCs) as integration of PA diagnostics, real-time highly localized (i.e., not harmful for surrounding normal blood cells) PT killing using the same laser, and control efficiency of therapy through decrease PA signal amplitudes and CTC counts (29, 31); 5) PA detection of disseminated tumor cells in lymphatics as the earliest prognostic marker of metastasis, compared to sentinel lymph node and blood assessment (30); 6) use of magnetic NPs as multifunctional PA-PT-magnetic resonance imaging contrast agents for in vivo magnet-induced NP clustering, nanobubble-related signal amplification, imaging, and cell enrichment/sorting/separation (15, 31); and 7) the use of PA FCM both with noninvasive transcutaneous laser irradiation and minimally-invasive approach using tiny needle or catheter for delivery of laser radiation theoretically in any vessels inside the body (25, 29). Recent applications include real-time monitoring of blood rheology parameters and its change during pathological processes (review in this issue [38)]), label-free detection of circuiting clots using negative PT and PA contrasts for early cardiovascular disease diagnosis and potentially prevention stroke (this issue [39]), in vivo real-time monitoring of dye-cell interaction, circulating dead cells, and potential blood volume (this issue [41]), (and cell identification based on their different velocity in circulation (42). One of the most important clinical applications in future should be high sensitive detection of CTCs (melanoma and breast) with unprecedented threshold sensitivity as 1 CTC/mL on animal model with potential to improve this threshold by two orders of magnitude on humans (15, 39), detection of the extremely rare circulating cancer stem cells (31), and dangerous clots (39).
In the initial study of Lin’s group fluorescent detection mode was used (43–48). RBCs were isolated from the animal, fluorescently labeled ex vivo, and then reinjected into the animal. In contrast, WBCs were labeled in vivo by injecting a WBC surface antigen-specific antibody. It was observed that the number of circulating RBCs remained constant for at least 3 days, while the number of WBCs decreased significantly in a few hours compared to the much longer life of non-labeled cells. This finding resulted possible from the influence of the tags on genuine cell interactions in flow. These experiments also revealed the dependence of the kinetics of circulating cancer cells, in particular, the dependence of the rate of cell elimination from blood flow, on the type of cancer cells and the host environment (44). Fluorescent techniques in animal models have shown promises for detecting labeled hematopoietic stem cells and green fluorescent protein–expressing cells (49, 52, 55). The methods used to label circulating cells for fluorescence detection by in vivo FCM which are useful for cell tracking in small animals, and are particularly useful for the detection of circulating cancer cells and quantification of circulating immune cells are overviewed in this issue (57).
No doubts that in vivo FCM is currently a fast growing area of research with large horizon of new opportunities in biological sciences and medical fields including combination of different detection methods (21,22).
This special issue is mostly focused on the state-of-the-art in the field of in vivo FCM and its applications with particular emphasising the biophotonic methods, disease diagnosis, and monitoring of disease treatment at single cell level in flow conditions. The use of photonic technologies in medicine is a rapidly emerging and potentially powerful approach for disease detection and treatment. Therefore, the collection of papers from the leading groups in the field seeks to advance scholarly research that spans from fundamental interactions between light, cells, vascular tissue, and labeling particles, to strategies and opportunities for preclinical and clinical research. The general topics are fast video-imaging microscopic, polarization sensitive, laser-scanning, light scattering, confocal microscopic, single and multiphoton fluorescence, PT and PA FCM methods for cellular diagnostics and monitoring of disease treatment in living organisms and plants.
It is opened by a few comprehensive reviews by Galanzha and Zharov on the analysis of the capability of PT and PT FCMs for monitoring multiple blood rheology parameters including identification of sickle cells in a mouse model of human sickle cell disease (38), by Pitsillides et al. on the analysis of cell labeling approaches for fluorescence-based in vivo FCM (57), by Morgan on optical techniques for in vivo imaging of the microcirculation and FCM with focus of its capability to assess sickle cells (17), by Tkaczyk ER and Tkaczyk AH on strategies and applications of multiphoton FCM (76), by Niesner and Hauser on recent advances of dynamic intravital multi-photon microscopy (78), and Wlodkowic et al. on innovative technologies for in situ analysis of small multicellular organisms (137). Most reviews contain carefully selected and deeply analyzed data which are typically summarized in the convenient way for readers mostly in well-designed tables. The table with listed methods of cell labeling for in vivo FCM as well as the cell types studied, can be found in paper (57). The table summarizing optical techniques for in vivo imaging of the microcirculation, such as multiphoton microscopy (MPM), second harmonic generation (SHG), fluorescence lifetime imaging microscopy (FLIM), CARS, Doppler optical coherence tomography (DOCT), optical microangiography (OMAG), PA microscopy (PAM), PT microscopy, laser speckle contrast analysis (LASCA), and hyperspectral imaging (HIS) is presented in paper (17). Paper (76) contains a table with overview of cytometry variations incorporating multiphoton excitation, including techniques that have been used in vivo. Imaging techniques, their lateral and axial resolution, imaging depth, intravitality/in vivo (cell culture/organ models) investigative capabilities are summarized in the table of paper (77). The following techniques are analyzed: MRI, PET/CT, PA microscopy, optical coherence tomography (OCT), wide-field microscopy, confocal microscopy, multi-photon microscopy, confocal or multi-photon endoscopic microscopy, 4Pi-microscopy, stimulated emission depletion microscopy (STED-microscopy), stochastic optical reconstruction microscopy (STORM), photo-activated localization microscopy (PALM) and some others.
