Abstract
14C-Indoleacetic acid was applied to coleoptiles of corn (Zea mays) and oat (Avena sativa). The coleoptiles were detached from the endosperms at 6-minute intervals after indoleacetic acid application, and the radioactivity was determined in successive 2-millimeter regions. The rate (per cent per minute) of basipetal transport of indoleacetic acid is periodic in various regions of the coleoptile, with a period of about 20 minutes. The possible relation of this cyclic phenomenon to other rhythmic processes of similar periodicities is discussed. A distinct acropetal transport (against the concentration gradient) from the subapical region to the apical 2-millimeter region of the coleoptile was detected.
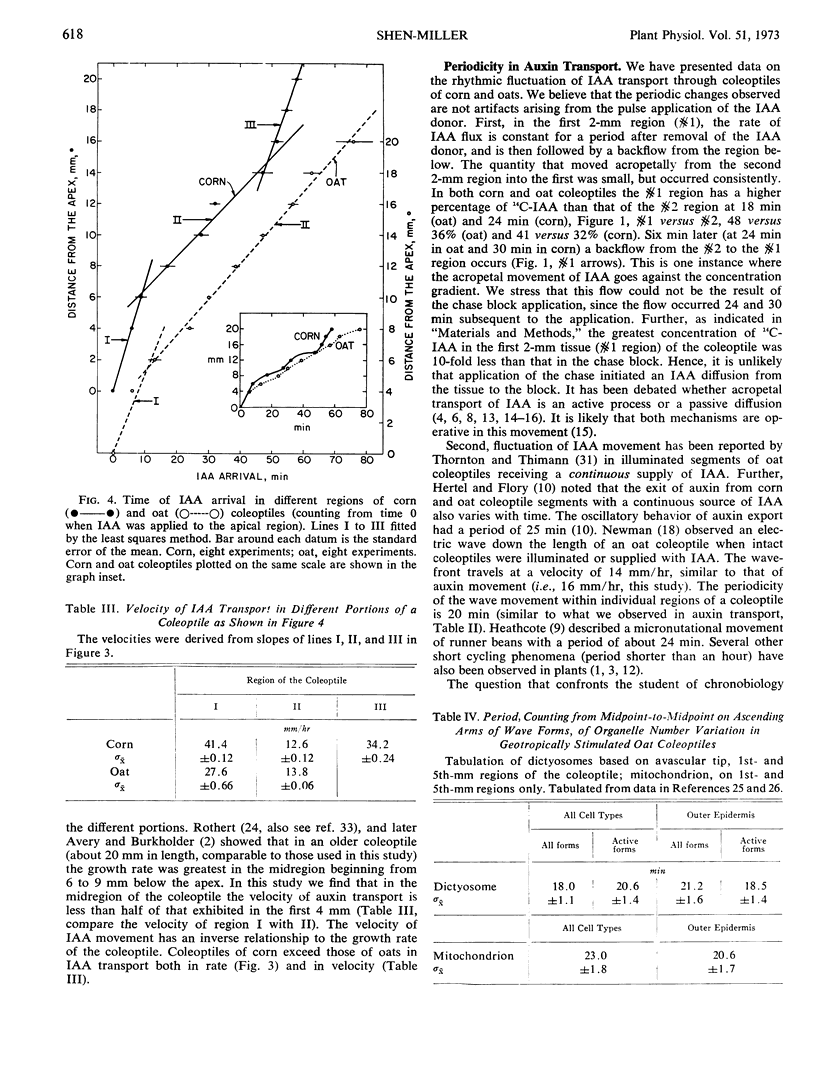
The velocity of indoleacetic acid transport differs in different regions of the coleoptile. Within an entire coleoptile the velocity can be divided into three classes for corn, 41 millimeters per hour (apical), 13 millimeters per hour (mid), and 34 millimeters per hour (base), and 2 classes for oats, 28 millimeters per hour (apical) and 14 millimeters per hour (remainder). An inverse relationship between the velocity of indoleacetic acid transport, and the growth rate of the coleoptile is discussed. Corn coleoptiles exceed oat coleoptiles both in rate and in velocity of IAA transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford D. K., Tibbitts T. W. Endogenous Short Period Rhythms in the Movements of Unifoliate Leaves of Phaseolus angularis Wight. Plant Physiol. 1971 Jan;47(1):68–70. doi: 10.1104/pp.47.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret C. F., Trucco E. Molecular models for the circadian clock. I. The chronon concept. J Theor Biol. 1967 May;15(2):240–262. doi: 10.1016/0022-5193(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. H. Separation of transit of auxin from uptake: average velocity and reversible inhibition by anaerobic conditions. Science. 1967 May 5;156(3775):661–663. doi: 10.1126/science.156.3775.661. [DOI] [PubMed] [Google Scholar]
- HIGGINS J. A CHEMICAL MECHANISM FOR OSCILLATION OF GLYCOLYTIC INTERMEDIATES IN YEAST CELLS. Proc Natl Acad Sci U S A. 1964 Jun;51:989–994. doi: 10.1073/pnas.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitt G. W., Jr, Baker R. A. Acropetal movement of auxin: dependence on temperature. Science. 1967 Jun 9;156(3780):1380–1381. doi: 10.1126/science.156.3780.1380. [DOI] [PubMed] [Google Scholar]
- LEOPOLD A. C. THE POLARITY OF AUXIN TRANSPORT. Brookhaven Symp Biol. 1964 Mar;16:218–234. [PubMed] [Google Scholar]
- Naqvi S. M., Gordon S. A. Auxin Transport in Zea mays L. Coleoptiles I. Influence of Gravity on the Transport of Indoleacetic Acid-2-C. Plant Physiol. 1966 Sep;41(7):1113–1118. doi: 10.1104/pp.41.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTENDRIGH C. S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pye K., Chance B. Sustained sinusoidal oscillations of reduced pyridine nucleotide in a cell-free extract of Saccharomyces carlsbergensis. Proc Natl Acad Sci U S A. 1966 Apr;55(4):888–894. doi: 10.1073/pnas.55.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Gordon S. A. Hormonal Relations in the Phototropic Response: III. The Movement of C-labeled and Endogenous Indoleacetic Acid in Phototropically Stimulated Zea Coleoptiles. Plant Physiol. 1966 Jan;41(1):59–65. doi: 10.1104/pp.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J. Kinetics of stress relaxation properties of oat coleoptile cell wall after geotropic stimulation. Plant Physiol. 1973 Mar;51(3):464–467. doi: 10.1104/pp.51.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Miller C. Distribution and activation of the Golgi apparatus in geotropism. Plant Physiol. 1972 Apr;49(4):634–639. doi: 10.1104/pp.49.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Miller J., Miller C. Intracellular distribution of mitochondria after geotropic stimulation of the oat coleoptile. Plant Physiol. 1972 Jul;50(1):51–54. doi: 10.1104/pp.50.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton R. M., Thimann K. V. Transient effects of light on auxin transport in the Avena coleoptile. Plant Physiol. 1967 Feb;42(2):247–257. doi: 10.1104/pp.42.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman E. D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T. F., Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971 Jul;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- de la Fuente R. K., Leopold A. C. Kinetics of polar auxin transport. Plant Physiol. 1966 Nov;41(9):1481–1484. doi: 10.1104/pp.41.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]


