Abstract
Honokiol ((3’,5-di-(2-propenyl)-1,1’-biphenyl-2,2’-diol) is a bioactive natural product derived from Magnolia spp. Recent studies have demonstrated anti-inflammatory, anti-angiogenic, anti-oxidative and anti-cancer properties of honokiol in vitro and in preclinical models. Honokiol targets multiple signaling pathways including nuclear factor kappa B (NF-κB), signal transducers and activator of transcription 3 (STAT3), epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (m-TOR), which have great relevance during cancer initiation and progression. Furthermore, pharmacokinetic profile of honokiol has revealed a desirable spectrum of bioavailability after intravenous administration in animal models, thus making it a suitable agent for clinical trials. In this review, we discuss recent data describing the molecular targets of honokiol and its anti-cancer activities against various malignancies in pre-clinical models. Evaluation of honokiol in clinical trials will be the next step towards its possible human applications.
Keywords: Honokiol, chemoprevention, chemotherapy, natural agent
1.0. Introduction
Honokiol is a bioactive compound obtained from several species of the genus Magnolia (officinalis, obovata, and grandiflora) of Magnoliaceae family (1). The Magnolia genus is distributed throughout the world with main center in east and south-east Asia. The bark, cones, and leaves from Magnolia officinalis plant have long been used in traditional Chinese medicine, where it is known as “houpa”. In Japan, Magnolia obovata has been used in a similar manner and is known as “koboku”. Recent studies in animals and in vitro models have demonstrated multiple biological properties of honokiol including anti-arrhythmic (2), anti-inflammatory (3), anti-thrombocytic (4), anti-angiogenesis (5, 6), anti-tumor (7, 8), anxiolytic (9), anti-oxidative (10) activities. Honokiol has also been found to exert potent broad-spectrum antimicrobial (11), antifungal (12) and anti-HIV (human immunodeficiency viruses) activities (13).
Cancer is major health problem world-wide. It is the second-leading cause of disease-related deaths in the United States after the heart disease (14). While the mortality from heart disease continues to decrease both in younger and older patients, not much improvement in life expectancy has been achieved in patients diagnosed with cancer (15). To make significant progress in fight against cancer, advances in cancer diagnosis and development of effective preventive and therapeutic strategies are needed. As exemplified by other agents from aspirin to taxol, the use of natural products has been successful approach towards managing, this devastating health problem. The present review discusses the accumulating literature on the anti-tumor activity of honokiol against various malignancies, its mechanism of action, pharmacokinetics and safety aspects for possible human applications.
2.0. Honokiol and its anti-tumor derivatives
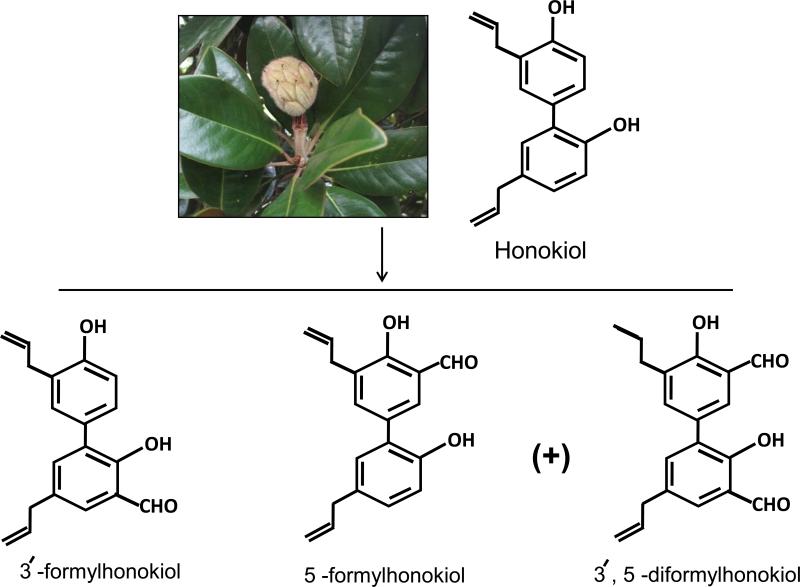
Honokiol is a small bi-phenolic lignan with molecular formula C18H18O2 and molecular weight of 266 g/mole (Figure 1). It consists of a para-allyl-phenol and an ortho-allyl-phenol that are linked together through ortho-, para-C-C-coupling. Previously, it has been shown that, ether derivative and 3,3′-diallyl derivative also have significant growth inhibitory activity against Hep-G2 cells (human hepatocellular carcinoma), suggesting that the 3′-allyl group of honokiol plays an important role in inducing cytotoxicity (16). The 4′-hydroxy and the 5-allyl groups are responsible for honokiol-mediated neurite growth-promoting activity (1). Three novel anti-tumor derivatives of honokiol namely 3’- formylhonokiol, 5-formylhonokiol and 3’, 5-diformylhonokiol have also been developed (Figure 1) (17). Out of these derivatives, 3’- formyl-honokiol and 5-formyl-honokiol are actually isomers with latter possessing the strongest inhibitory activity against K562 (human myelogenous leukemia), A549 (human lung adenocarcinoma) and SPC-A1 (human lung adenocarcinoma) tumor cell lines (17). The formyl group at C-5 position poses the enhanced inhibitory effect through formation of intra-molecular hydrogen bond, whereas a formyl group at C-3 position decreases anti-tumor activity. In a recent study, a derivative of honokiol namely, 3′,5-diallyl-2,4′-dihydroxy-[1,1′-biphen-yl]-3,5′-dicarbaldehyde was found to suppress the newly-grown segmental vessels from the dorsal aorta of zebrafish, and to prevent inappropriate vascularisation as well as exhibit more potent growth inhibitory effects on human umbilical vein endothelial cells (HUVECs), A549, HepG2, and LL/2 (mouse Lewis lung carcinoma) cells as compared to honokiol (18). The above structure–activity relationship (SAR) studies suggest that the presence of hydroxyl and allylic groups on a biphenolic moiety of honokiol are responsible for growth inhibitory effect of honokiol (18, 19)
Figure 1.
