Abstract
Purpose
This study aimed to evaluate the correlation between associating factors of moderate to severe asthma with obstructive sleep apnea (OSA).
Materials and Methods
One hundred and sixty-seven patients who visited the pulmonary and sleep clinic in Severance Hospital presenting with symptoms of sleep-disordered breathing were evaluated. All subjects were screened with ApneaLink. Thirty-two subjects with a high likelihood of having OSA were assessed with full polysomnography (PSG).
Results
The mean age was 58.8±12.0 years and 58.7% of subjects were male. The mean ApneaLink apnea-hypopnea index (AHI) was 12.7±13.0/hr. The mean ApneaLink AHI for the 32 selected high risk patients of OSA was 22.3±13.2/hr, which was lower than the sleep laboratory-based PSG AHI of 39.1±20.5/hr. When OSA was defined at an ApneaLink AHI ≥5/hr, the positive correlating factors for OSA were age, male gender, and moderate to severe asthma.
Conclusion
Moderate to severe asthma showed strong correlation with OSA when defined at an ApneaLink AHI ≥5/hr.
Keywords: Apnea-hypopnea index, ApneaLink, asthma, obstructive sleep apnea, sleep-disordered breathing
INTRODUCTION
Obstructive sleep apnea syndrome (OSA) is characterized by repeated episodes of upper airway obstruction that results in brief periods of breathing cessation (apnea) or a marked reduction in airflow (hypopnea) during sleep. OSA is the most severe form of obstructive sleep-disordered breathing (SDB). OSA is a common disorder with a prevalence estimated at 10-20%.1,2 Risk factors for OSA include male gender, age, obesity, and nocturnal nasal congestion.2 A population-based study in Korea reported the prevalence of OSA was 4.5% and 3.2% in men and women, respectively.3
Previous epidemiologic studies demonstrated that patients with asthma have an increased risk of OSA.4-6 In an Australian longitudinal study, asthma was an independent risk factor for the development of habitual snoring.7 A prospective cohort study showed a high prevalence of OSA in patients with difficult-to-control asthma.8 On the contrary, OSA may aggravate asthma because treatment for OSA has been shown to improve asthma symptoms.9 The National Asthma Education and Prevention Program Expert Panel Report recommends OSA evaluation because it is a potential contributor to poor asthma control.10 For such reasons, a more specific understanding of what increases a predisposition for OSA would be useful.
It has been suggested that gastroesophageal reflux disease (GERD), postnasal drip syndrome, and obesity may contribute to the development of OSA, but the role of each of these conditions in OSA has not been studied.11,12 To date, many studies have investigated the risk or prevalence of OSA in asthma patients; however, there is lack of data concerning the prevalence of asthma in high-risk OSA patients. Therefore, we investigated the predisposing factors of moderate to severe asthma in high OSA risk patients and assessed possible associations to predict other risk factors for OSA.
Although sleep laboratory-based polysomnography (PSG) is currently regarded as the gold standard for the diagnosis of sleep apnea,13 it is labor-intensive, costly, and has limited availability.14 The use of a portable recording device such as the ApneaLink for screening sleep apnea has some potential advantages in terms of cost, convenience, and better sleep quality for patients.15 Portable recording devices may be used as an alternative to PSG for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA.16 Recently, two studies validated the use of ApneaLink for the screening of sleep apnea compared with PSG.17,18 As such, we used ApneaLink for OSA screening to estimate the predisposing factors of moderate to severe asthma in high OSA risk patients and to assess possible associations to predict other risk factors for OSA.
