Abstract
Purpose
We undertook an observational study to investigate the effects of immunosuppressive treatment on proteinuria and renal function in 179 Korean idiopathic membranous nephropathy patients with nephrotic syndrome.
Materials and Methods
The primary outcome was regarded as the first appearance of remission and the secondary outcomes as a decline in estimated glomerular filtration rate (eGFR) >50% or initiation of dialysis, and all-cause mortality. Seventy-two (40.2%) and 50 (27.9%) patients were treated with corticosteroids alone (C) and corticosteroids plus cyclosporine (C+C), respectively, whereas 57 (31.8%) did not receive immunosuppressants (NTx). Cyclosporine was added if there was no reduction in proteinuria of >50% from baseline by corticosteroids alone within 3 months.
Results
There were no differences in baseline renal function and the amount of proteinuria among the three groups. Overall, complete remission (CR) was achieved in 88 (72.1%) patients by immunosuppressants. In a multivariate analysis adjusted for covariates associated with adverse renal outcome, the probability of reaching CR was significantly higher in the C [hazard ratio (HR), 4.09; p<0.001] and C+C groups (HR, 2.57; p=0.003) than in the NTx group. Kaplan-Meier analysis revealed that 5-year CR rates of C, C+C, and NTx groups were 88.5%, 86.2%, and 56.7% (p<0.001). Ten-year event-free rates for the secondary endpoints in these three groups were 91.7%, 79.9%, and 57.2% (p=0.01).
Conclusion
Immunosuppressive treatment was effective in inducing remission and preserving renal function in these patients. Therefore, stepwise treatment using corticosteroids alone and in combination with cyclosporine is warranted in these patients.
Keywords: Corticosteroids, cyclosporine, idiopathic membranous nephropathy, nephrotic syndrome, remission
INTRODUCTION
Membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults. Even though MN can be accompanied by a wide spectrum of disease, including tumors, infections, autoimmune diseases, and drugs, the idiopathic form remains the most common. The natural course of idiopathic MN (IMN) varies from a spontaneous remission to progression to end-stage renal disease (ESRD). Approximately one-third of patients experience spontaneous remission, another third show persistent proteinuria, and the remaining third progress to ESRD.1 These variable courses of IMN cause physicians great difficulty in deciding whether to treat and, if so, what kind of drugs should be used.
Previous studies have shown that heavy proteinuria, arterial hypertension, renal insufficiency at presentation, and severe histologic lesions are associated with renal survival in IMN patients with nephrotic syndrome.1-3 Among these risk factors, persistent heavy proteinuria is the most reliable predictor of life-threatening complications and poor renal outcome in these patients.1-3 Therefore, aggressive treatments to induce complete or partial remission or to reduce the amount of proteinuria have been tested. Previous randomized control trials found that corticosteroids alone were not effective in IMN patients of Western patients with nephrotic syndrome in terms of preserving renal function and long term proteinuria reduction.4,5 These results led to the suggestion that a 6-month combination therapy with alternating corticosteroids and alkylating agents, either chlorambucil or cyclophosphamide, should be considered as the first-line of treatment in IMN patients with nephrotic syndrome.6-9 However, many physicians feel constrained when prescribing these cytotoxic agents due to the potential risks of marrow toxicity, gonadal dysfunction, and malignancy.10-12
Compared to studies on Western patients, the prognosis of IMN and response to drugs seem to be more favorable in East Asian patients. One study demonstrated that renal survival rates were relatively high in Japanese IMN patients with nephrotic syndrome. In addition, another study showed that treatment responses to corticosteroids alone or with other immunosuppressants were more favorable in Japanese and Chinese IMN patients.13-15 In both of these studies, the majority of patients received cyclophosphamide as the adjunctive drug to corticosteroids. In addition, since the randomized trials by Cattran, et al.16,17 revealed that cyclosporine was effective in inducing remission in steroid-resistant MN with nephrotic syndrome and in MN patients with progressive renal insufficiency and heavy proteinuria, the beneficial effect of cyclosporine in MN has been frequently reported. However, there has been no investigation on the impact of corticosteroids with or without cyclosporine on the outcome of IMN in East Asian patients. In this study, we aimed to elucidate the remission and renal survival rates in adult Korean patients with biopsy proven IMN, who presented with nephrotic syndrome and were followed up for more than 1 year, according to a three stage therapeutic strategy; conservative treatment, corticosteroids alone, and corticosteroids plus cyclosporine.
MATERIALS AND METHODS
Ethics statement
This study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Yonsei University College of Medicine Clinical Trial Center. All patients participated in the current study were aware of this investigation. However, since this study was a retrospective medical record-based study and the study subjects were de-identified, the IRB waived the need for written consent from the patients.
