Abstract
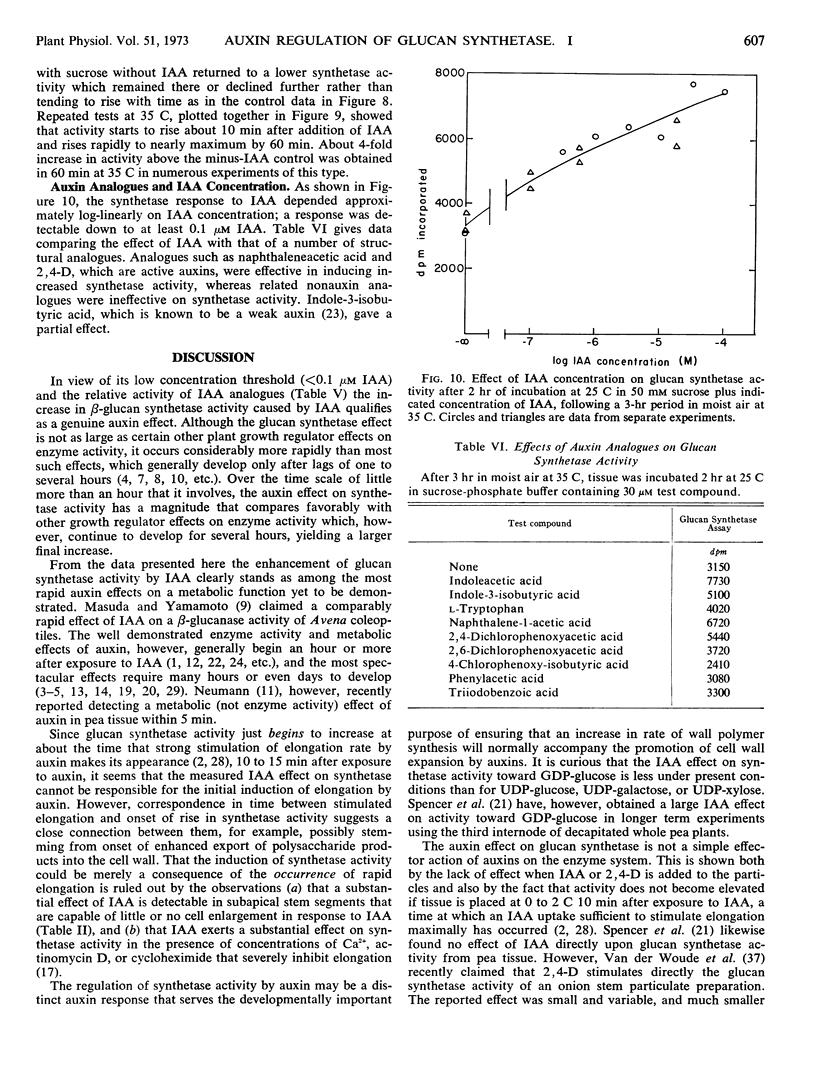
Treatment of pea stem segments with indoleacetic acid (IAA) causes within 1 hour a 2- to 4-fold increase in activity of particulate uridine diphosphoglucose-dependent β-glucan synthetase obtainable from the tissue. The IAA effect is observable in tissue from all parts of the elongation zone of the pea stem, and also in older tissue that is not capable of a cell enlargement response to IAA. A large increase in activity is caused by IAA only if synthetase activity in the isolated tissue has first been allowed to fall substantially below the intact plant level, and only if sucrose is supplied along with IAA. Treatment of tissue with sucrose alone after a period of sugar starvation causes a transient rise of synthetase activity. The decline in synthetase activity in absence of IAA, the rise caused by IAA, and the transient rise caused by sucrose are all strongly temperature-dependent. IAA and sucrose do not affect the activity of isolated synthetase particles. Synthetase activity in vivo is sensitive to as low as 0.1 μm IAA and is increased by IAA analogues that are active as auxins on elongation but not by nonauxin analogues. Activity begins to rise 10 to 15 minutes after exposure to IAA, which places this among the most rapid enzyme effects of a plant growth regulator heretofore demonstrated, and among the most rapid known metabolic effects of auxins. The effect is seen also with polysaccharide synthetase activity using uridine diphosphate-galactose or uridine diphosphate-xylose as substrates, and to a lesser extent with guanosine diphosphoglucose-dependent glucan synthetase activity. Glucan synthetase from IAA-treated tissue appears to have a higher affinity for uridine diphosphate-glucose than the control.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley G. M., Evans M. L. Timing of the auxin response in etiolated pea stem sections. Plant Physiol. 1970 Feb;45(2):143–147. doi: 10.1104/pp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Maclachlan G. A. Indoleacetic Acid and the synthesis of glucanases and pectic enzymes. Plant Physiol. 1968 May;43(5):735–742. doi: 10.1104/pp.43.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Thimann K. V. The Effect of Auxin on Growth and Respiration of Artichoke Tissue. Proc Natl Acad Sci U S A. 1952 Sep;38(9):770–775. doi: 10.1073/pnas.38.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H., Yang S. F. Ethylene-enhanced Synthesis of Phenylalanine Ammonia-Lyase in Pea Seedlings. Plant Physiol. 1971 Jun;47(6):765–770. doi: 10.1104/pp.47.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- NEWCOMB E. H. Effect of auxin on ascorbic oxidase activity in tobacco pith cells. Proc Soc Exp Biol Med. 1951 Mar;76(3):504–509. doi: 10.3181/00379727-76-18538. [DOI] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Ordin L., Hall M. A. Cellulose synthesis in higher plants from UDP glucose. Plant Physiol. 1968 Mar;43(3):473–476. doi: 10.1104/pp.43.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Regulation of beta-Glucan Synthetase Activity by Auxin in Pea Stem Tissue: II. Metabolic Requirements. Plant Physiol. 1973 Apr;51(4):609–614. doi: 10.1104/pp.51.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F. S., Ziola B., Maclachlan G. A. Particulate glucan synthetase activity: dependence on acceptor, activator, and plant growth hormone. Can J Biochem. 1971 Dec;49(12):1326–1332. doi: 10.1139/o71-192. [DOI] [PubMed] [Google Scholar]
- Thimann K. V. Auxin Activity of Some Indole Derivatives. Plant Physiol. 1958 Sep;33(5):311–321. doi: 10.1104/pp.33.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A. J. Effect of IAA on RNA and protein synthesis. Effects of 3-indolylacetic acid on the metabolism of ribonucleic acid and protein in etiolated subapical sections of Pisum sativum. Arch Biochem Biophys. 1968 Feb;123(2):324–335. doi: 10.1016/0003-9861(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Solubilization and Separation of Uridine Diphospho-d-glucose: beta-(1 --> 4) Glucan and Uridine Diphospho-d-glucose:beta-(1 --> 3) Glucan Glucosyltransferases from Coleoptiles of Avena sativa. Plant Physiol. 1971 Jun;47(6):740–744. doi: 10.1104/pp.47.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli V., Moyed H. S. The role of 3-methyleneoxindole in auxin action. J Biol Chem. 1969 Sep 25;244(18):4916–4920. [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]
- Warner H. L., Leopold A. C. Timing of growth regulator responses in peas. Biochem Biophys Res Commun. 1971 Aug 20;44(4):989–994. doi: 10.1016/0006-291x(71)90809-6. [DOI] [PubMed] [Google Scholar]


