ABSTRACT
The discovery of bacterial conductive structures, termed nanowires, has intrigued scientists for almost a decade. Nanowires enable bacteria to transfer electrons over micrometer distances to extracellular electron acceptors such as insoluble metal oxides or electrodes. Nanowires are pilus based and in Geobacter sulfurreducens are composed of the type IV pilin subunit PilA. Multiheme c-type cytochromes have been shown to attach to nanowire pili. Two hypotheses have been proposed for electron conduction in nanowires. The first (termed the metal-like conductivity or MLC hypothesis) claims that the pilus itself has the electron-conductive properties and the attached cytochromes mediate transfer to the final electron acceptor, whereas the second hypothesis (termed the superexchange conductivity or SEC hypothesis) suggests that electrons are “hopping” between heme groups in cytochromes closely aligned with the pilus as a scaffold. In their recent article in mBio, Vargas et al. [M. Vargas, N. S. Malvankar, P.-L. Tremblay, C. Leang, J. A. Smith, P. Patel, O. Snoeyenbos-West, K. P. Nevin, and D. R. Lovley, mBio 4(2):e00210-13, 2013] address this ambiguity through an analysis of strain Aro-5, a G. sulfurreducens PilA mutant lacking aromatic residues in the nonconserved portion of PilA. These residues were suspected of involvement in electron transport according to the MLC hypothesis. The G. sulfurreducens mutant had reduced conductive properties, lending important support to the MLC hypothesis. The data also highlight the need for further and more conclusive evidence for one or the other hypothesis.
THE QUEST FOR A CLEAR NANOWIRE ELECTRON TRANSPORT MECHANISM
Dissimilatory metal-reducing bacteria (DMRB) may generate nanowire structures that help them exploit insoluble Mn and Fe oxides as electron acceptors for the oxidation of organic matter in anoxic soils and sediment (1). Understanding how protein-based nanowires are able to conduct electrons is intriguing, as proteins are generally considered to be electrical insulators. DMRB have been employed in microbial fuel cells and in bioremediation techniques, and bioengineered nanowires have been proposed for future use in nanobioelectronics (2). Recently, a potential role for nanowires in pathogenesis was shown when nanowire-producing bacterial biofilms were identified in bone samples from patients suffering from bisphosphonate-related osteonecrosis of the jaw (3). Hence, a complete understanding and control of electron transport in nanowires could have great implications for the management of environmental processes, the construction of bioelectrochemical systems, the fighting of pathogenesis, and the development of the next generation of electronics.
METAL-LIKE OR SUPEREXCHANGE NANOWIRE CONDUCTIVITY—AROMATIC RESIDUES VERSUS MULTIHEME CYTOCHROMES
Nanowire conductivity was first demonstrated in Geobacter sulfurreducens by conducting-probe atomic force microscopy of individual nanowire pili (2). In addition, the conductive properties of G. sulfurreducens biofilms were measured with microbial fuel cells. In situ measurements showed a conductivity of 5 mS cm−1, which is comparable to that of synthetic organic metallic conductors such as polyaniline (4, 5). Sheared off G. sulfurreducens nanowires displayed a conductivity of 4 µS cm−1 and had a temperature dependence similar to that of organic metals (5). By using scanning tunneling microscopy, nanofabricated electrodes, and conducting-probe atomic force microscopy, a number of other studies have demonstrated nanowire conductivity of 1 S cm−1 in Shewanella oneidensis pili (1, 6). The metal-like conductivity (MLC) and SEC hypotheses were put forward to explain the mechanism of electron transport in nanowires, and a great deal of effort has since been put into providing conclusive evidence to support one or the other hypothesis (5, 7). One group of scientists suggested that PilA itself has the propensity to transport electrons through stacking of aromatic residues, aligning the pi orbitals in the quaternary structure of the pilus, and allowing the electrons to be conducted through these pi-pi interchain stackings, conferring metal-like properties (5). According to this hypothesis, it was suggested that the role of attached multiheme cytochromes is to mediate direct contact with the electron acceptors and transfer the electrons from the pilus nanowire to the metal (5). Other evidence supported the superexchange conductivity (SEC) hypothesis. It was observed that acetate oxidation was carried out at a finite rate and that biofilms of finite thickness would grow on anodes. These data supported a finite rate of electron transfer between aligned cytochromes, as described by the SEC hypothesis (7). Further reinforcement of this hypothesis was obtained from indications of reversible oxidation and reduction of biofilm-associated redox factors based on spectroelectrochemical, conductivity, and cyclic voltammetry measurements (7). The debate has been going on without any conclusive or overwhelming evidence excluding either hypothesis (8–10).