Study subjects and data collection
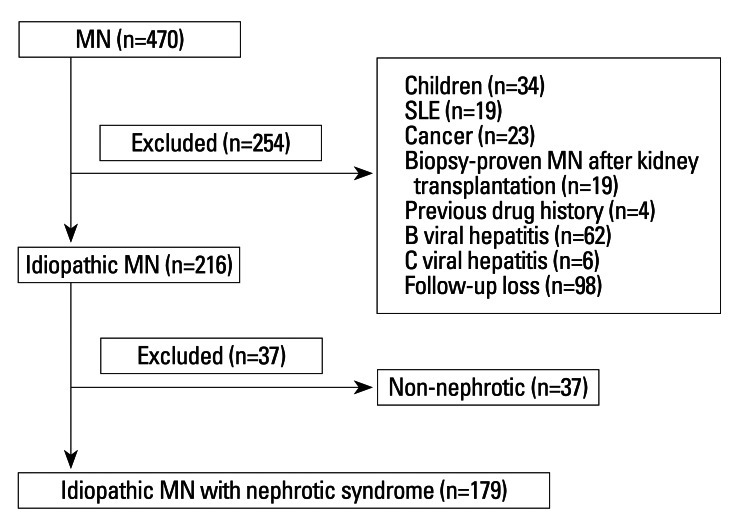
Between January 1990 and December 2009, 470 patients were diagnosed as having MN by renal biopsy at Yonsei University Severance Hospital in Seoul, Korea. Of the initial 470 MN patients, we excluded patients younger than 18 years old, patients with other diseases including systemic lupus erythematosus, diabetes mellitus, malignancy, and systemic infection, patients with a previous history of kidney transplantation or exposure to drugs associated with MN, such as gold, penicillamine, and captopril, patients with positive hepatitis B virus antigen or hepatitis C virus antibody, and patients with a follow-up duration of less than 1 year. Patients with proteinuria of a non-nephrotic range were also excluded (Fig. 1). Nephrotic syndrome was diagnosed based on heavy proteinuria of more than 3.5 g/day, hypoalbuminemia of less than 3.5 g/dL, the presence of edema, and hypercholesterolemia. Demographic and clinical data were reviewed retrospectively for age, gender, medical history, presenting symptoms, medications, response to treatment, time to remission, and follow-up duration and were recorded from the time of renal biopsy until the development of ESRD or the end of follow-up. The following laboratory data at the time of renal biopsy were collected: urinalysis, 24-hour urinary protein excretion, urinary protein-to-creatinine ratio, hemoglobin, blood urea nitrogen, serum creatinine, albumin, and total cholesterol levels. The estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease study formula. The stages of MN, which were reported from I to IV, were also reviewed.18
Fig. 1.
Flow-chart of the enrolment procedure. A total of 470 patients were diagnosed as membranous nephropathy (MN). Patients with an age of less than 18 years, patients with other diseases including systemic lupus erythematosus, diabetes mellitus, and malignancy, patients with previous history of kidney transplantation or exposure to drugs associated with MN, such as gold, penicillamine, and captopril, patients with positive hepatitis B virus antigen or hepatitis C virus antibody, and patients with a follow-up duration of less than 1 year were excluded. Finally, patients with proteinuria of non-nephrotic range were also excluded.
Treatment of patients
Conservative treatment, including dietary salt restriction, renin-angiotensin system (RAS) blockades or other anti-hypertensive medications, diuretics, and/or HMG co-A reductases (statins), was given to nearly all patients. In general, immunosuppressive therapy was initiated in patients with nephrotic range proteinuria for more than 3 months, serious complications of nephrotic syndrome such as venous thromboemboli and intractable edema, or rapidly deteriorating renal function. The first-line immunosuppressive drug was corticosteroids at a dose of 1 mg/kg/day. If there was no reduction in proteinuria of more than 50% from the baseline value by corticosteroids within 3 months, cyclosporine A was added along with 0.5 mg/kg/day corticosteroids. The initial cyclosporine A dose of 3 mg/kg/day was later adjusted aiming at 12-hour trough levels between 100 and 150 µg/L. In a minority of patients, cyclophosphamide at a dose of 2 mg/kg/day or mycophenolate mofetil at doses up to 2 g/day was used as a second-line drug.
Outcomes
The primary outcome was the first appearance of complete remission (CR) or partial remission (PR). Complete remission was defined as the absence of proteinuria (urinary protein excretion less than 0.3 g/day or a urinary protein-to-creatinine ratio of less than 0.3) and trace or negative urinary albumin on a dipstick test with the disappearance of edema and normalization of biochemical findings such as hypoalbuminemia and hypercholesterolemia. Partial remission was defined as a reduction in proteinuria by more than 50% from baseline and <3.5 g/day or urinary protein-to-creatinine ratio of <3.5. If neither of these remissions was achieved, the results were defined as no response to treatment. Relapse was defined as the reappearance of significant proteinuria of more than 3.5 g/day and edema in patients on remission. Meanwhile, secondary outcomes were considered as a decline in eGFR of more than 50% of baseline value or a requirement of renal replacement therapy, and all-cause mortality. During follow-up, complete or partial remission, spontaneous remission, relapse, deterioration of renal function, ESRD, and death were recorded.