Honokiol and its derivatives. Three novel anti-tumor derivatives of honokiol namely 3’- formylhonokiol, 5-formylhonokiol and 3’, 5-diformylhonokiol are prepared after treating the honokiol with chloroform in 35% sodium hydroxide solution in presence of tetrabutyl ammonium bromide as phase transfer catalyst under 50 °C for 3 h. Out of these derivatives 3’- formylhonokiol and 5-formylhonokiol are isomers. Among honokiol and its three C-formylation derivatives, 5-formylhonokiol displayed the strongest inhibitory ability on tumor cell lines.
3.0. Molecular targets and mechanisms of action
Cancer progresses through a series of genetic and epigenetic aberrations that cause dysregulation of key cell signaling pathways involved in growth, malignant behavior and therapy-resistance (20). Studies have shown that honokiol is able to target many such pathologically relevant pathways. While novel modes of honokiol action continue to emerge, we discuss some of the important molecular targets of honokiol (Figure. 2), with emphasis on its translational relevance in cancer prevention and therapy.
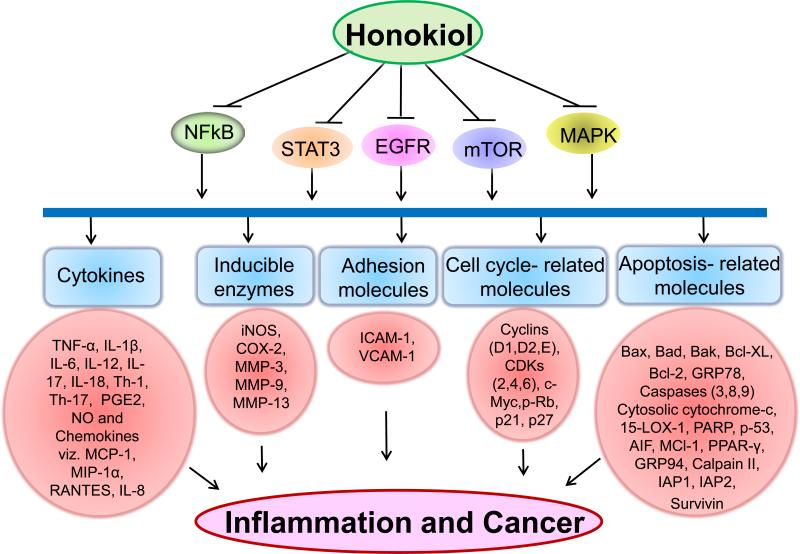
Figure 2.
A schematic diagram depicts the mechanism of action of honokiol through various molecular targets that results in chemopreventive and chemotherapeutic activity. Honokiol inhibits nuclear factor-κB (NF-κB), signal transducers and activator of transcription (STAT-3), epidermal growth factor receptor (EGFR), mammalian target of rapamycin (m-TOR) or Mitogen-activated protein kinases (MAPK) followed by downstream inhibition of cytokines, inducible enzymes, adhesion molecules, cell cycle and apoptosis-related molecules involved in inflammation, proliferation, angiogenesis, invasion and metastasis.
3.1. Nuclear factor kappa B (NF-κB) and signal transducers and activator of transcription (STAT3)
NF-κB and STAT3 are two ubiquitously expressed transcription factors controlling the expression of a wide array of genes involved in numerous physiological processes including development, differentiation, immunity, metabolism and cancer (21). Aberrant activation of these transcription factors is common in many malignancies, and is pathologically significant in early and late developmental steps of cancer (21). Some of the gene targets are common, while others are unique to these transcription factors. For example, both NF-κB and STAT3 upregulate the expression of proteins that regulate apoptosis (Bcl-xL, Bcl-2, c-IAP2), cell cycle (cyclins D and B) and proliferation c-Myc, Cox2) (22, 23) On the other hand, A1/Bfl-1(Bcl-2 family member) and c-FLIP are mostly NF-κB-dependent, whereas Mcl-1 and Survivin are STAT3-dependent (21, 22). Both NF-κB and STAT3 are also shown to be involved in regulation of HIF1α and VEGF gene expression, involved in invasion and angiogenesis, respectively (24). Some of these genes, in fact, require transcriptional cooperation between STAT3 and NF-κB (21). All these observations clearly suggest that targeting of NF-κB and STAT3 could yield preventive and therapeutic effects in cancer.
Honokiol has been shown to inhibit NF-κB activation through suppression of Akt and activation of IKK (inhibitor kinase), which then leads to phosphorylation and subsequent IκBα degradation (21, 22). IκBα keeps NF-κB sequestered in the cytoplasm in an inactive state and its degradation leads to nuclear translocation of NF-κB and transcriptional activation (22, 24). STAT3, among various STAT proteins, is constitutively activated in many human cancer cells (21) and can be inhibited by honokiol (25). Honokiol suppresses STAT3 activity induced by IL-6, one of the many growth factors that activate STAT3 (25). Furthermore, STAT3 inhibition by honokiol has also been correlated with the repression of upstream protein tyrosine kinases c-Src, JAK1 and JAK2 (26). Although not yet demonstrated, suppression of STAT3 and NF-κB activation by honokiol could also be inter-linked. The p65 subunit of NF-κB has been shown to interact with STAT3 (27) and it is reported that STAT3 prolongs NF-κB nuclear retention through acetylation (28). Activation of both STAT3 and NF-κB in tumor cells by factors in tumor microenvironment (interleukins and chemokines) and release of cytokines and chemokines by tumor cells as a response (21, 28) also suggest their role in mediating the cross-talk between tumor and its microenvironment. All these observations clearly imply that NF-κB and STAT3 inhibition by honokiol can have a multifaceted impact on the growth and spread of the tumor cells.