SITE-DIRECTED MUTAGENESIS OF PilA LEADS THE WAY
The work presented by Vargas et al. (11) is based on a quintuple-mutant PilA protein from G. sulfurreducens. Vargas et al. targeted the five aromatic residues in the C-terminal part of PilA for alanine substitutions on the basis of the hypothesis that the aromatic residues of the G. sulfurreducens PilA C-terminal region are involved in pi-pi stacking and electron conductivity, reasoning that the conductive properties would be impaired by mutagenesis. PilA from G. sulfurreducens deviates from known structures of type IV pilins (T4Ps) in the apparent lack of a C-terminal head domain. The PilA mutant was shown to have proper decoration (similar to the wild type) with multiheme c-type cytochrome OmcS, as demonstrated by immunoelectron microscopy. The mutant showed diminished conductivity and a lowered ability to reduce Fe(III) oxide, demonstrating that association of cytochromes is not enough for efficient reduction to occur. Only 10% of the current production of the control was observed in mutant biofilms when using a graphite anode as an electron acceptor, and current levels of the mutant were comparable to that of a pilA-deficient mutant strain. Likewise, conductivity measurements in biofilms grown on gold electrodes showed a 10-fold reduction in conductivity compared to that of the control. Taken together, these data suggest that pili have to be conductive for effective long-range electron transfer, which supports the MLC hypothesis. This is consistent with the hypothesis that the aromatic amino acids replaced with alanine in the mutant account for the conductivity observed in nanowires. Structural studies have been initiated in the Lovley lab to further substantiate this proposal.
ONE MORE STEP IS TAKEN, BUT ADDITIONAL STEPS ARE NEEDED
Although the analyses presented by Vargas et al. (11) are important contributions to the elucidation of electron conductivity in nanowires and the results can be interpreted as supporting the MLC hypothesis, some important issues need to be addressed. First, the brute force approach of mutating five amino acids in a small protein might have unintentional effects on the structural integrity of the protein studied. Aromatic residues are normally important for stabilization of the hydrophobic core of protein domains, and mutagenesis could also affect the correct packing in the hydrophobic core of the pilus. The data presented indicate that the mutant produces structurally intact nanowires and that multiheme cytochromes can attach to the nanowires, but the alignment of associated cytochromes might well be impaired by the mutations. Careful analysis of the functional roles of the individual aromatic residues mutated in the study by Vargas et al. is necessary to follow up on these results. Second, as mentioned by Vargas et al., structural studies resulting in an atomic model for PilA and the nanowire pilus are essential to fully explain the role of aromatic residues in pilus conductivity and could provide a satisfying mechanism for electron transfer through nanowires. Although this is presumably a hard task, it is a necessary next step. G. sulfurreducens PilA aligns quite well with Pseudomonas aeruginosa PAK pilin, for which a high-resolution crystal structure is known (12). On the basis of the sequence alignment presented by Vargas et al., four of the five mutated aromatic residues can be identified in the known crystal structure (Fig. 1). By inspection of the positions of these amino acids in the context of the monomeric pilin and in the polymeric pilus, it can be seen that two of the aromatic residues (corresponding to F53 and Y56 in unprocessed G. sulfurreducens PilA) are located at the base of the head domain and point toward the hydrophobic core of the pilus (13). The two remaining residues (corresponding to Y61 and F80 in unprocessed G. sulfurreducens PilA) are located at the bottom and top, respectively, of the head domain of PAK pilin and point outward from the pilus. These residues could have an “anchoring” function in the head domain. All four residues are located in the highly conserved T4P “core” structure, and they do not show a continuous stacking of the identified aromatic residues in either the monomer or the pilus polymer. This raises the intriguing possibility that conducting nanowire pilus structures deviate substantially from known T4Ps. We thus eagerly await the determination of the first nanowire pilin structure.
FIG 1 .
