Abstract
Osmotic shock severely reduces the ability of aged strips of Phaseolus vulgaris leaves to take up α-aminoisobutyric acid, an amino acid analogue which is known to be transported by a specific mechanism. Cold osmotic shock, i.e., transfer from 0.5 m sucrose at 25 C to H2O at 2 C, decreases α-aminoisobutyric acid uptake almost to zero. Substitution of 10−3m ethylenediaminetetraacetate for the sucrose, i.e., treatment which does not involve plasmolysis, produces a similar, but less severe, effect.
About 3.5% of the total cell protein is released as a result of cold osmotic shock, by far the greater proportion being liberated into the water during the second stage of the shock treatment. Ethylenediaminetetraacetate and other shock treatments also bring about protein release, and the amount released is correlated with degree of depression of subsequent α-aminoisobutyric acid uptake.
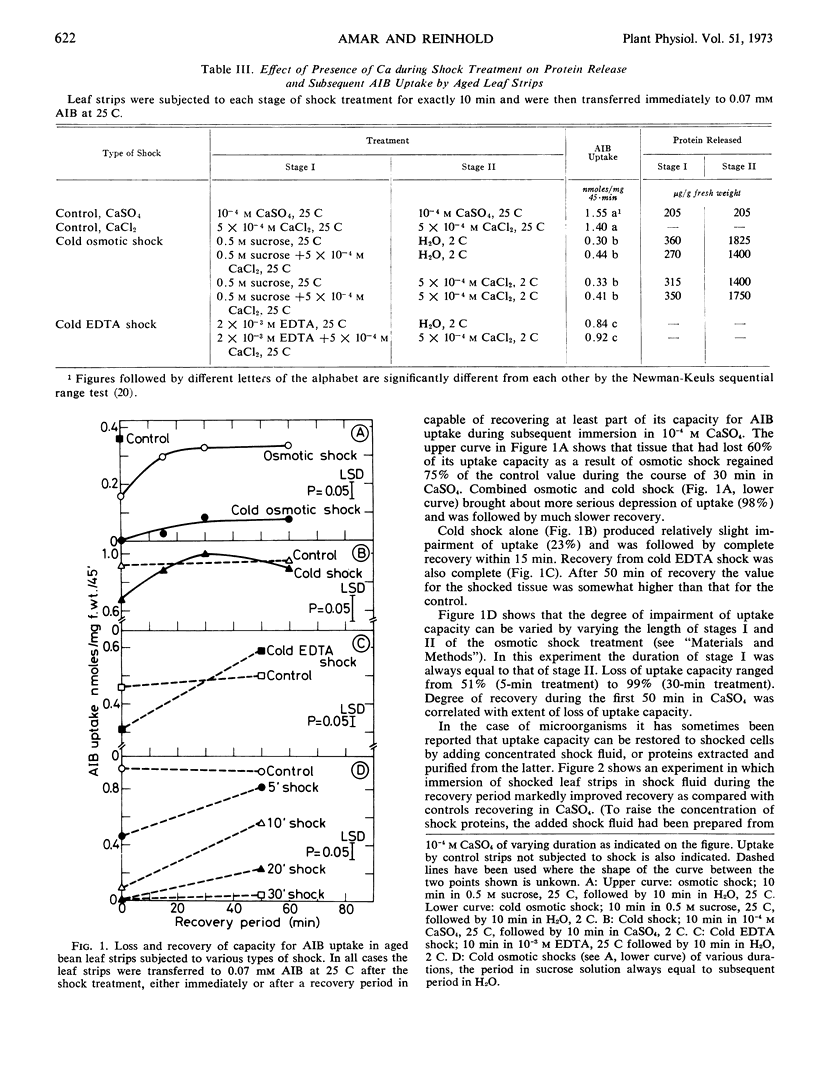
Shock tissue is capable of recovering a large proportion of its uptake capacity during subsequent immersion in 10−4m CaSO4.