3.2. Epidermal growth factor receptor (EGFR) and downstream signaling
EGFR or ErbB1 is one of the four members of ErbB receptor tyrosine kinase family (29). EGFR activation occurs upon binding to its ligands, which then leads to its homo- or hetero-dimerization with other members of the ErbB family, and subsequent activation of downstream signaling cascades. There are multiple ligands reported for EGFR including epidermal growth factor (EGF), transforming growth factor-a (TGF-a), amphiregulin, epiregulin, betacellulin and heparin-binding epidermal growth factor (HB-EGF) (29). EGFR is overexpressed in a variety of cancers and its aberrant activation is shown to promote cell survival and proliferation through induction of phosphatidyl-inositol 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), and STAT3 (30). EGFR can also be activated in a ligand-independent manner by cellular Src (c-Src), a non-receptor tyrosine kinase. c-Src is also upregulated in many human malignancies and promotes activation of mitogenic signaling through EGFR (30). An earlier study reported that the growth inhibitory effect of honokiol was associated with downmodulation of EGFR signaling in human breast cancer cells (31). Honokiol has been shown to downregulate the expression and phosphorylation of both c-Src and EGFR, causing reduced activation of downstream signaling molecules. Honokiol also inhibited EGFR signaling in head and neck squamous cell carcinoma cells (HNSCC) (32). Furthermore, honokiol also promoted the growth inhibitory activity of the EGFR-inhibiting agents erlotinib and lapatinib in HNSCC and Her-2 over-expressing breast cancer cells, respectively (32, 33). These observations provide clear evidence for the suppressive effects of honokiol on EGFR and its downstream signaling in cancer cells.
3.3. Mammalian target of rapamycin (m-TOR)
The mammalian target of rapamycin (mTOR) is a protein kinase that is centrally involved in the control of cell metabolism, growth and proliferation (34). Aberrant activation of mTOR in most cancer cells is primarily caused by PI3K–Akt pathway, which is activated through a variety of mechanisms (34). mTOR controls translational initiation of many survival proteins via activating p70 S6 kinase (S6K) and inhibition of eIF4E inhibitor 4E-BP1(35). mTOR signaling pathway is dysregulated in premalignant or early malignant human tissues and is highly implicated in the carcinogenic process(35). Honokiol suppresses the activation of mTOR and its signaling mediators (4E-BP1 and p70 S6 kinase) by inhibiting ERK and Akt pathways (31) or by upregulation of PTEN (Phosphatase and Tensin homolog) expression (33). Combination of honokiol with the mTOR inhibitor rapamycin resulted in synergistic apoptosis induction in breast cancer cells (33). Furthermore, honokiol decreased PI3K/mTOR pathway mediated immunoresistance of glioma, breast and prostate cancer cell lines without affecting proinflammatory T cell functions (36).
4.0. Chemopreventive/ chemotherapeutic effects of honokiol in various malignancies: preclinical studies
4.1. Skin cancer
Importance of skin cancer can be judged from its incidence, which is almost equal to incidence of cancers in all other organs in the United States (37). Moreover, its incidence is expected to increase substantially, because of continuous depletion of the ozone layer that allows more solar ultraviolet (UV) radiation to reach to the earth's surface, and changing life style and dietary behavior (38). Solar UV radiations particularly UVB (290-320 nm), can induce both direct and indirect biological effects including DNA damage, cancer and premature aging signs (38). In a recent study, effect of honokiol was assessed on ultraviolet (UV) radiation-induced skin tumorigenesis using the SKH-1 hairless mouse (a model for dermatological studies) (39). It was found that honokiol treatment (3 mg per mouse applied topically) resulted in 80% reduction in tumor size and 62% reduction in malignant progression of papillomas to carcinomas in UVB-irradiated mice as compared to untreated group. At molecular level, honokiol was found to inhibit UVB-induced expression of cyclooxygenase-2, prostaglandin E2, proliferating cell nuclear antigen and pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β and IL-6 in the skin as well as in skin tumors (39). Chilampalli and colleagues also reported the reduction (45%) in tumor multiplicity by honokiol treatment one hour prior to UVB irradiation in SKH-1 mice (39). They observed the release of caspases-3, -8 and -9as well as poly (ADP-ribose) polymerase (PARP) cleavage and p53 activation upon honokiol treatment that led to DNA fragmentation and apoptosis. Cytotoxicity of honokiol was also associated with translocation of cytochrome c to cytosol in human melanoma cell lines (SK-MEL2 and MeWo) (40). Altogether, these studies indicate that honokiol is a promising candidate for the development of new strategies for the prevention of UV radiation-induced skin cancers in humans by targeting inflammatory mediators, cell cycle regulators, and cell survival signals in UVB-exposed skin.
4.2. Lung cancer
In both economically developed and developing countries, lung cancer is the most frequently diagnosed cancer and the leading cause of cancer related deaths (14). Honokiol was tested for its radio-sensitization potential in lung carcinoma (6). Lewis lung carcinoma cells (LL/2) treated with liposomal honokiol for 24 h showed a higher radiation enhancement ratio (~ two-fold) as compared to the radiation alone, indicating increased sensitivity to radiation-induced cytotoxicity when co-treated with honokiol. In an animal model (Lewis lung carcinoma-bearing C57BL/6 mice), combination treatment (radiation and honokiol) caused greater reduction in tumor volume (78%) as compared to radiation alone (42%). In another study, it was shown that honokiol alone had antitumor activity in A549 lung cancer xenograft model and the combination of honokiol with cisplatin reduced the tumor volume (3.59-fold) as compared to cisplatin alone on day 40 (41).
4.3. Breast cancer
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death among females in the United States (14). It accounts for nearly 23% of the total cancer cases and 14% of the cancer deaths in females (14). In many research studies, honokiol has been evaluated for its anti-cancer potential against breast cancer (31, 42-44). Honokiol was shown to inhibit the growth in breast cancer cell lines (MDA-MB-231, SK-BR-3, MDA-MB-436, ZR-75-1and T47-D, MCF-7, 4T1) in a dose dependent manner regardless of their HR, HER2 or p53 status (31, 43, 44). Furthermore, in 4T1 bearing-BALB/c mice, systemic administration of liposomal honokiol (20 mg/kg) and adriamycin (5 mg/kg) was more effective as compared to adriamycin alone increasing the life span of animals by 8 days (42). Growth inhibitory effects of honokiol in breast cancer were shown to occur through arrest of cell cycle at the G0/G1 phase and induction of apoptosis (33, 42, 44). Honokiol also caused suppression of Ras activation induced by EGFR in and Ras dependent PLD (phospholipase D) activity in MDA-MB-231 breast cancer cells suggesting that it can serve as a valuable therapeutic agent for all those cancers that depend on Ras and PLD (42). Furthermore, it was shown that growth inhibitory effect of honokiol was mediated, in part, by downmodulation of c-Src/EGFR-mediated signaling (31). Inhibition of NF-κB, COX-2 and PGE2 was also observed in breast cancer after treatment with honokiol. Honokiol also sensitized the cancer cells for drug therapy by downregulating the expression of P-glycoprotein. These studies indicate that honokiol could be used, alone or in combination with other drugs, for breast cancer therapy.