The remaining papers are original ones: they demonstrate the cutting edge of research and developments in in vivo FCM (37, 39–41, 62) and present techniques prospective for in vivo and microfluidic-based FCM (65,66). Four original papers are from Zharov’s group. The paper by Nedosekin et al. (40) demonstrates the ultra-fast in vivo PA FCM of circulating human melanoma cells either with label-free detection or with molecular targeting and magnetic enrichment using conjugated magnetic NPs; paper by Proskurnin et al. (41) shows potentialities of PT and PA FCM with multicolor dyes for in vivo real-time assessment of circulation, dye-cell interaction, and blood volume; the paper by Galanzha et al. (39) presents successful application of PT and PA negative contrasting for cytometry of in vivo circulating clots; and paper by Nedosekin et al (37) proposes the real-time noninvasive FCM for study of transported nanomaterials in xylem and phloem plant vascular systems.
One paper by Li et al. (62) is from Wei’s group, where authors using fluorescence in vivo FCM quantified the number and flow characteristics of labeled cells and on this basis investigated the relationship between metastatic potential and depletion kinetics of circulating tumor cells. The two last papers from Georgakoudi’s group by Greiner et al. (65,66) describe in detail fundamentals, instrumentation and applications of confocal backscattering spectroscopy for leukemic and normal blood cell discrimination in flowing blood samples.
Acknowledgments
Editors do appreciate extremely valuable contribution of all authors for the special issue. For studies presented in this mini-review VPZ was supported in part by the National Institutes of Health, grant numbers: R01CA131164; R01EB009230; R01EB000873; R21CA139373; the National Science Foundation, grant number: DBI-0852737, and the Department of Defense, grant numbers: W88XWH-10-2-0130, W81XWH-10-BCRP-CA, and W81XWHAQ711-1-0129. VVT was supported in part by RFBR grant No. 10-07-00526-a, grant No 224014 PHOTONICS4LIFE of FP7-ICT-2007-2, projects No 1.4.09, 2.1.1/4989 and 2.2.1.1/2950 of RF Ministry of Education and Science, RF Governmental contracts 02.740.11.0484, 02.740.11.0770, and 02.740.11.0879; FiDiPro, TEKES Program, Finland (3081/31/2010, § 32.20.40.2.4/11).
Contributor Information
Attila Tárnok, Email: tarnok@medizin.uni-leipzig.de.
Vladimir P. Zharov, Email: zharovvladimirp@uams.edu.
References
- 1.Shapiro HM. Practical Flow Cytometry. 4. N.Y: Wiley-Liss; 2003. [Google Scholar]
- 2.Robinson JP. Reference Books in Cytometry. Purdue University Cytometry Laboratories; Aug 11, 2011. http://www.cyto.purdue.edu/flowcyt/books/bookindx.htm. [Google Scholar]
- 3.Sack U, Tárnok A, Rothe G, editors. Cellular Diagnostics: Basic Principles, Methods and Clinical Applications of Flow Cytometry. Basel, Freiburg, Paris: Karger; 2008. [Google Scholar]
- 4.Ormerod MG. Flow Cytometry - A Basic Introduction, De Novo software. 2008 the electronic version of the book: http://flowbook.denovosoftware.com/; page last modified 08:42, 12 Aug 2010 by Ormerod MG; August 11, 2011.
- 5.Tuchin VV, editor. Advanced Optical Flow Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. [Google Scholar]
- 6.Watson DA, Gaskill DF, Brown LO, Doorn SK, Nolan JP. Spectral measurements of large particles by flow cytometry. Cytometry A. 2009;75A:460–464. doi: 10.1002/cyto.a.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan JP, Sebba DS. Surface-enhanced Raman scattering (SERS) cytometry. Methods Cell Biol. 2011;102:515–32. doi: 10.1016/B978-0-12-374912-3.00020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goddard G, Martin JC, Graves SW, Kaduchak G. Ultrasonic particle-concentration for sheathless focusing of particles for analysis in a flow cytometer. Cytometry A. 2006;69A:66–74. doi: 10.1002/cyto.a.20205. [DOI] [PubMed] [Google Scholar]
- 9.Abkarian M, Faivre M, Stone HA. High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc Natl Acad Sci USA. 2006;103:538–542. doi: 10.1073/pnas.0507171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KC, Di Berardino M, Schade-Kampmann G, Hebeisen M, Pierzchalski A, Bocsi J, Mittag A, Tárnok A. Microfluidic impedance-based flow cytometry. Cytometry A. 2010;77A:648–666. doi: 10.1002/cyto.a.20910. [DOI] [PubMed] [Google Scholar]
- 11.Takao M, Takeda K. Enumeration, characterization, and collection of intact circulating tumor cells by cross contamination-free flow cytometry. Cytometry A. 2011;79A:107–117. doi: 10.1002/cyto.a.21014. [DOI] [PubMed] [Google Scholar]
- 12.Frankowski M, Bock N, Kummrow A, Schädel-Ebner S, Schmidt M, Tuchscheerer A, Neukammer J. A microflow cytometer exploited for the immunological differentiation of leukocytes. Cytometry A. 2011;79A:613–624. doi: 10.1002/cyto.a.21083. [DOI] [PubMed] [Google Scholar]