MATERIALS AND METHODS
Subjects
All subjects were adults age ≥18 years that visited the pulmonary and sleep clinic at Severance hospital from February 2007 to August 2008 with symptoms of daytime sleepiness, choking and gasping during sleep, recurrent awakenings from sleep, unrefreshed sleep, daytime fatigue, and impaired concentration.13,19
The enrolled patients were checked for diagnoses of lung and other comorbidities. Patients diagnosed with asthma were categorized by severity according to the American Thoracic Society criteria20 and difficult asthma study.21
Severe asthma required at least one of the following major criteria: daily oral steroid use for >50% of the previous 12 months or a high-dose inhaled steroid (≥1000 mg/day fluticasone or equivalent) and at least one additional continuous add-on therapy for ≥12 months. Severe asthma also required at least two of the following minor criteria: use of a daily short-acting β-agonist, a persistent forced expiratory volume in one second (FEV1) <70%, a predicted forced vital capacity (FEV1/FVC) of <80%, at least one urgent visit or at least three steroid bursts in the last 12 months, prompt deterioration with <25% steroid dose reduction, or a previous near-fatal asthma attack within the last three years.
Moderate asthma was defined as well-controlled asthma symptoms, the use of a long-acting β-agonist at ≥200 mg/day and fluticasone (or an equivalent) at ≤1000 mg/day, at least two steroid bursts in the past year and none within the last three months, <30 total days on oral steroids within the last 12 months, a predicted FEV1 of >70%, and at least one unscheduled clinical visit within the previous 12 months.
Asthma was defined as a positive response to 1) being diagnosed with asthma, 2) having wheezed in the last 12 months, 3) taking corticosteroids and objective evidence of variable airway obstruction, following the diagnostic criteria of Global Initiative for Asthma (GINA) guidelines. Additionally a demonstration of a positive bronchodilator test (increase in FEV1 ≥12% and 200 mL) or a positive methacholine challenge test within the previous year was required.
Pregnant women and patients with significant cognitive impairment, a poorly controlled psychiatric disorder, or who had previous treatment for OSA were excluded.
All subjects provided informed consent to join the study. The Institutional Review Board of the Clinical Research Institute of Severance Hospital, Yonsei University College of Medicine, approved the study protocol (IRB number: 4-2011-0448).
Design
This was a cross-sectional study. Patients presented with symptoms of SDB were enrolled. All subjects agreed to use the ApneaLink device at home for one night after being instructed on the use of the device by an experienced sleep technician. Subjects returned the ApneaLink device to the pulmonary and sleep clinic the following morning, and the data were downloaded and analyzed. Patients with a high likelihood of having OSA were assessed with full PSG within 2 weeks after the ApneaLink study. We investigated all established diagnoses of lung and other comorbidities at enrollment, such as bronchial asthma, chronic obstructive pulmonary disease (COPD), GERD, rhinitis, diabetes, cardiovascular disease, and habitual snoring. Past smoking and body mass index (BMI) were also identified. In patients with asthma, current and previous asthma medications were recorded and recent spirometry data was collected to assess asthma severity.
Device
The ApneaLink™ device (ResMed, Sydney, Australia) is a portable single-channel screening tool for sleep apnea. The device consists of a nasal cannula attached to a small case that houses a pressure transducer. The ApneaLink software analyzes data generated by the flow signal, producing a report that provides information regarding snoring and inspiratory flow limitation; only the apnea-hypopnea index (AHI) information was used for this study. The ApneaLink default settings for apnea and hypopnea were used in this study. Apnea was defined as a decrease in airflow by 80% of baseline for at least 10 seconds and hypopnea was defined as a decrease in airflow by 50% to 80% of baseline for at least 10 seconds. The ApneaLink AHI was automatically analyzed by the ApneaLink software version 6.0.
The overnight in-lab diagnostic PSG (Comet-PLUS® XL, Grass Technologies, Warwick, RI, USA) included a recording electro-encephalogram (EEG), electrooculogram, submental electromyogram (EMG), bilateral anterior tibialis EMG, electrocardiogram, chest and abdominal wall movement by inductance plethysmography, and airflow measured by a nasal pressure transducer. The equipment was supplemented with an oral thermistor and finger pulse oximeter.19,22 PSG scoring was performed according to internationally agreed criteria.13 Apnea was defined as a cessation of nasal flow lasting 10 seconds or longer. Hypopnea was defined as a 50% decrease in nasal flow lasting at least 10 seconds or a discernible decrease leading to an at least 3% oxygen desaturation or an EEG arousal.