4.4. Ovarian cancer
Studies in a variety of ovarian cell lines (SKOV3, COC1, Angelen and A2780) have demonstrated the cytotoxicity of honokiol in vitro with IC50 reported to be in the range of 14 - 20 μg/mL after 24 h treatment (45). Administration of honokiol into tumor-bearing animals (xenograft model of SKOV3 cells in BALB/c mice) decreased microvessel density and inhibited tumor growth (~ 70%) as compared to the control (45). The anti-tumor activity of liposomal honokiol was also evaluated in nude mice bearing human ovarian cancer cells viz. A2780s (cisplatin-sensitive) and A2780cp (cisplatin-resistant). Administration of liposomal honokiol (10 mg/kg, intravenously twice per week) was shown to cause significant inhibition (84–88% relative to controls) in the growth of both A2780s and A2780cp tumor xenografts and prolong the survival of the treated mice (46). In another study, combination of pegylated liposomal honokiol and cisplatin produced synergistic growth-inhibitory effects in human ovarian carcinoma mouse xenograft model of SKOV3 cells (47). The combination treatment inhibited tumor growth by 91% as compared to either pegylated honokiol (66%) or Cisplatin (52%) alone. The mechanism responsible for anticancer effects of honokiol in ovarian cancer was suggested to be the induction of apoptosis and inhibition of angiogenesis.
4.5. Prostate cancer
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer related death in males in economically developed countries (14). Therefore, novel cancer prevention and therapeutic approaches are required to have an impact on the incidence and mortality from prostate cancer. In an earlier study, honokiol was shown to induce apoptosis in human prostate cancer cells irrespective of their androgen responsiveness or p53 status (48). The study used PC-3 (an androgen-independent cell line lacking functional p53), LNCaP (an androgen responsive cell line with wild-type p53), and C4-2 (an androgen-independent variant of the LNCaP cell line) (48). Suppression of growth by honokiol administration (2 mg/mouse thrice a week) in PC-3 xenograft mouse model correlated with induction of apoptosis (TUNEL positive apoptotic bodies) and decrease in proliferation index (PCNA staining)(48). Interestingly, honokiol was found to be more effective in inhibiting the growth of cancer-associated stromal fibroblasts as compared to that associated with normal/benign-disease (48). In mouse (BALB/c nude) xenograft model of C4-2 cells, honokiol alone (100 mg/kg/day for 6 weeks i.p.) and honokiol with docetaxel (5 mg/kg once a week), showed a significant decrease of serum PSA (prostate-specific antigen) in comparison with the control group after 6 weeks treatments (49). Therefore, honokiol may be effective in prevention and therapy of prostate cancer.
4.6. Gastrointestinal cancers
Honokiol has also been shown to be effective in three types of gastro-intestinal cancers viz., colorectal, gastric and pancreatic. Colorectal cancer is the third most commonly diagnosed cancer in males and the second in females in developed countries (14). Usefulness of honokiol against colorectal carcinoma has been shown in two independent studies. In the first study, honokiol was shown to impart anti-proliferative effect against RKO (Human colorectal carcinoma cells) with an IC50 of 12.47 μg/mL after 68 h treatment (50). Furthermore, a similar effect of honokiol was also observed in vivo in xenograft model of RKO cells in BALB/c nude mice. The mean survival time increased to 50.9 days in honokiol-treated group (i.p. injection, 80 mg/kg body weight/day) with significant inhibition of tumor growth as compared to that for vehicle treated group which had an average survival time of 29.7 days. In the second study, human colorectal carcinoma cell lines, RKO, SW480 and LS180, were treated with various doses of honokiol (51). Honokiol inhibited the growth with an IC50 of 10.33, 12.98, 11.16 μg/mL in RKO, SW480 and LS180 cells, respectively, after 68 h treatment.
Honokiol was also shown to be effective against gastric cancer cells. Honokiol induced apoptosis and downregulated the expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ) and COX-2 in gastric cancer cells (AGS, MKN45, N87 and SCM-1) (52). In another study, honokiol markedly decreased the levels of glucose-regulated protein (GRP94) in a dose (5-40 μM) – and time- (0.5-18 h) dependent manner in various gastric cancer cells (AGS, N87, MKN45 and SCM-1) (53). Significant anti-tumor activity was associated with decreased accumulation of GRP94 in tumors (xenograft model of MKN45 cells in BALB/c mice) after i.v. administration of honokiol (0.5 and 1.5 mg/kg body weight every day for 10 days).
In a recent study from our group, honokiol was shown to exhibit growth-inhibitory effects against two human pancreatic cancer cell lines (MiaPaCa and Panc1) (54). Honokiol altered the expression of many cell-cycle associated and survival proteins, in part, through inhibition of constitutively active NF-κB. Furthermore, we showed that honokiol potentiated the cytotoxic effect of gemcitabine by restricting gemcitabine-induced nuclear localization of NF-κB. As constitutive activation of NF-κB is reported in majority of pancreatic cancer cases and implicated in early and late development of the disease (54), honokiol could serve as a novel natural agent for pancreatic cancer prevention and therapy.