- 13.Meade SO, Godin J, Chen C-H, Cho SH, Tsai FS, Qiao W, Lo Y-H. Microfluidic flow cytometry: Advancements toward compact, integrated systems. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 273–310. [Google Scholar]
- 14.Swalwell JE, Petersen TW, van den Engh G. Virtual-core flow cytometry. Cytometry A. 2009;75A:960–965. doi: 10.1002/cyto.a.20792. [DOI] [PubMed] [Google Scholar]
- 15.Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nature Nanotechnology. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan SP, Stockford IM. Instrumentation for in vivo flow cytometry – a sickle cell anemia case study. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 433–461. [Google Scholar]
- 17.Morgan SP. Can new optical techniques for in vivo imaging and flow cytometry of the microcirculation benefit sickle cell disease research? Cytometry A. 2011 doi: 10.1002/cyto.a.21101. (this issue) [DOI] [PubMed] [Google Scholar]
- 18.Tuchin VV, Tárnok A, Zharov VP. Towards in vivo flow cytometry. J Biophoton. 2009;2:457–547. doi: 10.1002/jbio.200910546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zharov VP, Galanzha EI, Tuchin VV. Photothermal imaging of moving cells in lymph and blood flow in vivo. Proc SPIE. 2004;5320:256–263. [Google Scholar]
- 20.Zharov VP, Galanzha EI, Tuchin VV. Photothermal image flow cytometry in vivo. Opt Lett. 2005;30:628–630. doi: 10.1364/ol.30.000628. [DOI] [PubMed] [Google Scholar]
- 21.Zharov VP, Galanzha EI, Tuchin VV. Integrated photothermal flow cytometry in vivo. J Biomed Opt. 2005;10:51502. doi: 10.1117/1.2070167. [DOI] [PubMed] [Google Scholar]
- 22.Zharov VP, Galanzha EI, Tuchin VV. In vivo photothermal flow cytometry: imaging and detection of cells in blood and lymph flow (review/prospect) J Cell Biochem. 2006;97(5):916–932. doi: 10.1002/jcb.20766. [DOI] [PubMed] [Google Scholar]
- 23.Zharov VP, Galanzha EI, Shashkov EV, Khlebtsov NG, Tuchin VV. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt Lett. 2006;31:3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]
- 24.Zharov VP, Galanzha EI, Tuchin VV. Photothermal flow cytometry in vitro for detection and imaging of individual moving cells. Cytometry A (review) 2007;71A:191–206. doi: 10.1002/cyto.a.20384. [DOI] [PubMed] [Google Scholar]
- 25.Zharov VP, Galanzha EI, Shashkov EV, Kim J-W, Khlebtsov NG, Tuchin VV. Photoacoustic flow cytometry: principle and application for real-time detection of circulating single nanoparticles, pathogens, and contrast dyes in vivo. J Biomed Opt. 2007;12:0551503. doi: 10.1117/1.2793746. [DOI] [PubMed] [Google Scholar]
- 26.Galanzha EI, Shashkov EV, Tuchin VV, Zharov VP. In vivo multispectral multiparameter photoacoustic lymph flow cytometry with natural cell focusing, label-free detection and multicolor nanoparticle probes. Cytometry A. 2008;73A:884–894. doi: 10.1002/cyto.a.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olszewski WL, Tárnok A. Photoacoustic listening of cells in lymphatics: Research art or novel clinical noninvasive lymph test. Cytometry A. 2008;73A:1111–1113. doi: 10.1002/cyto.a.20654. [DOI] [PubMed] [Google Scholar]
- 28.Biris AS, Galanzha EI, Li Z, Mahmood M, Xu Y, Zharov VP. In vivo Raman flow cytometry for real-time detection of carbon nanotube kinetics in lymph, blood, and tissues. J Biomed Opt. 2009;14(2):021006. doi: 10.1117/1.3119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galanzha EI, Shashkov EV, Spring P, Suen JY, Zharov VP. In vivo noninvasive label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69:7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galanzha EI, Kokoska MS, Shashkov EV, Kim J-W, Tuchin VV, Zharov VP. In vivo fiber-based multicolor photoacoustic detection and photothermal purging of metastasis in sentinel lymph nodes targeted by nanoparticles. J Biophotonics. 2009;2:528–539. doi: 10.1002/jbio.200910046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanzha EI, Kim J-W, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J Biophotonics. 2009;2:725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J-W, Galanzha EI, Shashkov EV, Moon H-M, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nature Nanotechnology. 2009;4:688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shashkov EV, Galanzha EI, Zharov VP. Photothermal and photoacoustic Raman cytometry in vitro and in vivo. Opt Exp. 2010;18(7):6929–6944. doi: 10.1364/OE.18.006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nedosekin DA, Sarimollaoglu M, Shashkov EV, Galanzha EI, Zharov VP. Ultra-fast photoacoustic flow cytometry with a 0.5 MHz pulse repetition rate nanosecond laser. Opt Exp. 2010;18(8):8605–8620. doi: 10.1364/OE.18.008605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuchin VV, Galanzha EI, Zharov VP. In vivo photothermal and photoacoustic flow cytometry. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 501–571. [Google Scholar]