Statistical analysis
Statistical analyses were performed using the statistical package SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as means±SD or as percentages. Continuous data were analyzed using one-way ANOVA or Kruskal-Wallis test according to treatment modalities, and significant differences were further confirmed by the Student's t-test or Mann-Whitney U test, respectively. For categorical variables, the chi-square or Fisher's exact test was used for multiple comparison. To identify independent factors associated with CR and CR+PR, multivariate Cox regression analysis was performed including all covariates with a p-value of <0.1 on univariate analysis. Even though a p-value was ≥0.1, potential confounding factors that were traditionally known as significant prognostic determinants of IMN1-3 were also included in the multivariate analysis. In result, covariates such as age, sex, blood pressure, baseline eGFR, proteinuria, RAS blockades use, and pathologic stages were entered in the multivariate models. Kaplan-Meier analyses and log rank tests were used to compare the differences in reaching CR or PR, and the secondary outcomes. p-values less than 0.05 were considered statistically significant.
RESULTS
Baseline characteristics of patients
A total of 179 IMN patients with nephrotic syndrome were included in the present study. The mean age of patients at the time of renal biopsy was 52.1 years, 102 patients (57.0%) were males, and the mean follow-up duration was 56.9±52.5 months. Twenty-five patients (14.0%) had hypertension and the mean serum creatinine, albumin, and cholesterol concentrations were 1.1±0.5, 2.7±0.7, and 306.4±99.5 mg/dL, respectively. The mean 24-hour urinary protein excretion values were 7.5±4.2 g/day. RAS blockades such as angiotensin converting enzyme inhibitors or angiotensin II receptor blockers were prescribed in 161 patients (89.9%) and statins in 146 patients (81.6%). Renal biopsy findings revealed MN with stage I, II, III, and IV in 51 (28.0%), 84 (46.9%), 42 (23.5%), and 2 patients (1.1%), respectively (Table 1).
Table 1.
Baseline Characteristics According to Treatment Modalities
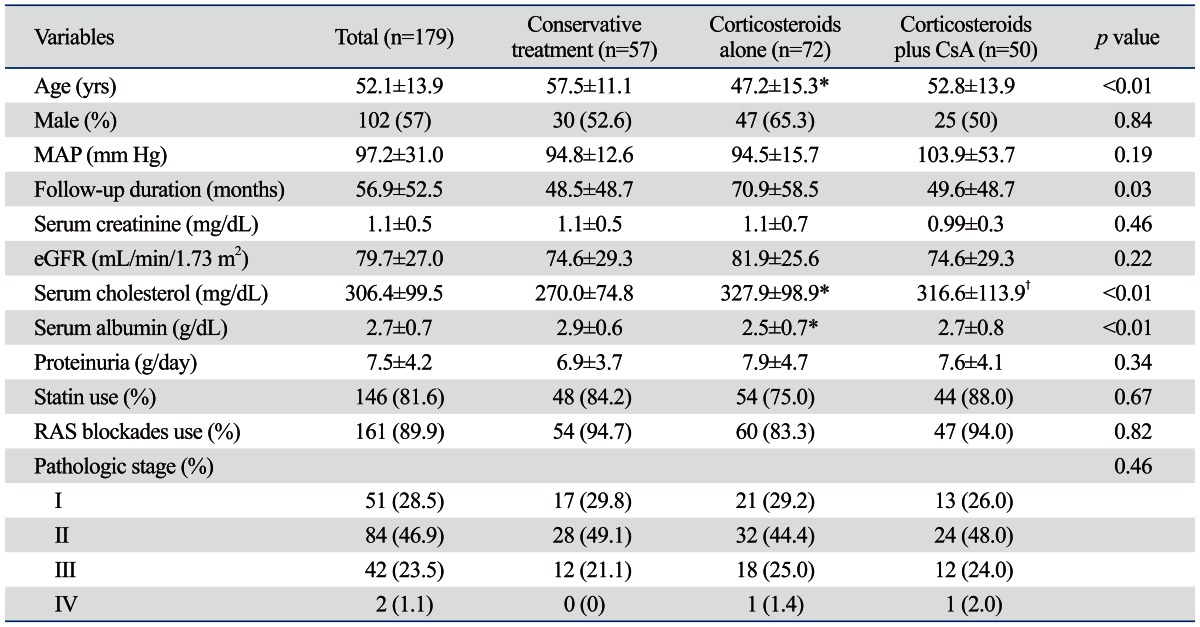
CsA, cyclosporine A; MAP, mean arterial pressure; eGFR, estimated glomerular filtration rate (by MDRD-4 equation); RAS, renin-angiotensin system; MDRD, Modification of Diet in Renal Disease study.
Values are expressed as mean±standard deviation or number (percentage).
*p<0.01 vs. conservative treatment.
†p<0.05 vs. conservative treatment.
Comparison of clinical and laboratory parameters according to treatment modality
Among the 179 patients, 72 (40.2%) received only corticosteroids treatment, while 50 patients (27.9%) received both cyclosporine and corticosteroids. Cyclophosphamide or mycophenolate mofetil combined with corticosteroids were given in 4 patients (0.02%) and 1 patient (0.01%), respectively. The remaining 57 patients (31.8%) did not receive any immunosuppressants during the follow-up.