4.7. Other cancers
Honokiol has also proven its therapeutic potential as anti-cancer agent for various other solid and hematological malignancies. One category of such cancers is malignant gliomas, which accounts for approximately 70% of the malignant primary brain tumors that are diagnosed in adults in the United States each year (55). Brain tumors are particularly hard to treat, because the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) limit the delivery of both conventional and targeted therapeutics. Interestingly, it was recently shown that honokiol could effectively cross BBB and BCSFB and inhibit brain tumor growth (7). In this study, two in vivo model systems viz. Rat 9L (intracerebral gliosarcoma model in Fisher 344 rats) and human U251 xenograft glioma model in nude mice were used. Intravenous injection of honokiol (twice a week for 3 weeks) caused significant reduction in tumor volume (52% and 50% in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model, respectively). It was shown that approximately 10% of honokiol in plasma crossed BCSFB and entered into cerebrospinal fluid (CSF). This study is indicative of the potential of honokiol as a novel therapeutic candidate for treatment of gliosarcoma. In another study, honokiol was shown to repress PI3K/mTOR pathway- mediated immunoresistance in glioma cell lines (U87 and U251) without affecting critical proinflammatory T cell functions (36). Thus, honokiol may also be a candidate to overcome immunoresistance in glioma, without interfering with T cell functions.
Dose- and time- dependent cytotoxic effects of honokiol were also observed in oral sqamous carcinoma cells (HSC-3 and HSC-4) (56). Furthermore, growth inhibitory effects of honokiol have also reported in human head and neck squamous cell carcinoma (HNSCC) cell lines (Cal-33, Cal-1483, 686LN and 686LNR30 cells (32). These effects were associated with inhibition of EGFR and its downstream effectors, including mitogen-activated protein kinase, Akt, and signal transducer and activator of transcription 3 (STAT3), and reduced expression of target genes, Bcl-XL and cyclin D1. In these studies, honokiol was also shown to inhibit growth of erlotinib-resistant HNSCC cells.
With regard to hematological malignancies, honokiol has been shown to be effective in leukemia cell lines viz. B-CLL and Molt 4B (57-59). Honokiol also induced apoptosis in human multiple myeloma cell lines including dexamethasone-sensitive (MM.1S) and dexamethasone-resistant (MM.1R, RPMI-8226, U266 and SU-DHL-4) cells and in tumor cells collected from patients with relapsed refractory multiple myeloma (60). Honokiol also enhanced doxorubicin accumulation by 24% in human doxorubicin-resistant uterine sarcoma cells (MES-SA/Dx5), thus indicating its usefulness as an adjuvant (60, 61). Honokiol also inhibited the growth of chondrosarcoma cell lines, JJ012 and SW1353, with an IC50 of 27 μM and 27.3 μM, respectively (62). Furthermore, honokiol led to a reduction in tumor volume (~53%) in BALB/c nude mouse xenograft model of JJ012 cells (1.5 mg/kg body weight i.p. every day for 21 days) (62). Honokiol also exhibited growth inhibitory effects against primary human endothelial cells, fibroblasts, mouse endothelium cells (SVR) and anti-tumor effects in SVR bearing tumor model in BALB/c mice (5). Honokiol inhibited the migration of human fibrosarcoma cells (HT-1080) at 100 μM (63). All these studies indicate that honokiol could be an effective therapeutic agent against multiple cancer types.
5.0. Pharmacokinetics of honokiol
Pharmacokinetics includes the study of absorption, distribution, metabolism and excretion of the administered drug (ADME). Honokiol demonstrates a biphasic kinetic profile, consisting of a rapid distribution phase followed by a slower elimination phase (elimination t1/2 = 49.2 and 56.2 min for 5 and 10 mg of honokiol, respectively) following intravenous (i.v.) administration (64). In another study, elimination t1/2 was found to be 526.6 min after a single oral dose of honokiol in Houpu decoction (a compound prescription of honokiol; 5g/Kg body weight) in Wistar rats. This study suggested that rhubarb and immature orange fruit extract in the decoction influenced the absorption, distribution and elimination of honokiol (65). The maximal plasma concentration of honokiol following rectal administration of Houpo extract at a dose of 245 mg/ kg (equivalent to 13.5 mg/kg of honokiol) in Wistar rats was approximately six times to that administered orally at an identical dose (66) indicating that rectal dosing avoids first-pass metabolism to some extent. Honokiol was shown to undergo metabolism through glucuronidation and sulfation in liver before elimination in humans and rats (67).
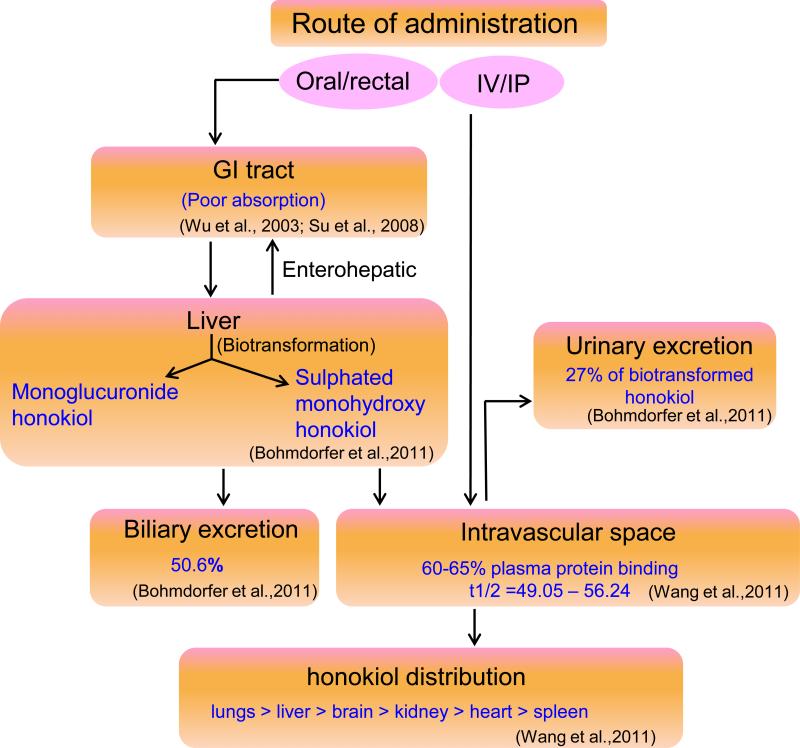
Due to the concerns about poor aqueous solubility, liposomal formulations of honokiol have been developed and tested for their pharmacokinetics. PEGylated (polyethylene glycol coated) liposomal honokiol was shown to enhance the serum honokiol concentration and decreased clearance. PEGylated liposomal honokiol had higher t1/2β (time it takes for plasma levels of the drug to decrease to half) ratio (26 min) as compared to free honokiol (13 min) after single i.v. administration (20 mg/kg body weight) in Balb/c mice (68). Approximately, 60-65% honokiol was protein-bound as revealed by equilibrium dialysis. In another comparative study, plasma honokiol concentrations was maintained above 30 and 10 μg/mL for 24 and 48 hours, respectively, in liposomal honokiol-treated mice, whereas it fell quickly (less than 5 μg/mL) by 12 hours in free honokiol-treated mice bearing A549 xenograft tumors (41). In a recent study, organ distribution of honokiol was revealed to be in the following order: lungs > plasma > liver > brain > kidney > heart > spleen, following treatment of animal models (rat 9L intracerebral gliosarcoma model in Fisher 344 rats and human U251 xenograft glioma model in nude mice) (7). This study also demonstrated that honokiol crossed blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) after i.v. administration. Pharmacokinetics of honokiol (Figure 3) can be summarized as: free honokiol has poor GIT absorption, bio-transformed in liver to mono-glucuronide honokiol and sulphated mono-hydroxyhonokiol, ~ 50% is secreted in bile, ~ 60-65% plasma protein bound with elimination half life of (t1/2) of 49.05 – 56.24 minutes.