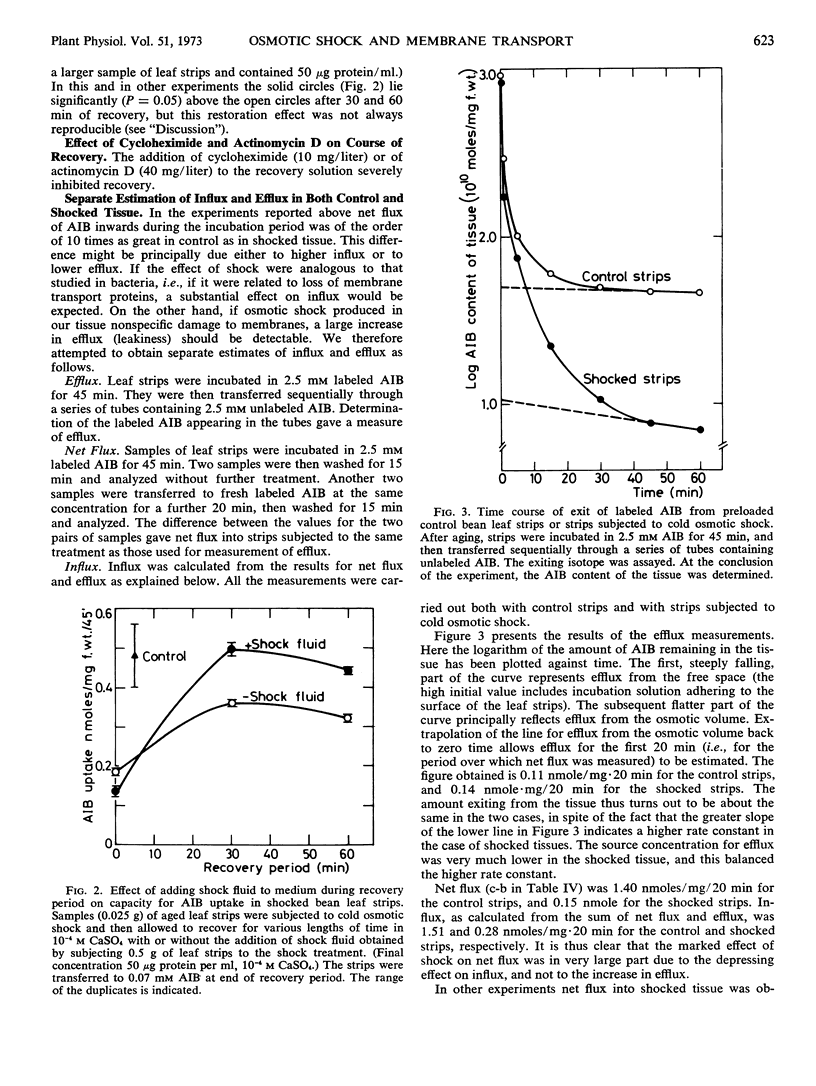
Separate estimation of α-aminoisobutyric acid influx and efflux showed that the marked effect of shock on net flux is largely attributable to a reduction in influx, and not to an increase in efflux. This and other results indicate that the shock effect on net flux is not due to nonspecific damage to membranes bringing about “leakiness.”
The fact that α-aminoisobutyric acid uptake is reduced to near zero by treatment which allows the cells to retain over 95% of their protein suggests that the shock phenomenon is analogous to that in bacteria, and that the small fraction of protein lost may be closely involved in the transport mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J Biol Chem. 1967 Mar 10;242(5):793–800. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. 3. Studies on the restoration of active transport. J Biol Chem. 1968 Jun 10;243(11):3128–3135. [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. II. Properties of galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3123–3127. [PubMed] [Google Scholar]
- Brockman R. W., Heppel L. A. On the localization of alkaline phosphatase and cyclic phosphodiesterase in Escherichia coli. Biochemistry. 1968 Jul;7(7):2554–2562. doi: 10.1021/bi00847a016. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakane P. K., Nichoalds G. E., Oxender D. L. Cellular localization of leucine-binding protein from Escherichia coli. Science. 1968 Jul 12;161(3837):182–183. doi: 10.1126/science.161.3837.182. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nieman R. H., Willis C. Correlation between the Suppression of Glucose and Phosphate Uptake and the Release of Protein from Viable Carrot Root Cells Treated with Monovalent Cations. Plant Physiol. 1971 Sep;48(3):287–293. doi: 10.1104/pp.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Pardee A. B. Membrane transport proteins. Proteins that appear to be parts of membrane transport systems are being isolated and characterized. Science. 1968 Nov 8;162(3854):632–637. doi: 10.1126/science.162.3854.632. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Pardee A. B., Watanabe K. Location of sulfate-binding protein in Salmonella typhimurium. J Bacteriol. 1968 Oct;96(4):1049–1054. doi: 10.1128/jb.96.4.1049-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Wiley W. R. Tryptophan transport in Neurospora crassa: a tryptophan-binding protein released by cold osmotic shock. J Bacteriol. 1970 Sep;103(3):656–662. doi: 10.1128/jb.103.3.656-662.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Arginine transport and metabolism in osmotically shocked and unshocked cells of Escherichia coli W. J Biol Chem. 1969 May 25;244(10):2737–2742. [PubMed] [Google Scholar]