Baseline clinical and laboratory parameters were compared among the three groups; conservative treatment, corticosteroids alone, and corticosteroids plus cyclosporine groups. Compared to the conservative treatment group, the mean age was significantly younger (p<0.001), serum cholesterol concentrations were significantly higher (p=0.003), and serum albumin levels were significantly lower (p=0.003) in patients treated with corticosteroids alone. There were also significant differences in serum cholesterol concentrations between the conservative treatment and corticosteroids plus cyclosporine groups (p=0.04). However, post-hoc tests revealed that serum cholesterol and albumin levels were comparable between patients with corticosteroids alone and with corticosteroids plus cyclosporine. On the other hand, there were no significant differences in the proportion of male patients, serum creatinine concentrations, eGFR, 24-hour urinary protein excretion, and the stage of MN among the three groups (Table 1).
Response to treatment in terms of nephrotic syndrome
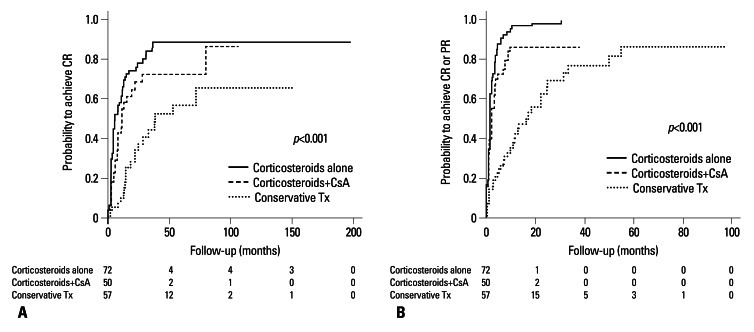
Kaplan-Meier analysis revealed that cumulative probabilities to achieve CR and to achieve CR or PR were significantly higher in the corticosteroids alone and corticosteroids plus cyclosporine groups compared to patients with conservative treatment. The 1-year CR rates in the conservative treatment, corticosteroids alone, and corticosteroids plus cyclosporine groups were 10.5%, 61.5%, and 42.9%, respectively (p<0.001). In addition, the 5-year CR rates in these three groups were 56.7%, 88.5%, and 86.2%, respectively (p<0.001) (Fig. 2A). Considering either CR or PR, the overall 1-year remission rates according to three therapeutic modalities were 42.4%, 97.0%, and 86.2%, respectively (p<0.001) (Fig. 2B).
Fig. 2.
Kaplan-Meier plots for cumulative probabilities to achieve complete remission (CR) and to achieve CR or partial remission (PR) according to treatment modalities. (A) Probability to achieve CR was significantly higher in patients treated with corticosteroids alone or with cyclosporine A (CsA) compared to patients with conservative treatment (Tx). (B) Probability to achieve CR or PR was significantly higher in patients treated with corticosteroids alone or with CsA compared to patients with conservative Tx.
In a multivariate analysis adjusted for age, sex, blood pressure, baseline eGFR, proteinuria, RAS blockades use, and pathologic stages, the probability of reaching CR was significantly higher in the corticosteroids alone [hazard ratio (HR), 4.09; 95% CI, 2.34-7.15; p<0.001] and corticosteroids plus cyclosporine groups (HR, 2.57; 95% CI, 1.45-4.57; p=0.003) compared to patients with conservative treatment (Table 2). Corticosteroids alone (HR, 4.74; 95% CI, 2.94-7.64; p<0.001) and corticosteroids plus cyclosporine treatment (HR, 3.51; 95% CI, 2.16-5.71; p<0.001) were also significantly associated with an increased probability of reaching CR or PR (Table 2).
Table 2.
Multiple Cox Regression Analysis for CR, and CR or PR Adjusted for Covariates
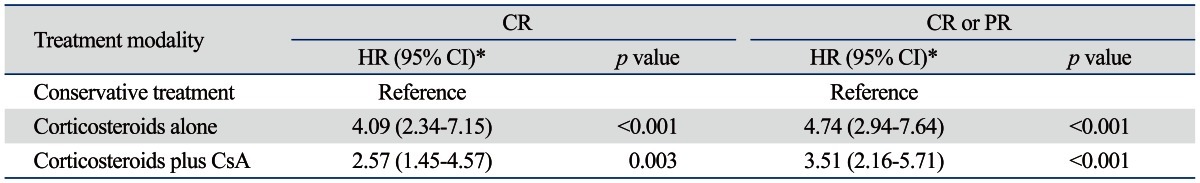
CR, complete remission; PR, partial remission; CsA, cyclosporine A; eGFR, estimated glomerular filtration rate.
*Adjusted for age, sex, blood pressure, baseline eGFR, proteinuria, renin-angiotensin system blockades use, and pathologic stage.