- 36.De la Zerda A, Kim JW, Galanzha EI, Gambhir SS, Zharov VP. Advanced contrast nanoagents for photoacoustic molecular imaging, cytometry, blood test, and photothermal theranostics. Contrast Media & Molecular Imaging. doi: 10.1002/cmmi.455. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedosekin DA, Khodakovskaya MV, de Silva K, Dervishi E, Biris AS, Galanzha EI, Zharov VP. In vivo plant flow cytometry: a first proof-of-concept. Cytometry A. 2011 doi: 10.1002/cyto.a.21128. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galanzha EI, Zharov VP. In vivo photoacoustic and phototohermal flow cytometry for monitoring mutiple blood rheology parameters. Cytometry A. 2011 doi: 10.1002/cyto.a.21133. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galanzha EI, Sarimollaoglu M, Nedosekin DA, Keyrouz SG, Mehta JL, Zharov VP. In vivo flow cytometry of circulating clots using negative phototothermal and photoacoustic contrasts. Cytometry A. 2011 doi: 10.1002/cyto.a.21106. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nedosekin DA, Sarimollaoglu M, Ye J-H, Galanzha EI, Zharov VP. In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers. Cytometry A. 2011 doi: 10.1002/cyto.a.21102. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proskurnin M, Galanzha EI, Mock DM, Zharov VP. In vivo photothermal and photoacoustic flow cytometry with multicolor dyes: a potential for real-time assessment of circulation, dye-cell interaction, and blood volume. Cytometry A. 2011 doi: 10.1002/cyto.a.21127. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarimollaoglu M, Nedosekin DA, Simanovsky Y, Galanzha EI, Zharov VP. In vivo photoacoustic time-of-flight velocity measurement of single cells and nanoparticles. Opt Lett. doi: 10.1364/OL.36.004086. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novak J, Georgakoudi I, Wei X, Prossin A, Lin CP. An in vivo flow cytometer for real-time detection and quantification of circulating cells. Opt Lett. 2004;29:77–79. doi: 10.1364/ol.29.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgakoudi I, Solban N, Novak J, Rice WL, Hasan T, Lin CP. In vivo flow cytometry: A new method for the quantification of circulating tumor cells. Cancer Res. 2004;64:5044–5047. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- 45.Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumor engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei X, Sipkins DA, Pitsillides CM, Novak J, Georgakoudi I, Lin CP. Real-time detection of circulating apoptotics cells by in vivo flow cytometry. Mol Imaging. 2005;4(4):415–416. doi: 10.2310/7290.2005.05148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H, Alt C, Pitsillides C, Puoris’haag M, Lin CP. In vivo imaging flow cytometer. Opt Exp. 2006;14(17):7789–7800. doi: 10.1364/oe.14.007789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alt C, Veilleux I, Lee H, Pitsillides CM, Côté D, Lin CP. Retinal flow cytometer. Opt Lett. 2007;32(23):3450–3452. doi: 10.1364/ol.32.003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boutrus S, Greiner C, Hwu D, Chan M, Kuperwasser C, Lin CP, Georgakoudi I. Portable two-color in vivo flow cytometer for real-time detection of fluorescently-labeled circulating cells. J Biomed Opt. 2007;12(2):020507. doi: 10.1117/1.2722733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsayed Y, Ngo H, Runnels J, Leleu X, Singha OK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM, Jia X, McMillin D, Lu G, Timm M, Kumar A, Hedin KE, Cote D, Veilleux I, Roodman GD, Witzig TE, Kung A, Mitsiades C, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12) dependent migration and homing in Multiple Myeloma. Blood. 2007;109:2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leleu X, Jia X, Runnels J, Ngo HT, Moreau AS, Farag M, Spencer JA, Pitsillides CM, Hatjiharissi E, Roccaro A, O’Sullivan G, McMillin DW, Moreno D, Kiziltepe T, Carrasco R, Treon SP, Hideshima T, Anderson KC, Lin CP, Ghobrial IM. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110(13):4417–4426. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu XP, Xiaoying J, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial I. The CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azab AK, Azab F, Blotta S, Pitsillides CM, Thompson B, Runnels JM, Roccaro AM, Ngo HT, Melhem MR, Sacco A, Jia X, Anderson KC, Lin CP, Rollins BJ, Ghobrial IM. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood. 2009;114(3):619–629. doi: 10.1182/blood-2009-01-199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roccaro AM, Sacco A, Husu EN, Pitsillides C, Vesole S, Azab AK, Azab F, Melhem M, Ngo HT, Quang P, Maiso P, Runnels J, Liang M-C, Wong K-K, Lin C, Ghobrial IM. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood. 2010;115:559–569. doi: 10.1182/blood-2009-07-235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan Z, Spencer JA, Lu Y, Pitsillides CM, Singh G, Kim P, Yun SH, Toxavidis V, Strom TB, Lin CP, Koulmanda M. In vivo tracking of “color-coded” effector, natural and induced regulatory T cells in the allograft response. Nat Med. 2010;16:718–722. doi: 10.1038/nm.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Runnels JM, Carlson AL, Pitsillides C, Thompson B, Wu J, Spencer JA, Kohler JMJ, Azab AK, Moreau AS, Rodig SJ, Kung A, Anderson KC, Ghobrial IM, Lin CP. Characterization of multiple myeloma engraftment, growth and response to therapy using complementary in vivo optical technologies. J Biomed Opt. 2011;16(1):011006. doi: 10.1117/1.3520571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitsillides CM, Runnels JM, Zhi L, Wu M, Lin CP. Cell labeling approaches for fluorescence-based in vivo flow cytometry. Cytometry A. 2011 doi: 10.1002/cyto.a.21125. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- 59.Paques M, Tadayoni R, Sercombe R, Laurent P, Genevois O, Gaudric A, Vicaut E. Structural and hemodynamic analysis of the mouse retinal microcirculation. Invest Ophthalmol Vis Sci. 2003;44:4960–4967. doi: 10.1167/iovs.02-0738. [DOI] [PubMed] [Google Scholar]
- 60.Laemmel E, Genet M, Le Goualher G, Perchant A, Le Gargasson JF, Vicaut E. Fibered confocal fluorescence microscopy (Cell-viZio) facilitates extended imaging in the field of microcirculation. A comparison with intravital microscopy. J Vasc Res. 2004;41:400–411. doi: 10.1159/000081209. [DOI] [PubMed] [Google Scholar]
- 61.Novak J, Puoris’haag M. Two-color, double-slit in vivo flow cytometer. Opt Lett. 2007;32:2993–2995. doi: 10.1364/ol.32.002993. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Guo J, Wang C, Fan Z, Liu G, Wang C, Gu Z, Damm D, Mosig A, Wei X. Circulation times of prostate cancer and hepatocellular carcinoma cells by in vivo flow cytometry. Cytometry A. 2011 doi: 10.1002/cyto.a.21134. (this issue) [DOI] [PubMed] [Google Scholar]
- 63.Greiner C, Georgakoudi I. Advances in fluorescence-based in vivo flow cytometry for cancer applications. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 463–500. [Google Scholar]
- 64.Hwu D, Boutrus S, Greiner C, DiMeo T, Kuperwasser C, Georgakoudi I. Assessment of the role of circulating breast cancer cells in tumor formation and metastatic potential using in vivo flow cytometry. J Biomed Opt. 2011;16(4):040501. doi: 10.1117/1.3560624. [DOI] [PubMed] [Google Scholar]
- 65.Greiner C, Hunter M, Huang P, Rius F, Georgakoudi I. Confocal backscattering spectroscopy for leukemic and normal blood cell discrimination. Cytometry A. 2011 doi: 10.1002/cyto.a.21095. (this issue) [DOI] [PubMed] [Google Scholar]
- 66.Greiner C, Hunter M, Rius F, Huang P, Georgakoudi I. Confocal backscattering-based detection of leukemic cells in flowing blood samples. Cytometry A. 2011 doi: 10.1002/cyto.a.21086. (this issue) [DOI] [PubMed] [Google Scholar]
- 67.Padera TP, Stoll BR, So PT, Jain RK. Conventional and high-speed intravital multiphoton laser scanning microscopy of microvasculature, lymphatics, and leukocyte-endothelial interactions. Mol Imaging. 2002;1:9–15. doi: 10.1162/15353500200200004. [DOI] [PubMed] [Google Scholar]
- 68.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantification of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci USA. 2007;104(28):11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong C, Ye J, Myc A, Cao Z, Kukowska J, Baker J, Norris T. In vivo flow cytometry. Optical Society of America Frontiers in Optics. 2004:FTuE5. [Google Scholar]
- 70.Zhong CF, Ye JY, Myc A, Thomas TP, Bielinska AU, Baker JR, Jr, Norris TB. Two-photon flow cytometry. Proc SPIE. 2005;5700:78–89. [Google Scholar]
- 71.Tkaczyk E, Zhong C, Ye J, Katnik S, Myc A, Thomas T, Luker K, Luker G, Jr, Norris T. Two-photon, two-color in vivo flow cytometry to noninvasively monitor multiple circulating cell lines. SPIE. 2007;6631:66310T. [Google Scholar]
- 72.Tkaczyk ER, Zhong CF, Ye JY, Myc A, Thomas T, Cao Z, Duran-Struuck R, Luker KE, Luker GD, Norris TB, Baker JR. In vivo monitoring of multiple circulating cell populations using two-photon flow cytometry. Opt Commun. 2008;281:888–894. doi: 10.1016/j.optcom.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong CF, Tkaczyk ER, Thomas T, Ye JY, Myc A, Bielinska AU, Cao Z, Majoros I, Keszler B, Baker JR, Norris TB. Quantitative two-photon flow cytometry-in vitro and in vivo. J Biomed Opt. 2008;13:034008-1–19. doi: 10.1117/1.2931077. [DOI] [PubMed] [Google Scholar]