Statistical analysis
Data was analyzed using SPSS for windows, version 18.0 (SPSS Inc., Chicago, IL, USA). A Bland-Altman plot is a graphic representation of the observed differences between paired measurements. The differences between ApneaLink and PSG were plotted against the averages of the two methods. Results showing a mean difference close to zero indicate little systemic bias. Correlation analysis was carried out using Pearson correlation coefficients. The p-values of <0.05 were considered statistically significant. Multivariate logistic regression analysis was used to predict independent risk factors for OSA (age, gender, asthma, diabetes, cardiovascular disease, habitual snoring, and obesity) that demonstrated statistically significant univariate relationships. We defined mild OSA as 15>AHI≥5/hr, and moderate OSA as 30>AHI≥15/hr, and severe OSA as AHI≥30/hr, according to the severity criteria by an American Academy of Sleep Medicine.13
RESULTS
Patient characteristics
One hundred and sixty-seven subjects who visited the pulmonary and sleep clinics as well as complained of habitual snoring, OSA, and excessive daytime sleepiness completed the ApneaLink study. Of the 167 patients, 32 (19.2%) patients with a high likelihood of having OSA were assessed with full PSG within 2 weeks after the ApneaLink study. Thirty-two who patients performed PSG were selected by physician's decision according to definite symptoms of OSA without objective criteria. The characteristics of the 167 patients who were screened with the ApneaLink study are shown in Table 1.
Table 1.
Demographics and Characteristics of ApneaLink Screened 167 Patients
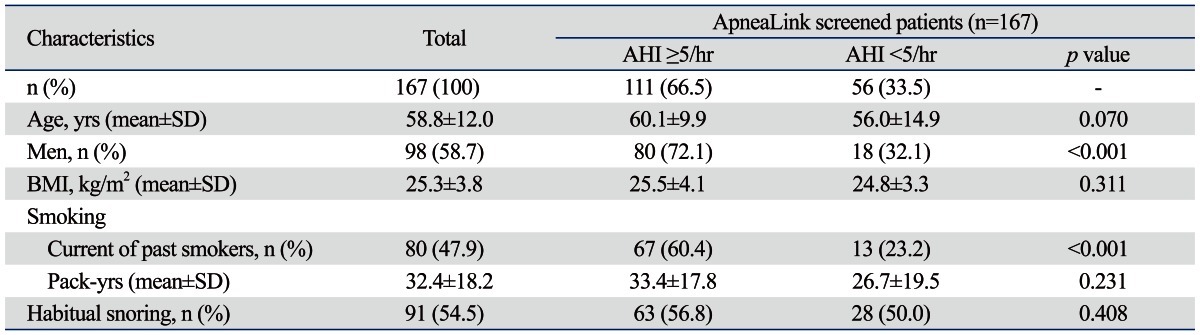
AHI, apnea-hypopnea index; BMI, body mass index; Pack-years, (Packs smoked per day) × (years as a smoker); SD, standard deviation.
Data are presented as the mean±SD and No. (%).
The mean age was 58.8±12.0 years and 58.7% were male. BMI scores were not different according to the presence of OSA. Of the 167 patients, 80 patients (47.9%) were either current or past smokers. The mean number of pack-years was 32.4±18.2. Of the current or past smokers, 67 patients (60.4%) had OSA (AHI ≥5/hr) and 13 patients (23.2%) did not. The presence of smoking history showed a difference between the OSA and non-OSA groups, but there was no difference in pack-years between groups. The prevalence of OSA depended on AHI cut-off values; AHI values of ≥5/hr, ≥10/hr, ≥15/hr and ≥30/hr corresponded to 111 (66%), 71 (42.5%), 52 (31%) and 20 (12%) patients, respectively. The mean ApneaLink AHI for the 167 screened patients was 12.7±13.0/hr. The mean ApneaLink AHI for the 32 selected high risk patients of OSA was 22.3±13.2/hr, which was lower than the sleep laboratory-based PSG AHI of (39.1±20.5/hr). The most common comorbidities in decreasing order were cardiovascular disease (50.3%), asthma (38.9%), COPD (21%), diabetes (14.4%), obesity (10.2%), postnasal drip syndrome (7.8%), and GERD (4.8%) (Table 2).