Response to treatment in terms of renal outcome and all-cause mortality
The numbers of patients who experienced renal outcome, which was defined as a decline in eGFR of more than 50% of baseline value or a requirement of renal replacement therapy, were 10 (17.5%) in the conservative treatment, 5 (6.9%) in the corticosteroids alone, and 5 (10.0%) in the corticosteroids plus cyclosporine group. During the follow-up period 2 patients (3.5%) in the conservative treatment group died of acute myocardial infarction and cerebral infarction, and 2 deaths due to acute myocardial infarctions were observed in the corticosteroids plus cyclosporine group, while no mortality was noticed in patients treated with corticosteroids alone. Even though the 5-year event-free rates for the composite secondary endpoints of renal outcome and all-cause mortality were comparable among the three groups (conservative treatment, 82.6%; corticosteroids alone, 91.7%; and corticosteroids plus cyclosporine, 86.5%; p=0.41), the 10-year event-free rates for the secondary endpoints were significantly higher in patients treated with corticosteroids alone (91.7%) and with corticosteroids plus cyclosporine (79.9%) compared to the conservative treatment group (57.2%; p=0.01) (Fig. 3).
Fig. 3.
Kaplan-Meier plots for the composite secondary endpoints, defined as renal outcome and all-cause mortality, according to treatment modalities. The cumulative event-free rates were significantly higher in patients treated with corticosteroids alone or with cyclosporine A (CsA) compared to patients with conservative treatment (Tx).
Adverse events
Cushingoid appearance was observed in all patients treated with corticosteroids. A life-threatening infection developed in 1 patient in the corticosteroids plus cyclosporine group, and newly-developed diabetes or impaired glucose tolerance was seen in 5 patients in the corticosteroids alone and 1 patient in the corticosteroids plus cyclosporine group. In addition, 1 patient had transiently elevated liver enzyme and 2 patients experienced mild nausea after treatment with corticosteroids plus cyclosporine. Moreover, mild gum hypertrophy was observed in 1 patient treated with corticosteroids plus cyclosporine (Table 3).
Table 3.
Adverse Events According to Treatment Modalities
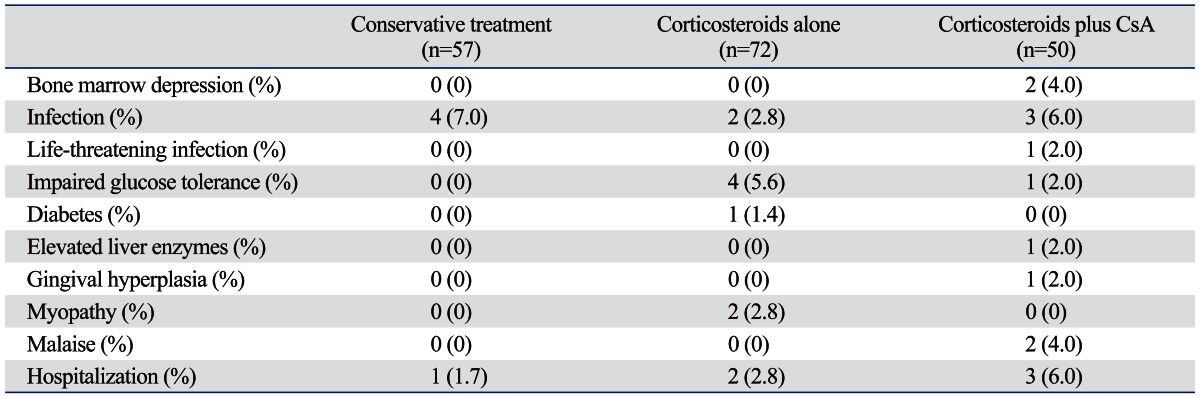
CsA, cyclosporine A.
Characteristics of patients who experienced relapses
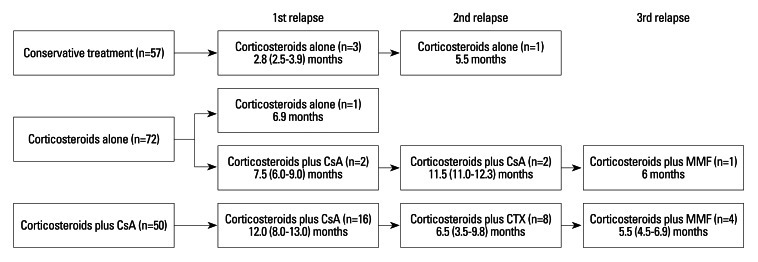
Table 4 shows the characteristics of patients who experienced relapses according to three treatment modalities. Of all patients, 38 relapses occurred in 22 patients (11 patients with one relapse, 6 patients with two relapses, and 5 patients with three relapses); 3 (5.3%) in the conservative treatment group, 3 (4.2%) in the corticosteroids alone group, and 16 patients (32.0%) in the corticosteroid plus cyclosporine group. During the follow-up, 17 patients (77.2%) achieved CR or PR. All patients in the conservative treatment group were treated with corticosteroids alone at the time of relapses. In the corticosteroids alone group, corticosteroids plus cyclosporine were used in 2 patients at the time of 1st and 2nd relapses and corticosteroids plus mycophenolate mofetil were used in 1 patient at the time of 3rd relapse. All patients in the corticosteroids plus cyclosporine group were treated with corticosteroids plus cyclosporine at the time of 1st relapse, while corticosteroids plus cyclophosphamide and corticosteroids plus mycophenolate mofetil were used in 8 patients at the time of 2nd relapse and in 4 patients at the time of 3rd relapse, respectively (Fig. 4).