Figure 3.
Schematic representation of pharmacokinetic profile of honokiol. Honokiol has poor GIT absorption, bio-transformed in liver to mono-glucuronide honokiol and sulphated mono-hydroxyhonokiol, ~ 50% is secreted in bile, ~ 60-65% plasma protein bound and ~ 27% is excreted in the urine with elimination half life of (t1/2) of 49.05 – 56.24 minutes.
6.0. Safety issues
Although most natural products are generally considered to be safe, it is important to establish the toxicological profile of honokiol before its use in humans. There has been only one direct toxicological study on honokiol, while other studies have been conducted on Magnolia bark extract (containing honokiol and magnalol as active compounds) or Magnolol alone (biologically active structural isomer of honokiol). Toxicity of honokiol was evaluated in rats by i.v. administration in the dose range of 20-80mg/Kg body weight, once a day for 14 days in Sprague-Dawley rats. No significant differences were observed in body weight, mean daily food intake, hematological values, serum biochemical values and tissue pathologic changes between honokiol-treated group and vehicle-treated group (7) suggesting potential safety of honokiol. The potential genotoxic effects of Magnolia bark extract were evaluated in Chinese hamster ovary cells (CHO) and in Chinese hamster lung cells (V79) using chromosomal aberration assays (69). No mutagenic activity was observed, indicating that honokiol is not genotoxic. Furthermore, Magnolia bark extract treatment also did not affect the proportions of immature to total erythrocytes, nor did it increase the number of micronuclei in the immature erythrocytes of Swiss albino mice. In a short-term toxicity study, magnolia bark extract was shown to produce no clinically significant changes (macroscopic or microscopic hematological, clinical chemistry, urinalysis, organ and total body weight) in Sprague-Dawley rats after oral administration (70). Sub-chronic toxicity studies by the same group for 90-days indicated no mortality, ophthalmic abnormalities or treatment related changes in hematology, coagulation or organ weight. In fact, in another study, Magnolol was shown to inhibit mutagenicity induced by several indirect mutagens (71). These studies suggest that honokiol either alone or as a part of magnolia bark extract does not induce toxicity in animal models and thus could be clinically safe.
7.0. Conclusions
Honokiol has generated an extensive interest due to its various beneficial pharmacological properties. Besides its potent anti-inflammatory effects, honokiol has shown promising activities against multiple malignancies. Honokiol inhibits the production of numerous cytokines, inducible enzymes, and adhesion molecules associated with tumor initiation, promotion, progression, metastasis and therapy-resistance. Therefore, it has significant potential to serve as a novel agent for cancer prevention and therapy. Among the important chemopreventive and therapeutic target pathways are NF-κB, STAT3, EGFR, mTOR and MAPK. Honokiol, however, has poor aqueous solubility, which adversely affect its intestinal absorption. This limitation has been addressed by developing liposomal formulations. Biologically effective serum concentrations of honokiol can be achieved following intravenous administration; glucuronidation and sulfation are the main metabolic pathways for its clearance. Toxicological studies on honokiol and Magnolia bark extract have not shown any pathologic changes in the liver, lung, kidney, spleen, brain, heart, pancreas, intestines, or bone marrow after systemic or oral administration, suggesting its safety. Future studies are warranted to further broaden our understanding of the mechanistic basis of honokiol's biological action. Nonetheless, honokiol appears to be a promising natural agent for cancer prevention and therapy, and its evaluation in human clinical trials will be the next step towards its possible human applications.
Acknowledgments
Grant support: NIH/NCI (CA137513), DOD /US Army (W81XWH-09-1-0137) and USAMCI.