Table 2.
Comorbidities of ApneaLink Screened 167 Patients
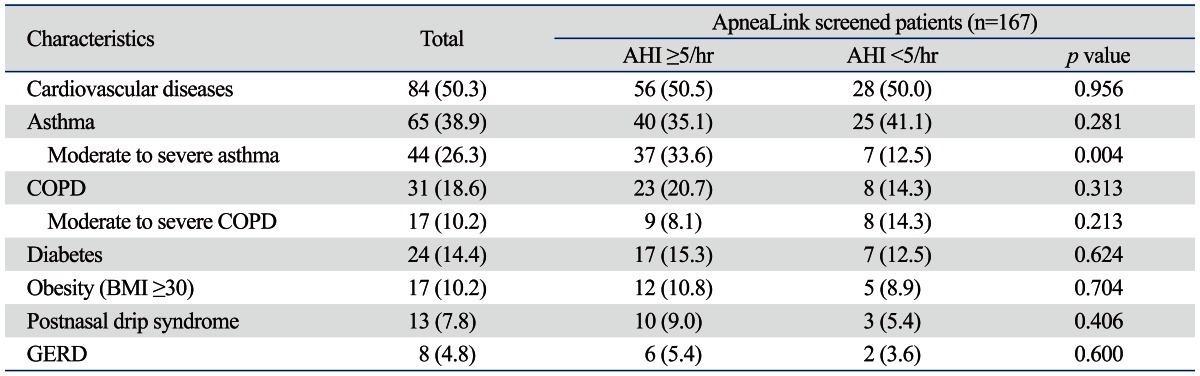
AHI, apnea-hypopnea index; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; BMI, body mass index.
Data are presented as No. (%).
Associations of comorbidities and clinical parameters with OSA
Variables found to be associated with OSA on both univariate and multivariate analyses are shown in Table 3. When OSA was defined as AHI ≥5/hr, age, male gender, and moderate to severe asthma showed positive correlations with OSA on univariate and multivariate analyses (Table 3). All asthma severity groups, including mild asthma did not show correlations with OSA. Only moderate to severe asthma showed strong correlation with OSA. COPD was not associated with OSA, regardless of its severity according to FEV1 (data not shown). Diabetes and habitual snoring were not found to be predictors. Although cardiovascular disease was the most common comorbid condition in this study, it was not associated. Postnasal drip syndrome and GERD were rare comorbid diseases in our study and consequently not showed positive correlations.
Table 3.
Univariate and Multivariate Analysis of Affecting Factors for the Presence of OSA (AHI ≥5/hr)
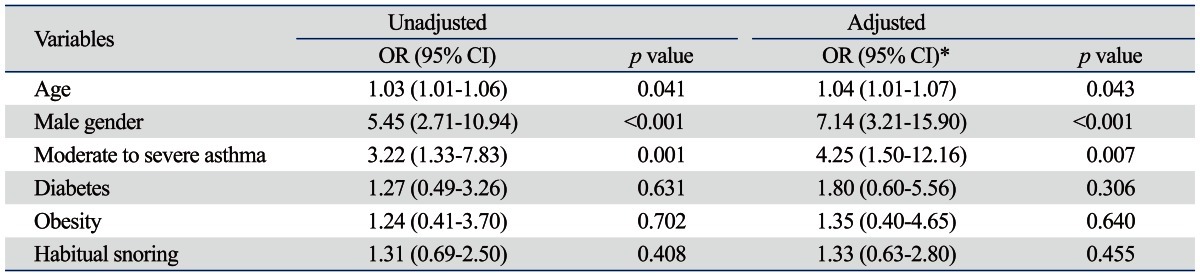
OR, odds ratio; CI, confidence intervals; OSA, obstructive sleep apnea; AHI, apnea-hypopnea index.