Table 4.
Characteristics of Patients, Who Experienced Relapses, According to Treatment Modalities
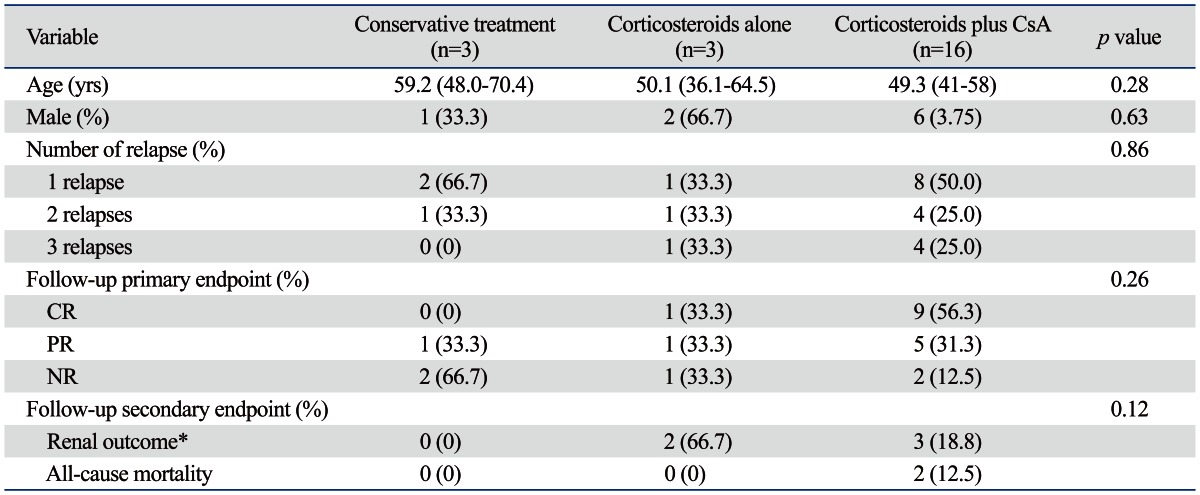
CsA, cyclosporine A; CR, complete remission; PR, partial remission; NR, no response; eGFR, estimated glomerular filtration rate; IQR, InterQuartile Rate. Values are expressed as median (IQR) or number (percentage).
*Defined as a decline in eGFR of more than 50% of baseline value or a requirement of renal replacement therapy.
Fig. 4.
Flow-chart of the treatment regimens and therapeutic duration in patients who experienced relapses. Values are expressed as median (IQR). CsA, cyclosporine A; CTx, cyclophosphamide; MMF, mycophenolate mofetil; IQR, InterQuartile Rate.
DISCUSSION
The results of this retrospective study demonstrate that corticosteroids alone or with cyclosporine were effective in inducing remission in Korean IMN patients with nephrotic syndrome. These treatment regimens were also independently associated with an increased probability of achieving remission. In addition, the cumulative renal survival was significantly higher in patients treated with immunosuppressants compared to patients who received only conservative treatment.
Numerous studies on the alternative treatment regimens for IMN have been conducted because treatment with corticosteroids alone in IMN patients with nephrotic syndrome of Western countries is not as effective as in nephrotic patients with minimal changes disease (MCD) in terms of long-term proteinuria reduction and renal function preservation.4,5,19 The results of these studies were used to define the widely accepted Ponticelli regimen, a 6-month combination therapy with corticosteroids and cytotoxic agents such as chlorambucil and cyclophosphamide, which has been considered the first-line treatment regimen for IMN patients with nephrotic syndrome.6-9 In addition, two meta-analyses and a systematic review confirmed that only the treatment including cytotoxic agents increased the chance of remission of nephrotic syndrome in IMN patients.20-22 However, the side effects of alkylating agents make physicians hesitate when prescribing these drugs. Approximately 10% of patients have reported discontinuing treatment because of adverse effects.12 Even though the incidence of malignancy in patients receiving cytotoxic drugs has been known to be comparable to that of the general Western population; however, leukopenia and infertility, of which Korean are more critically receptive, are additional problems. Due to the high cost of granulocyte colony stimulating factor (G-CSF) and a strict indication for insurance coverage, the use of G-CSF has been limited in Korea. In addition, Koreans consider infertility a serious disability even in the elderly. For these reasons, cyclosporine has been largely substituted for alkylating agents in Korea to treat nephrotic syndrome secondary to IMN.