Footnotes
Conflicts of Interest: No potential conflict of interest to disclose
References
- 1.Esumi T, Makado G, Zhai H, Shimizu Y, Mitsumoto Y, Fukuyama Y. Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg Med Chem Lett. 2004;14:2621–5. doi: 10.1016/j.bmcl.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 2.Tsai SK, Huang CH, Huang SS, Hung LM, Hong CY. Antiarrhythmic effect of magnolol and honokiol during acute phase of coronary occlusion in anesthetized rats: influence of L-NAME and aspirin. Pharmacology. 1999;59:227–33. doi: 10.1159/000028324. [DOI] [PubMed] [Google Scholar]
- 3.Liou KT, Lin SM, Huang SS, Chih CL, Tsai SK. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003;69:130–4. doi: 10.1055/s-2003-37707. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Zhang XX, Wang YY, Chen SZ. Honokiol inhibits arterial thrombosis through endothelial cell protection and stimulation of prostacyclin. Acta Pharmacol Sin. 2005;26:1063–8. doi: 10.1111/j.1745-7254.2005.00164.x. [DOI] [PubMed] [Google Scholar]
- 5.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–7. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Chen LJ, Liu L, Chen X, Chen PL, Yang G, et al. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp Mol Med. 2008;40:617–28. doi: 10.3858/emm.2008.40.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Duan X, Yang G, Zhang X, Deng L, Zheng H, et al. Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in rat 9L intracerebral gliosarcoma model and human U251 xenograft glioma model. PLoS One. 2011;6:e18490. doi: 10.1371/journal.pone.0018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SE, Hsieh MT, Tsai TH, Hsu SL. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem Pharmacol. 2002;63:1641–51. doi: 10.1016/s0006-2952(02)00894-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuribara H, Stavinoha WB, Maruyama Y. Honokiol, a putative anxiolytic agent extracted from magnolia bark, has no diazepam-like side-effects in mice. J Pharm Pharmacol. 1999;51:97–103. [PubMed] [Google Scholar]
- 10.Zhao C, Liu ZQ. Comparison of antioxidant abilities of magnolol and honokiol to scavenge radicals and to protect DNA. Biochimie. 2011;93:1755–1760. doi: 10.1016/j.biochi.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Park J, Lee J, Jung E, Park Y, Kim K, Park B, et al. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol. 2004;496:189–95. doi: 10.1016/j.ejphar.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 12.Ho KY, Tsai CC, Chen CP, Huang JS, Lin CC. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother Res. 2001;15:139–41. doi: 10.1002/ptr.736. [DOI] [PubMed] [Google Scholar]
- 13.Amblard F, Delinsky D, Arbiser JL, Schinazi RF. Facile purification of honokiol and its antiviral and cytotoxic properties. J Med Chem. 2006;49:3426–7. doi: 10.1021/jm060268m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 15.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 16.Kong ZL, Tzeng SC, Liu YC. Cytotoxic neolignans: an SAR study. Bioorg Med Chem Lett. 2005;15:163–6. doi: 10.1016/j.bmcl.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Xu Y, Chen L, Luo H, Peng C, Fu J, et al. Preparative purification of antitumor derivatives of honokiol by high-speed counter-current chromatography. J Chromatogr A. 2008;1178:160–5. doi: 10.1016/j.chroma.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Chen J, Wang X, Liang X, Luo Y, Zhu W, et al. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: the evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship. J Med Chem. 2011;54:6469–81. doi: 10.1021/jm200830u. [DOI] [PubMed] [Google Scholar]
- 19.Kim KR, Park KK, Chun KS, Chung WY. Honokiol inhibits the progression of collagen-induced arthritis by reducing levels of pro-inflammatory cytokines and matrix metalloproteinases and blocking oxidative tissue damage. J Pharmacol Sci. 2010;114:69–78. doi: 10.1254/jphs.10070fp. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–61. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 23.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 24.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–9. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajendran P, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, et al. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J Cell Physiol. 2011;10 doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- 26.Campbell GS, Yu CL, Jove R, Carter-Su C. Constitutive activation of JAK1 in Src-transformed cells. J Biol Chem. 1997;272:2591–4. doi: 10.1074/jbc.272.5.2591. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–23. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–10. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park EJ, Min HY, Chung HJ, Hong JY, Kang YJ, Hung TM, et al. Down-regulation of c-Src/EGFR-mediated signaling activation is involved in the honokiol-induced cell cycle arrest and apoptosis in MDA-MB-231 human breast cancer cells. Cancer Lett. 2009;277:133–40. doi: 10.1016/j.canlet.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, et al. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res. 2010;16:2571–9. doi: 10.1158/1078-0432.CCR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Zang C, Emde A, Planas-Silva MD, Rosche M, Kuhnl A, et al. Anti-tumor effect of honokiol alone and in combination with other anti-cancer agents in breast cancer. Eur J Pharmacol. 2008;591:43–51. doi: 10.1016/j.ejphar.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Crane C, Panner A, Pieper RO, Arbiser J, Parsa AT. Honokiol-mediated inhibition of PI3K/mTOR pathway: a potential strategy to overcome immunoresistance in glioma, breast, and prostate carcinoma without impacting T cell function. J Immunother. 2009;32:585–92. doi: 10.1097/CJI.0b013e3181a8efe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaid M, Sharma SD, Katiyar SK. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: development of topical formulation. Carcinogenesis. 2010;31:2004–11. doi: 10.1093/carcin/bgq186. [DOI] [PubMed] [Google Scholar]
- 38.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5:243–53. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 39.Chilampalli S, Zhang X, Fahmy H, Kaushik RS, Zeman D, Hildreth MB, et al. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010;30:777–83. [PubMed] [Google Scholar]
- 40.Mannal PW, Schneider J, Tangada A, McDonald D, McFadden DW. Honokiol produces anti-neoplastic effects on melanoma cells in vitro. J Surg Oncol. 2011;104:260–264. doi: 10.1002/jso.21936. [DOI] [PubMed] [Google Scholar]