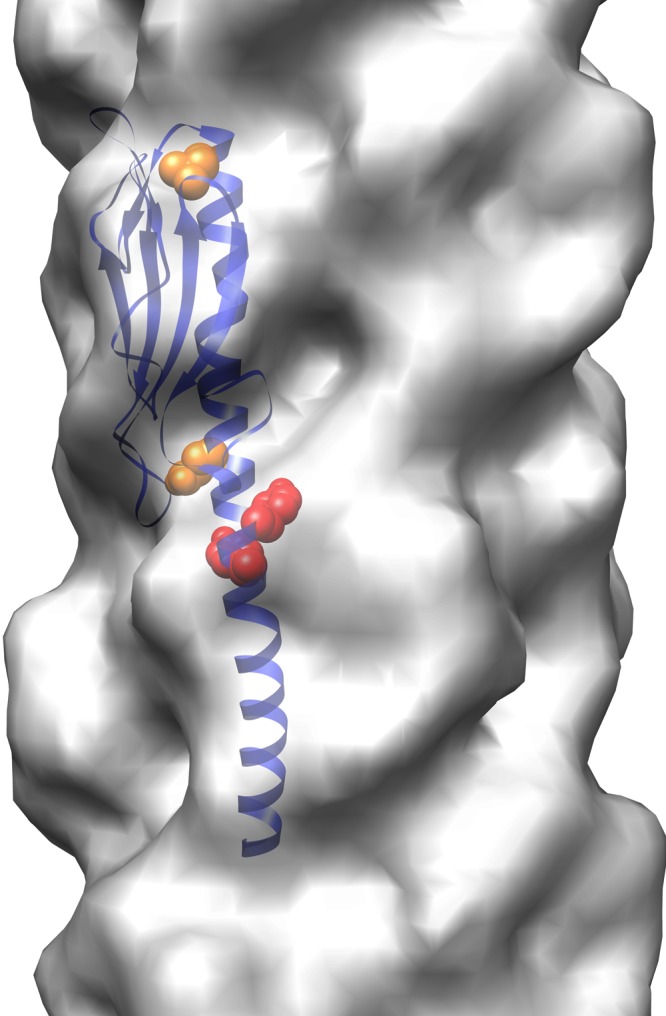
Cartoon representation of the P. aeruginosa PAK pilin structure (blue) docked into the cryoelectron microscopy density map (gray) of the type IV pilus of Neisseria gonorrhoeae to show the positions of the residues corresponding to four of the five mutated aromatic residues in G. sulfurreducens PilA in the context of the pilus. Residues corresponding to F53 and Y56 are shown in space fill and are colored red. These residues point toward the hydrophobic core of the pilus. Residues corresponding to Y61 and F80 are shown in space fill and are colored orange. These residues are located at the bottom and top of the head domain of the pilin, and they are pointing outward from the pilus. The figure was made with UCSF Chimera (14) and Protein Data Bank entries 2HIL and 1OQW, as well as EMDB entry EM-1236.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Citation Boesen T, Nielsen LP. 2013. Molecular dissection of bacterial nanowires. mBio 4(3):e00270-13. doi:10.1128/mBio.00270-13.
REFERENCES
- 1. Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii S, Logan B, Nealson KH, Fredrickson JK. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. U. S. A. 103:11358–11363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101 [DOI] [PubMed] [Google Scholar]
- 3. Wanger G, Gorby Y, El-Naggar MY, Yuzvinsky TD, Schaudinn C, Gorur A, Sedghizadeh PP. 2013. Electrically conductive bacterial nanowires in bisphosphonate-related osteonecrosis of the jaw biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 115:71–78 [DOI] [PubMed] [Google Scholar]
- 4. Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72:7345–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovley DR. 2011. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6:573–579 [DOI] [PubMed] [Google Scholar]
- 6. El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA. 2010. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. U. S. A. 107:18127–18131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bond DR, Strycharz-Glaven SM, Tender LM, Torres CI. 2012. On electron transport through Geobacter biofilms. ChemSusChem 5:1099–1105 [DOI] [PubMed] [Google Scholar]
- 8. Strycharz-Glaven SM, Tender LM. 2012. Reply to the ‘Comment on “On electrical conductivity of microbial nanowires and biofilms”’ by N. S. Malvankar, M. T. Tuominen and D. R. Lovley. Energy Environ. Sci. 5:6250–6255 [Google Scholar]
- 9. Malvankar NS, Tuominen MT, Lovley DR. 2012. Comment on “On electrical conductivity of microbial nanowires and biofilms”. by S. M. Strycharz-Glaven, R.M. Snider, A. Guiseppi-Elie and L.M. Tender. Energy Environ. Sci 5:6247–6249 [Google Scholar]
- 10. Polizzi NF, Skourtis SS, Beratan DN. 2012. Physical constraints on charge transport through bacterial nanowires. Faraday Discuss. 155:43–62; discussion, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas M, Malvankar NS, Tremblay P-L, Leang C, Smith JA, Patel P, Snoeyenbos-West O, Nevin KP, Lovley DR. 2013. Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4(2):e00210-13 http://dx.doi.10.1128/mBio.00105-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craig L, Taylor RK, Pique ME, Adair BD, Arvai AS, Singh M, Lloyd SJ, Shin DS, Getzoff ED, Yeager M, Forest KT, Tainer JA. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 11:1139–1150 [DOI] [PubMed] [Google Scholar]
- 13. Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. 2006. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23:651–662 [DOI] [PubMed] [Google Scholar]
- 14. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612 [DOI] [PubMed] [Google Scholar]