Cyclosporine has been widely used in nephrotic patients with MCD or focal segmental glomerulosclerosis, particularly in patients who are steroid-resistant or steroid-dependent, or in patients experiencing frequent relapses.23 In addition, Cattran, et al.16,17 demonstrated that cyclosporine significantly abrogated the rate of deterioration of renal function and reduced the amount of proteinuria in IMN patients at a high risk of progression and persistent nephrotic range proteinuria. Alexopoulos, et al.24 also found that cyclosporine with or without corticosteroids was effective in inducing remission in the majority of nephrotic patients with IMN and well-preserved renal function. Based on these findings, the 'Cyclosporine in Idiopathic Nephrotic Syndrome' working group recommended that cyclosporine could be tried as a first-line option in IMN patients with nephrotic syndrome either as monotherapy or in combination with corticosteroids.23 In the present study, however, patients at medium or high risk were not initially treated with cyclosporine. Instead, corticosteroids were used as the first-line drug for these patients. Unlike patients of Western countries, East Asian patients with IMN responded well to corticosteroids alone.14 Tang, et al.15 demonstrated that treatment with corticosteroids alone for 6 months induced remission in 71% (5/7) of Chinese IMN patients with nephrotic syndrome. In addition, a retrospective multicenter study from Japan showed that corticosteroids alone induced CR and PR of proteinuria in 47.9% and 39.3% of IMN patients with nephrotic syndrome, respectively.14 The results of our study also found that 70 out of 122 patients (57.4%) responded to the initial treatment of corticosteroids alone. These findings suggest that biological origin may influence the therapeutic responsiveness to corticosteroids and that corticosteroids alone can be tried first in East Asian IMN patients with nephrotic syndrome.
In this study, CR or PR occurred in 86.2% of our patients with conservative treatment, which was significantly higher compared to those found previously in Western studies.25 In contrast, even though the mean follow-up duration of this study was shorter than that of the multicenter study from Japan, the proportion of patients taking RAS blockades in the conservative treatment group was significantly higher (94.7% vs. 23.0%) in the present study, resulting in comparable spontaneous remission rates with the Japan study.14 Since spontaneous remission occurs progressively during the follow-up, and the use of RAS blockades and baseline proteinuria are significant independent predictors of spontaneous remission in IMN patients with nephrotic syndrome,25 we infer that these divergent results may be attributed to the differences in biological origin, follow-up duration, the proportion of patients on RAS blockades, and the severity of nephrotic syndrome in patients on conservative treatment. Meanwhile, since almost 20% of patients with conservative treatment still show progressive loss of renal function during follow-up despite an initial favorable prognosis, these patients would have benefited from immunosuppressive therapy as well.
A number of previous studies have revealed that old age, male gender, hypertension, heavy proteinuria, and renal insufficiency were significant risk factors for progression to ESRD in IMN patients with nephrotic syndrome.2,3 Cattran, et al.23 also found that the highest sustained 6-month period of proteinuria was the most important predictor of progression in patients with IMN. In addition, accumulating evidence suggests that IMN patients with CR or PR remission have a significantly favorable clinical course compared to those with persistent nephrotic range proteinuria.22 Therefore, reducing proteinuria as soon as possible may have a significant impact on the renal survival in these patients. In the current study, compared to the conservative treatment group, serum cholesterol levels were significantly higher and serum albumin concentrations were significantly lower in patients who were treated with corticosteroids alone or corticosteroids plus cyclosporine. Even though there was no statistical significance. Moreover, 24-hour urinary protein excretion was greater in these patients. These findings indicate that immunosuppressant treatment was given in more clinically severe patients. Nevertheless, CR or PR was more frequently observed in the immunosuppressive group. Immunosuppressive treatment was also found to be as the most significant independent predictor of achieving CR or PR. Furthermore, renal survival was better in patients who received immunosuppressive agents. Taken together, these findings indicate that corticosteroids alone or with cyclosporine treatment may be required not only to induce remission of proteinuria but also to preserve renal function in Korean IMN patients with severe nephrotic syndrome.
There are several limitations to our study. First, even though a relatively large number of IMN patients with nephrotic syndrome were included from a single center, this study was a retrospective cohort study without any intervention on treatment option. In addition, this nature of the present study could make inferences on the effect of corticosteroids treatment biased. Second, since there were no standardized indications for starting immunosuppressant drugs or continuing conservative treatment, the treatment decisions were dependent on the preferences of individual physician's decision. Third, immunosuppressive treatment was usually initiated within 3 months in the IMN patients with severe nephrotic syndrome. Recently, a retrospective multicenter study from Spain revealed that spontaneous remission occurred in 31.7% of IMN patients with nephrotic syndrome (104/328) and in 21.5% among those with proteinuria more than 12 g/day.25 Moreover, time required to achieve CR and PR in the Spanish study were 38.5±25.2 and 14.7±11.4 months, respectively, and the incidence of spontaneous remission was substantially increased during the follow-up period-up to 20 months.25 Therefore, an appreciable number of patients in the immunosuppressive treatment group of the current study might have a chance to achieve spontaneous remission. Despite these limitations, this study has a strong point of the inclusion of a large number of patients and only biopsy-proven IMN patients with nephrotic syndrome, who were indeed at a risk of renal progression. Furthermore, conservative treatment including RAS blockades was universally provided to most patients. Lastly, given the lack of data in Asian patients, our findings may provide clues for the treatment of IMN in this population.