*Adjusted for all variables shown.
DISCUSSION
There are little data showing a correlation between asthma and OSA. According to several studies, patients with asthma have a higher prevalence of snoring and witnessed apnea relative to patients without asthma,4,23 but the prevalence of asthma in patients with OSA was only investigated in one small and uncontrolled study.24
We investigated the associating factors thought to increase predisposition for OSA, including well-known factors such as male gender, age, and obesity and additional possible factors including asthma, GERD, postnasal drip syndrome, cardiovascular disease, diabetes, smoking, habitual snoring, and COPD. Factors with a positive correlation with OSA were age, male gender, and moderate to severe asthma when OSA was defined at an AHI ≥5/hr. We identified factors associated with moderate to severe asthma that showed positive correlations with OSA. Most patients with moderate to severe asthma had uncontrolled asthma. Irrespective of severity, asthma was not an independent predictor of OSA. Yigla, et al.8 found that 21 of 22 (96%) patients with severe asthma demonstrated OSA in complete PSG testing. Julien, et al.25 demonstrated a high prevalence of OSA among patients with severe asthma, compared to patients with moderate asthma or without asthma. Several plausible hypotheses have been proposed to explain the association of asthma and OSA including anatomic abnormalities of upper air way related to obesity, nasal disease or GERD related upper airway inflammation, chronic systemic corticosteroid use, and airway patency of lung volume reduction during sleep in asthmatics.12,26,27 However, these proposed mechanisms need to be studied further. The interaction of OSA and asthma could be reciprocal in that asthma-related factors could also contribute to OSA deterioration. Nocturnal hypoxemia could be aggravated by asthma-related lung function impairment. Poor sleep quality could also be a factor because it has been associated with severe asthma in previous studies.28,29 In our study, 32 subjects who performed PSG completed the sleep questionnaire of the Epworth Sleepiness Scale (ESS). The ESS mean score was over the upper limits of the normal range at 10.38±4.85, with approximately 62.5% showing excessive daytime sleepiness, defined as an ESS score greater than 10.30
COPD was not a predicting factor, regardless of severity, categorized by FEV1 (data not shown). Cardiovascular disease was also not associated with OSA, contrary to previous studies.31,32 We presumed that cardiovascular disease has not been well characterized in this study. Moreover, hypertension was the most common condition; not heart failure, stroke, or coronary heart disease (Table 4). Postnasal drip syndrome and GERD were comorbid in very small patients (9%, 5% respectively) in our study and showed no correlations with OSA. Obesity did not show positive correlations with OSA on univariate and multivariate analyses. Ip, et al.33 reported that obesity is prevalent and a strong risk factor for OSA in white populations but is relatively uncommon in Asians. They hypothesized that other strong OSA risk factors, such as craniofacial features, are prevalent in Asians, and clinical observational studies of Asian patients with OSA support this hypothesis.34-36 In this study, the number of obese patients was very small. The prevalence of obesity, defined as western cut-off (BMI ≥30 kg/m2), was only 4.6% in our population.3 Obesity was not correlated with OSA in our study and this is similar to a recent study of ethnic differences in craniofacial structures and obesity.37
Table 4.
Subgroup Analyses of 84 Patients with Cardiovascular Diseases
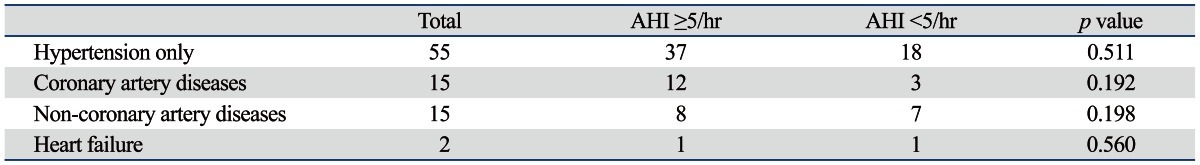
AHI, apnea-hypopnea index.