- 74.Tkaczyk ER, Tkaczyk AH, Katnik S, Ye JY, Luker KE, Luker GD, Myc A, Baker JR, Jr, Norris TB. Extended cavity laser enhanced two-photon flow cytometry. J Biomed Opt. 2008;13:041319-1–12. doi: 10.1117/1.2967983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang YC, Ye JY, Thomas TP, Cao Z, Kotlyar A, Tkaczyk ER, Baker JR, Jr, Norris TB. Fiber-optic multiphoton flow cytometry in whole blood and in vivo. J Biomed Opt. 2010;15:047004. doi: 10.1117/1.3463481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tkaczyk ER, Tkaczyk AH. Multiphoton flow cytometry strategies and applications. Cytometry A. 2011 doi: 10.1002/cyto.a.21110. (this issue) [DOI] [PubMed] [Google Scholar]
- 77.Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Niesner RA, Hauser AE. Recent advances in dynamic intravital multi-photon microscopy. Cytometry A. 2011 doi: 10.1002/cyto.a.21140. (this issue) [DOI] [PubMed] [Google Scholar]
- 79.Le TT, Huff TB, Cheng JX. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer. 2009;9:42. doi: 10.1186/1471-2407-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanev S, Sun W, Pond J, Tuchin VV, Zharov VP. Flow cytometry with gold nanoparticles and their clusters as scattering contrast agents. J Biophotonics. 2009;2:505–520. doi: 10.1002/jbio.200910039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanev S, Sun W, Pond J, Tuchin VV, Zharov VP. Optical Imaging of Cells with Gold Nanoparticle Clusters as Light Scattering Contrast Agents: A Finite-Difference Time-Domain Approach to the Modeling of Flow Cytometry Configurations. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 35–62. [Google Scholar]
- 82.Bednov AA, Brill GE, Tuchin VV, Ul’yanov SS, Zakharova (Galanzha) EI. Blood and lymph flow measurements in microressels using focused laser beam diffraction phenomenon. Proc SPIE. 1994;2370:379–383. [Google Scholar]
- 83.Ul’yanov SS, Tuchin VV, Bednov AA, Brill GE, Zakharova (Galanzha) EI. Speckle-interferometrical method in application to the blood and lymph flow monitoring in microvessels. Lasers Med Sci. 1996;11(2):97–107. [Google Scholar]
- 84.Bednov AA, Ul’yanov SS, Tuchin VV, Brill GE, Zakharova (Galanzha) EI. In vivo laser measurements of blood and lymph flow with a small number of scatterers. SPIE CIS Selected Papers. 1996;2732:27–33. [Google Scholar]
- 85.Bednov AA, Zakharova (Galanzha) EI, Brill GE, Tuchin VV. Investigation of statistical properties of lymph flow dynamics using speckle microscopy. Proc SPIE. 1997;2981:181–190. [Google Scholar]
- 86.Brill GE, Tuchin VV, Zakharova (Galanzha) EI, Ulyanov SS. Influence of low power laser irradiation on lymph microcirculation at the increasing of NO production. Proc SPIE. 1999;3726:157–162. [Google Scholar]
- 87.Galanzha EI, Brill GE, Tuchin VV, Ulyanov SS, Solov’eva AV, Sedykh AV. Analysis of lymph flow in microvessels by biomicroscopic and coherent optical methods. Proc SPIE. 2000;4001:166–173. [Google Scholar]
- 88.Starukhin P, Ulyanov S, Galanzha E, Tuchin V. Blood-flow measurements with a small number of scattering events. Appl Opt. 2000;39(10):2823–2829. doi: 10.1364/ao.39.002823. [DOI] [PubMed] [Google Scholar]
- 89.Galanzha EI, Brill GE, Ulyanov SS, Tuchin VV, Solov’eva AV, Sedykh AV. Peculiarities of lymph flow in microvessels. Proc SPIE. 2000;3923:149–154. [Google Scholar]
- 90.Galanzha EI, Ulyanov SS, Tuchin VV, Brill GE, Solov’eva AV, Sedykh AV. Comparison of lymph and blood flow in microvessels: coherent optical measurements. Proc SPIE. 2000;4163:94–98. [Google Scholar]
- 91.Galanzha EI, Ulyanov SS, Tuchin VV, Brill GE, Solov’eva AV. Imaging of lymph flow in single microvessels in vivo. Proc SPIE. 2000;4224:317–321. [Google Scholar]
- 92.Galanzha EI, Tuchin VV, Ulyanov SS, Solovieva AV, Luo Q, Cheng H. Optical properties of lymph flow in single microvessels: biomicroscopic, speckle-interferometric, and spectroscopic measurements. Proc SPIE. 2001;4434:197–203. [Google Scholar]
- 93.Galanzha EI, Solov’eva AV, Brill GE, Ulyanov SS, Tuchin VV. Monitoring of lymph flow in microvessels by biomicroscopy and speckle-interferometry. Proc SPIE. 2001;4251:210–214. [Google Scholar]
- 94.Brill GE, Galanzha EI, Ul’ianov SS, Tuchin VV, Stepanova TV, Solov’eva AV. Functional organization of lymphatic microvessels of the rat mesentery. Ross Fiziol Zh im I M Sechenova. 2001;87:600–607. [PubMed] [Google Scholar]
- 95.Galanzha EI, Fedosov IV, Solov’eva AV, Stepanova TV, Tuchin VV, Brill GE. In vivo lymph dynamic monitoring using speckle-correlation technique and light microscopy. Proc SPIE. 2002;4624:130–133. [Google Scholar]