- 41.Jiang QQ, Fan LY, Yang GL, Guo WH, Hou WL, Chen LJ, et al. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer. 2008;8:242.:242. doi: 10.1186/1471-2407-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou W, Chen L, Yang G, Zhou H, Jiang Q, Zhong Z, et al. Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytother Res. 2008;22:1125–32. doi: 10.1002/ptr.2472. [DOI] [PubMed] [Google Scholar]
- 43.Singh T, Katiyar SK. Honokiol, a phytochemical from Magnolia spp., inhibits breast cancer cell migration by targeting nitric oxide and cyclooxygenase-2. Int J Oncol. 2011;38:769–76. doi: 10.3892/ijo.2011.899. [DOI] [PubMed] [Google Scholar]
- 44.Wolf I, O'Kelly J, Wakimoto N, Nguyen A, Amblard F, Karlan BY, et al. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int J Oncol. 2007;30:1529–37. [PubMed] [Google Scholar]
- 45.Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang C, et al. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. Eur J Obstet Gynecol Reprod Biol. 2008;140:95–102. doi: 10.1016/j.ejogrb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 46.Luo H, Zhong Q, Chen LJ, Qi XR, Fu AF, Yang HS, et al. Liposomal honokiol, a promising agent for treatment of cisplatin-resistant human ovarian cancer. J Cancer Res Clin Oncol. 2008;134:937–45. doi: 10.1007/s00432-008-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Chen L, He X, Fan L, Yang G, Chen X, et al. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int J Gynecol Cancer. 2008;18:652–9. doi: 10.1111/j.1525-1438.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 48.Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14:1248–57. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 49.Shigemura K, Arbiser JL, Sun SY, Zayzafoon M, Johnstone PA, Fujisawa M, et al. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer. 2007;109:1279–89. doi: 10.1002/cncr.22551. [DOI] [PubMed] [Google Scholar]
- 50.Chen F, Wang T, Wu YF, Gu Y, Xu XL, Zheng S, et al. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10:3459–63. doi: 10.3748/wjg.v10.i23.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang T, Chen F, Chen Z, Wu YF, Xu XL, Zheng S, et al. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J Gastroenterol. 2004;10:2205–8. doi: 10.3748/wjg.v10.i15.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu SH, Shen CC, Yi YC, Tsai JJ, Wang CC, Chueh JT, et al. Honokiol inhibits gastric tumourigenesis by activation of 15-lipoxygenase-1 and consequent inhibition of peroxisome proliferator-activated receptor-gamma and COX-2-dependent signals. Br J Pharmacol. 2010;160:1963–72. doi: 10.1111/j.1476-5381.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheu ML, Liu SH, Lan KH. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One. 2007;2:e1096. doi: 10.1371/journal.pone.0001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6:e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 56.Chen XR, Lu R, Dan HX, Liao G, Zhou M, Li XY, et al. Honokiol: a promising small molecular weight natural agent for the growth inhibition of oral squamous cell carcinoma cells. Int J Oral Sci. 2011;3:34–42. doi: 10.4248/IJOS11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood. 2005;106:690–7. doi: 10.1182/blood-2004-11-4273. [DOI] [PubMed] [Google Scholar]
- 58.Hibasami H, Achiwa Y, Katsuzaki H, Imai K, Yoshioka K, Nakanishi K, et al. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 1998;2:671–3. doi: 10.3892/ijmm.2.6.671. [DOI] [PubMed] [Google Scholar]
- 59.Marin GH, Mansilla E. Apoptosis induced by Magnolia Grandi fl ora extract in chlorambucil-resistant B-chronic lymphocytic leukemia cells. J Cancer Res Ther. 2010;6:463–5. doi: 10.4103/0973-1482.77107. [DOI] [PubMed] [Google Scholar]
- 60.Ishitsuka K, Hideshima T, Hamasaki M, Raje N, Kumar S, Hideshima H, et al. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood. 2005;106:1794–800. doi: 10.1182/blood-2005-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angelini A, Di IC, Castellani ML, Conti P, Cuccurullo F. Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin-resistant sarcoma cells (MES-SA/DX-5): implications for natural sedatives as chemosensitizing agents in cancer therapy. J Biol Regul Homeost Agents. 2010;24:197–205. [PubMed] [Google Scholar]
- 62.Chen YJ, Wu CL, Liu JF, Fong YC, Hsu SF, Li TM, et al. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett. 2010;291:20–30. doi: 10.1016/j.canlet.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 63.Nagase H, Ikeda K, Sakai Y. Inhibitory effect of magnolol and honokiol from Magnolia obovata on human fibrosarcoma HT-1080. Invasiveness in vitro. Planta Med. 2001;67:705–8. doi: 10.1055/s-2001-18345. [DOI] [PubMed] [Google Scholar]
- 64.Tsai TH, Chou CJ, Cheng FC, Chen CF. Pharmacokinetics of honokiol after intravenous administration in rats assessed using high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;655:41–5. doi: 10.1016/0378-4347(94)00031-x. [DOI] [PubMed] [Google Scholar]
- 65.Su WJ, Huang X, Qin E, Jiang L, Ren P. [Pharmacokinetics of honokiol in rat after oral administration of Cortex of Magnolia officinalis and its compound preparation Houpu Sanwu Decoction]. Zhong Yao Cai. 2008;31:255–8. [PubMed] [Google Scholar]
- 66.Wu X, Chen X, Hu Z. High-performance liquid chromatographic method for simultaneous determination of honokiol and magnolol in rat plasma. Talanta. 2003;59:115–21. doi: 10.1016/s0039-9140(02)00470-8. [DOI] [PubMed] [Google Scholar]
- 67.Bohmdorfer M, Maier-Salamon A, Taferner B, Reznicek G, Thalhammer T, Hering S, et al. In vitro metabolism and disposition of honokiol in rat and human livers. J Pharm Sci. 2011;100:3506–16. doi: 10.1002/jps.22536. [DOI] [PubMed] [Google Scholar]
- 68.Wang XH, Cai LL, Zhang XY, Deng LY, Zheng H, Deng CY, et al. Improved solubility and pharmacokinetics of PEGylated liposomal honokiol and human plasma protein binding ability of honokiol. Int J Pharm. 2011;410:169–74. doi: 10.1016/j.ijpharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Maniatis T, Song Y, Zhang W, Zhang X, Li N, et al. Evaluation of magnolia bark extract in chromosomal aberration assays. Mutat Res. 2008;654:133–7. doi: 10.1016/j.mrgentox.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Zhang X, Cui W, Zhang X, Li N, Chen J, et al. Evaluation of short-term and subchronic toxicity of magnolia bark extract in rats. Regul Toxicol Pharmacol. 2007;49:160–71. doi: 10.1016/j.yrtph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Saito J, Sakai Y, Nagase H. In vitro anti-mutagenic effect of magnolol against direct and indirect mutagens. Mutat Res. 2006;609:68–73. doi: 10.1016/j.mrgentox.2006.06.021. [DOI] [PubMed] [Google Scholar]