Patients with one more cardiovascular disease are present. Non-coronary artery diseases are arrhythmia, hypertrophic cardiomyopathy and valvular heart disease.
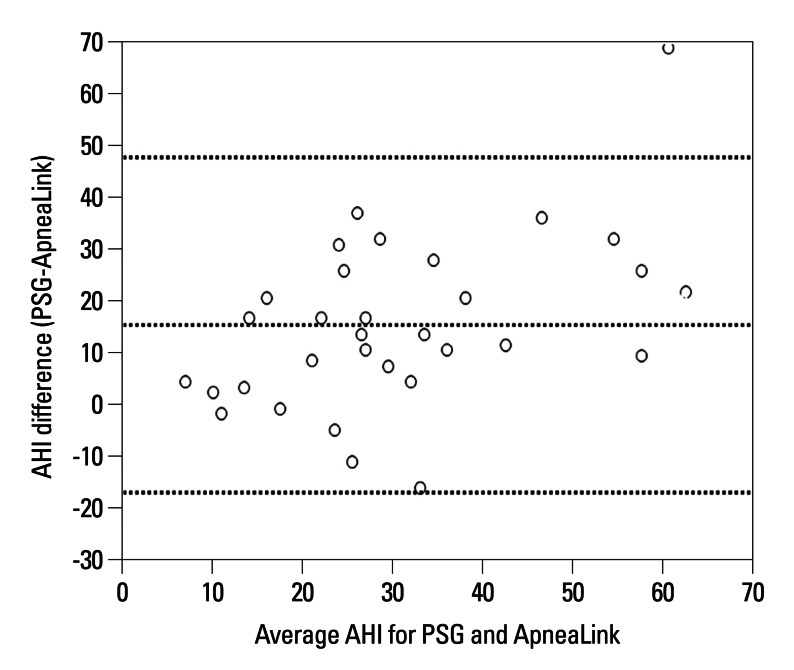
Previous studies have validated ApneaLink as an appropriate screening tool for OSA.17,18 Diagnostic accuracy of the ApneaLink for OSA detection was evaluated and compared with PSG. The ApneaLink device was highly sensitive and specific in quantifying AHI when compared with the AHI obtained from the lab-based PSG. In our study, 32 patients completed both ApneaLink and PSG studies, and ApneaLink AHI and PSG AHI were closely correlated (Pearson's correlation coefficient r=0.613) (Fig. 1). The ApneaLink tended to underestimate the AHI score, which is similar to previous studies.17,18 Erman, et al.18 applied ApneaLink a screening tool in comparison with the sleep-laboratory based PSG. ApneaLink was highly sensitive and specific in calculating AHI when compared with AHI obtained from PSG.18 Ng, et al.17 evaluated the diagnostic accuracy of the ApneaLink device in detecting sleep apnea compared to PSG and showed that the ApneaLink device was highly sensitive and specific at AHI values ≥10/hr and ≥20/hr when quantifying AHI in symptomatic OSA patients. These data support the use of ApneaLink for OSA screening studies in subjects with a high probability and suggest that ApneaLink is a useful tool for determining the presence or absence of sleep apnea. Our study has some limitations. First, a small number of subjects (32 patients), completed PSG along with ApneaLink, compared to 167 patients who were screened with ApneaLink. However, all subjects were at a high probability of having OSA when presenting with symptoms of SDB, and ApneaLink was well correlated with PSG in 32 patients. Second, this study was a cross-sectional study and the study population and clinical setting were selective and limited; therefore, it is difficult to generalize our results to other settings. Third, asthma severity was evaluated using clinical and spirometry data without an objective measurement for severity, such as an asthma control questionnaire.38,39
Fig. 1.
Bland-Altman plot of ApneaLink apnea-hypopnea index (AHI) and polysomnography (PSG) AHI data.
In summary, moderate to severe asthma showed strong correlations with OSA when defined at an AHI ≥5/hr in addition to the well-known predictors of age and male gender. ApneaLink was used for the screening of OSA in subjects with a high probability of having OSA instead of the laboratory-based PSG testing. Obesity was not associated with OSA in our study.