- 96.Galanzha EI, Brill GE, Aisu Y, Ulyanov SS, Tuchin VV. Speckle and Doppler methods of blood and lymph flow monitoring. In: Tuchin VV, editor. Handbook of Optical Biomedical Diagnostics. Bellingham, WA: SPIE Press; 2002. pp. 881–937. [Google Scholar]
- 97.Fedosov IV, Tuchin VV, Galanzha EI, Solov’eva AV, Stepanova TV. Recording of lymph flow dynamics in microvessels using correlation properties of scattered coherent radiation. Quantum Electronics. 2002;32(11):849–867. [Google Scholar]
- 98.Galanzha EI, Tuchin VV, Solovieva AV, Stepanova TV, Luo Q, Cheng H. Skin backreflectance and microvascular system functioning at the action of osmotic agents. J Phys D: Appl Phys. 2003;36:1739–1746. [Google Scholar]
- 99.Fedosov IV, Ulyanov SS, Galanzha EI, Galanzha VA, Tuchin VV. Laser Doppler and speckle techniques for bioflow measurements. In: Tuchin VV, editor. Handbook of Coherent-Domain Optical Methods: Biomedical Diagnostics, Environmental and Material Science. Vol. 1. Boston: Kluwer Academic Publishers; 2004. pp. 397–435. [Google Scholar]
- 100.Galanzha EI, Tuchin VV, Solov’eva AV, Stepanova TV, Brill GE, Zharov VP. Development imaging and experimental model for studying pathogenesis and treatment efficacy of postmastectomy lymphedema. Proc SPIE. 2002;4624:123–129. [Google Scholar]
- 101.Galanzha EI, Tuchin VV, Solov’eva AV, Zharov VP. Development of optical diagnostics of microlymphatics at the experimental lymphedema: comparative analysis. J X-Ray Sci and Technol. 2002;10:215–223. [PubMed] [Google Scholar]
- 102.Galanzha EI, Tuchin VV, Zharov VP, Solovieva AV, Stepanova TV, Brill GE. The diagnosis of lymph microcirculation on rat mesentery in vivo. Proc SPIE. 2003;4965:325–333. [Google Scholar]
- 103.Galanzha EI, Tuchin VV, Chowdhury P, Zharov VP. Monitoring of small lymphatics function under different impact on animal model by integrated optical imaging. Proc SPIE. 2004;5474:204–214. [Google Scholar]
- 104.Galanzha EI, Chowdhury P, Tuchin VV, Zharov VP. Monitoring of nicotine impact on microlymphatics of rat mesentery with time-resolved microscopy. Lymphology. 2005;38:181–192. [PubMed] [Google Scholar]
- 105.Galanzha EI, Tuchin VV, Zharov VP. In vivo integrated flow image cytometry and lymph/blood vessels dynamic microscopy. J Biomed Opt. 2005;10:54018. doi: 10.1117/1.2060567. [DOI] [PubMed] [Google Scholar]
- 106.Zharov VP, Galanzha EI, Menyaev YA, Tuchin VV. In vivo high-speed imaging of individual cells in fast blood flow. J Biomed Opt. 2006;11:054034. doi: 10.1117/1.2355666. [DOI] [PubMed] [Google Scholar]
- 107.Zharov VP, Menyaev Yu, Shashkov EV, Galanzha EI, Khlebtsov BN, Scheludko A, Zimnyakov DA, Tuchin VV. Fluctuations of probe beam in thermolens schematics as potential indicator of cell metabolism, apoptosis, necrosis and laser impact. Proc SPIE. 2006;6085:10–21. [Google Scholar]
- 108.Galanzha EI, Tuchin VV, Zharov VP. Optical monitoring of microlympatic disturbances at experimental lymphedema. Lymphat Res Biol. 2007;5:11–27. doi: 10.1089/lrb.2007.5103. [DOI] [PubMed] [Google Scholar]
- 109.Galanzha EI, Tuchin VV, Zharov VP. Advances in small animal mesentery models for in vivo flow cytometry, dynamic microscopy, and drug screening (invited review) World J Gastroenterol. 2007;13:192–218. doi: 10.3748/wjg.v13.i2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tuchin VV, Galanzha EI, Zharov VP. In vivo image flow cytometry. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 387–433. [Google Scholar]
- 111.Kalchenko V, Harmelin A, Fine I, Zharov V, Galanzha E, Tuchin V. Advances in intravital microscopy for monitoring cell flow dynamics in vivo. Proc SPIE. 2007;6436:64360D-1–15. [Google Scholar]
- 112.Kalchenko V, Brill A, Bayewitch M, Fine I, Zharov V, Galanzha E, Tuchin V, Harmelin A. In vivo dynamic light scattering imaging of blood coagulation. J Biomed Opt. 2007;12(5):052002-1–4. doi: 10.1117/1.2778695. [DOI] [PubMed] [Google Scholar]
- 113.Minamitani H, Tsukada K, Sekizuka E, Oshio C. Optical bioimaging: from living tissue to a single molecule: imaging and functional analysis of blood flow in organic microcirculation (Forum minireview) J Pharmacol Sci. 2003;93:227–233. doi: 10.1254/jphs.93.227. [DOI] [PubMed] [Google Scholar]
- 114.Japee SA, Pittman RN, Ellis CG. A new video image analysis system to study red blood cell dynamics and oxygenation in capillary networks. Microcirculation. 2005;12:489–506. doi: 10.1080/10739680591003332. [DOI] [PubMed] [Google Scholar]
- 115.Dixon JB, Zawieja DC, Gashev AA, Coté GL. Measuring microlymphatic flow using fast video microscopy. J Biomed Opt. 2005;10:064016. doi: 10.1117/1.2135791. [DOI] [PubMed] [Google Scholar]