In conclusion, the present data indicate that corticosteroids, either in combination with cyclosporine or alone, may induce rapid remission of proteinuria in Korean IMN patients with nephrotic syndrome. Additionally, renal survival in treated patients appears to be improved. Therefore, the results warrant a randomized trial of these treatments. The stepwise approach presented here may provide a helpful guide to physicians in the treatment of IMN.
ACKNOWLEDGEMENTS
This work was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0030711), and by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int. 2001;59:1983–1994. doi: 10.1046/j.1523-1755.2001.0590051983.x. [DOI] [PubMed] [Google Scholar]
- 2.Ponticelli C, Passerini P. Can prognostic factors assist therapeutic decisions in idiopathic membranous nephropathy? J Nephrol. 2010;23:156–163. [PubMed] [Google Scholar]
- 3.Muirhead N. Management of idiopathic membranous nephropathy: evidence-based recommendations. Kidney Int Suppl. 1999;70:S47–S55. doi: 10.1046/j.1523-1755.1999.07007.x. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JS, Healy MJ, Adu D The MRC Glomerulonephritis Working Party. The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. Q J Med. 1990;74:133–156. [PubMed] [Google Scholar]
- 5.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, et al. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 6.Glassock RJ. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis. 2004;44:562–566. [PubMed] [Google Scholar]
- 7.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–1604. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Zucchelli P, Passerini P, Cesana B The Italian Idiopathic Membranous Nephropathy Treatment Study Group. Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med. 1992;327:599–603. doi: 10.1056/NEJM199208273270904. [DOI] [PubMed] [Google Scholar]
- 10.Faurschou M, Sorensen IJ, Mellemkjaer L, Loft AG, Thomsen BS, Tvede N, et al. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35:100–105. [PubMed] [Google Scholar]
- 11.Wetzels JF. Cyclophosphamide-induced gonadal toxicity: a treatment dilemma in patients with lupus nephritis? Neth J Med. 2004;62:347–352. [PubMed] [Google Scholar]
- 12.du Buf-Vereijken PW, Branten AJ, Wetzels JF Membranous Nephropathy Study Group. Cytotoxic therapy for membranous nephropathy and renal insufficiency: improved renal survival but high relapse rate. Nephrol Dial Transplant. 2004;19:1142–1148. doi: 10.1093/ndt/gfh036. [DOI] [PubMed] [Google Scholar]
- 13.Eriguchi M, Oka H, Mizobuchi T, Kamimura T, Sugawara K, Harada A. Long-term outcomes of idiopathic membranous nephropathy in Japanese patients treated with low-dose cyclophosphamide and prednisolone. Nephrol Dial Transplant. 2009;24:3082–3088. doi: 10.1093/ndt/gfp251. [DOI] [PubMed] [Google Scholar]
- 14.Shiiki H, Saito T, Nishitani Y, Mitarai T, Yorioka N, Yoshimura A, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. 2004;65:1400–1407. doi: 10.1111/j.1523-1755.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Tang S, Chan TM, Cheng IK, Lai KN. Clinical features and treatment outcome of idiopathic membranous nephropathy in Chinese patients. QJM. 1999;92:401–406. doi: 10.1093/qjmed/92.7.401. [DOI] [PubMed] [Google Scholar]
- 16.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 17.Cattran DC, Greenwood C, Ritchie S, Bernstein K, Churchill DN, Clark WF, et al. Canadian Glomerulonephritis Study Group. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenreich T, Churg J. Pathology of membranous nephropathy. Pathol Annu. 1968;3:145–186. [Google Scholar]
- 19.Youn YS, Lim HH, Lee JH. The clinical characteristics of steroid responsive nephrotic syndrome of children according to the serum immunoglobulin E levels and cytokines. Yonsei Med J. 2012;53:715–722. doi: 10.3349/ymj.2012.53.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofstra JM, Wetzels JF. Alkylating agents in membranous nephropathy: efficacy proven beyond doubt. Nephrol Dial Transplant. 2010;25:1760–1766. doi: 10.1093/ndt/gfq017. [DOI] [PubMed] [Google Scholar]
- 21.du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis. 2005;46:1012–1029. doi: 10.1053/j.ajkd.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol. 2005;16:1188–1194. doi: 10.1681/ASN.2005010028. [DOI] [PubMed] [Google Scholar]
- 23.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, et al. Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: workshop recommendations. Kidney Int. 2007;72:1429–1447. doi: 10.1038/sj.ki.5002553. [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulos E, Papagianni A, Tsamelashvili M, Leontsini M, Memmos D. Induction and long-term treatment with cyclosporine in membranous nephropathy with the nephrotic syndrome. Nephrol Dial Transplant. 2006;21:3127–3132. doi: 10.1093/ndt/gfl360. [DOI] [PubMed] [Google Scholar]
- 25.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]