ACKNOWLEDGEMENTS
The authors are grateful for statistical support provided by the Biostatistics Collaboration Unit of Yonsei University College of Medicine.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 4.Larsson LG, Lindberg A, Franklin KA, Lundbäck B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 2001;95:423–429. doi: 10.1053/rmed.2001.1054. [DOI] [PubMed] [Google Scholar]
- 5.Janson C, De Backer W, Gislason T, Plaschke P, Björnsson E, Hetta J, et al. Increased prevalence of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9:2132–2138. doi: 10.1183/09031936.96.09102132. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick MF, Martin K, Fossey E, Shapiro CM, Elton RA, Douglas NJ. Snoring, asthma and sleep disturbance in Britain: a community-based survey. Eur Respir J. 1993;6:531–535. [PubMed] [Google Scholar]
- 7.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130:1779–1783. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 8.Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–871. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 9.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99:529–534. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 10.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Kasasbeh A, Kasasbeh E, Krishnaswamy G. Potential mechanisms connecting asthma, esophageal reflux, and obesity/sleep apnea complex--a hypothetical review. Sleep Med Rev. 2007;11:47–58. doi: 10.1016/j.smrv.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Arter JL, Chi DS, M G, Fitzgerald SM, Guha B, Krishnaswamy G. Obstructive sleep apnea, inflammation, and cardiopulmonary disease. Front Biosci. 2004;9:2892–2900. doi: 10.2741/1445. [DOI] [PubMed] [Google Scholar]
- 13.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 14.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–672. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 15.Whittle AT, Finch SP, Mortimore IL, MacKay TW, Douglas NJ. Use of home sleep studies for diagnosis of the sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:1068–1073. doi: 10.1136/thx.52.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 17.Ng SS, Chan TO, To KW, Ngai J, Tung A, Ko FW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39:757–762. doi: 10.1111/j.1445-5994.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 18.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–392. [PMC free article] [PubMed] [Google Scholar]
- 19.Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. 2006;61:1083–1090. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 21.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Hui DS, Ko FW, Fok JP, Chan MC, Li TS, Tomlinson B, et al. The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest. 2004;125:1768–1775. doi: 10.1378/chest.125.5.1768. [DOI] [PubMed] [Google Scholar]
- 23.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Correlates of daytime sleepiness in patients with asthma. Sleep Med. 2006;7:607–613. doi: 10.1016/j.sleep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The prevalence of asthma in patients with obstructive sleep apnoea. Prim Care Respir J. 2009;18:328–330. doi: 10.4104/pcrj.2009.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Bonekat HW, Hardin KA. Severe upper airway obstruction during sleep. Clin Rev Allergy Immunol. 2003;25:191–210. doi: 10.1385/CRIAI:25:2:191. [DOI] [PubMed] [Google Scholar]
- 27.Bohadana AB, Hannhart B, Teculescu DB. Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma. 2002;39:85–100. doi: 10.1081/jas-120002190. [DOI] [PubMed] [Google Scholar]
- 28.Catterall JR, Douglas NJ, Calverley PM, Brash HM, Brezinova V, Shapiro CM, et al. Irregular breathing and hypoxaemia during sleep in chronic stable asthma. Lancet. 1982;1:301–304. doi: 10.1016/s0140-6736(82)91567-7. [DOI] [PubMed] [Google Scholar]
- 29.Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM American Lung Association Asthma Clinical Research Centers. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–189. doi: 10.1080/02770900801890224. [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 31.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 32.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 33.Ip MS, Lam B, Lauder IJ, Tsang KW, Chung KF, Mok YW, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 34.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110(10 Pt 1):1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 35.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–410. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 36.Ong KC, Clerk AA. Comparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders center. Respir Med. 1998;92:843–848. doi: 10.1016/s0954-6111(98)90386-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee RW, Vasudavan S, Hui DS, Prvan T, Petocz P, Darendeliler MA, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 39.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]