- 116.Dixon JB, Greiner ST, Gashev AA, Coté GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 117.McNamara PM, O’Doherty J, O’Connell M-L, Fitzgerald BW, Anderson CD, Nilsson GE, Toll R, Leahy MJ. Tissue viability (TiVi) imaging: Temporal effects of local occlusion studies in the volar forearm. J Biophoton. 2010;3(1–2):66–74. doi: 10.1002/jbio.200910061. [DOI] [PubMed] [Google Scholar]
- 118.Leahy MJ, O’ Doherty J. Optical instrumentation for the measurement of blood perfusion, concentration, and oxygenation in living microcirculation. In: Tuchin VV, editor. Advanced Optical Cytometry: Methods and Disease Diagnoses. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. pp. 573–603. [Google Scholar]
- 119.Zhu Q, Stockford IM, Crowe JA, Morgan SP. An experimental and theoretical evaluation of rotating orthogonal polarization imaging. J Biomed Opt. 2009;14:034006. doi: 10.1117/1.3130268. [DOI] [PubMed] [Google Scholar]
- 120.Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zharov VP, Lapotko DO. Photothermal imaging of nanoparticles and cells (review) IEEE J Sel Topics Quant Electron. 2005;11:733–751. [Google Scholar]
- 122.Zharov VP, Galitovsky V, Lyle CS, Chambers TC. Superhigh-sensitivity photothermal monitoring of individual cell response to antitumor drug. J Biomed Opt. 2006;11:064034. doi: 10.1117/1.2405349. [DOI] [PubMed] [Google Scholar]
- 123.Wang LV. Multiscale photoacoustic microscopy and computed tomography. Nat Photonics. 2009;3:503–509. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zharov VP. Ultrasharp nonlinear photothermal and photoacoustic resonances and holes beyond the spectral limit. Nature Photonics. 2011;5:110–116. doi: 10.1038/nphoton.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee H, Alt C, Pitsillides CM, Lin CP. Optical detection of intracellular cavitation during selective laser targeting of the retinal pigment epithelium: dependence of cell death mechanism on pulse duration. J Biomed Opt. 2007;12:064034. doi: 10.1117/1.2804078. [DOI] [PubMed] [Google Scholar]
- 126.Khodakovskaya MV, Silva Ke, Nedosekin DA, Dervishi E, Biris AB, Shashkov EV, Galanzha EA, Zharov VP. Multiplex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. PNAS. 2011;108(3):1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Standard American National Standard for Safe Use of Lasers. ANSI Z136 1. 2000. [Google Scholar]
- 128.Hu S, Maslov K, Wang LV. Optical-resolution photoacoustic microscopy for in vivo volumetric microvascular imaging in intact tissues. In: Tuchin VV, editor. Handbook of Photonics for Biomedical Science. London: CRC Press, Taylor & Francis Group; 2010. pp. 361–375. [Google Scholar]
- 129.Petrov YY, Petrova IY, Patrikeev IA, Esenaliev RO, Prough DS. Multiwavelength optoacoustic system for noninvasive monitoring of cerebral venous oxygenation: a pilot clinical test in the internal jugular vein. Opt Lett. 2006;31:1827–1829. doi: 10.1364/ol.31.001827. [DOI] [PubMed] [Google Scholar]
- 130.Ermilov SA, Khamapirad T, Conjusteau A, Leonard MH, Lacewell R, Mehta K, Miller T, Oraevsky AA. Laser optoacoustic imaging system for detection of breast cancer. J Biomed Opt. 2009;14:024007. doi: 10.1117/1.3086616. [DOI] [PubMed] [Google Scholar]
- 131.Petrova IY, Esenaliev RO, Petrov YY, Brecht HPE, Svensen CH, Olsson J, Deyo DJ, Prough DS. Optoacoustic monitoring of blood hemoglobin concentration: a pilot clinical study. Opt Lett. 2005;30:1677–1679. doi: 10.1364/ol.30.001677. [DOI] [PubMed] [Google Scholar]
- 132.Zharov V, Galitovsky V, Viegas M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl Phys Lett. 2003;83(24):4897–4899. [Google Scholar]
- 133.Zharov VP, Kim J-W, Everts M, Curiel DT. Self-assembling nanoclusters in living systems: application for integrated photothermal nanodiagnostics and nanotherapy (review) Nanomedicine. 2005;1:326–345. doi: 10.1016/j.nano.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 134.Zharov VP, Galitovskaya EN, Jonson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Laser Surg Med. 2005;37:219–226. doi: 10.1002/lsm.20223. [DOI] [PubMed] [Google Scholar]
- 135.Khlebtsov B, Zharov V, Melnikov A, Tuchin V, Khlebtsov N. Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters. Nanotechnology. 2006;17:5167–5179. [Google Scholar]
- 136.Akchurin G, Khlebtsov B, Akchurin G, Jr, Tuchin V, Zharov V, Khlebtsov N. Gold nanoshell photomodification under single nanosecond laser pulse accompanied by color-shifting and bubble formation phenomena. Nanotechnology. 2008;19:015701-1–8. doi: 10.1088/0957-4484/19/01/015701. [DOI] [PubMed] [Google Scholar]
- 137.Wlodkowic D, Khoshmanesh K, Akagi J, Williams DE, Cooper JM. Wormometry-on-a-chip: Innovative technologies for in situ analysis of small multicellular organisms. Cytometry A. 2011 doi: 10.1002/cyto.a.21070. (this issue) [DOI] [PubMed] [Google Scholar]