Abstract
Purpose
The pulsatility index (PI), measured by transcranial Doppler (TCD), is a surrogate marker for distal vascular resistance in cerebral arteries, and elevated plasma total homocysteine (tHcyt) is regarded as a cause of ischemic stroke, including lacunar infarction. We investigated the relationship between the PI of cerebral arteries and plasma tHcyt in patients with lacunar infarction.
Materials and Methods
Plasma tHcyt level and TCD examination were performed in 94 patients with lacunar infarction. Mean flow velocity (MFV) and PI were assessed at the ipsilateral middle cerebral artery (MCA) and contralateral MCA, relative to the infarction, and the basilar artery (BA). Multivariate regression analysis was conducted between log-transformed tHcyt levels (logHcyt) and the PI of individual arteries.
Results
There was a significant correlation between logHcyt and the PI in all tested arteries (ipsilateral MCA: r=0.21, p=0.03; contralateral MCA: r=0.21, p=0.04; BA: r=0.35, p=0.01). In multivariate regression analysis, this significance remained unchanged after adjusting for vascular risk factors, creatinine, hematocrit and platelet count (ipsilateral MCA: β=0.26, p=0.01; contralateral MCA: β=0.21, p=0.04; BA: β=0.39, p=0.001). There was no significant association between logHcyt and MFV of individual arteries.
Conclusion
A significant association between plasma tHcyt and the PI of cerebral arteries indicates that homocysteine plays a role in the increase of distal arterial resistance in lacunar infarction.
Keywords: Homocysteine, pulsatility index, lacunar infarction
INTRODUCTION
Lacunar infarction is a subtype of ischemic stroke, resulting from occlusion of a perforating artery originating from a major cerebral artery. Distinct from other types of ischemic stroke, lacunar infarctions have different pathogeneses, including lipohyalinosis, microatheroma of the cerebral microvessels, and segmental demyelination of white matter in the brain.1 Recent studies have demonstrated that endothelial dysfunction has a pivotal role in the development of lacunar infarction.2
Hyperhomocysteinemia is an established risk factor for major vascular events, including stroke.3 Elevated plasma total homocysteine (tHcyt) levels increase oxygen free radical generation, inducing the acceleration of atherosclerosis and endothelial dysfunction through the reduction of bioavailability of endothelial nitric oxide synthetase (eNOS).4,5 The majority of previous studies have shown that elevated plasma tHcyt level is associated with the small vessel disease (SVD) type of ischemic stroke.6-8
The pulsatility index (PI), measured by transcranial Doppler (TCD), represents peripheral resistance downstream from tested arteries. Several studies have demonstrated that the PI is an independent predictor of SVD, including lacunar infarction.9,10 One previous study reported that elevated plasma tHcyt level is associated with the increased PI of cerebral arteries in patients with lacunar infarction, indicating close relationship between plasma tHcyt and PI in this disease.9 Because of the paucity of data, however, this relationship requires validation in another cohort. Previous studies have non-selectively included cases with anterior and posterior circulation infarcts, without considering the effect of plasma tHcyt on arteries relevant or irrelevant to the infarct lesion.
We, therefore, investigated the impact of plasma tHcyt on the PI of the middle cerebral artery (MCA), ipsilateral and contralateral to the infarct lesion, and on the basilar artery (BA) in patients with anterior circulation lacunar infarction.
MATERIALS AND METHODS
Patients and vascular risk factor assessment
This study included 94 patients with acute lacunar infarction who admitted to the Department of Neurology between January 2009 and December 2010. The Institutional Review Board at CHA Medical Center approved this study. All patients showed clinical symptoms of lacunar stroke within 24 hours of stroke onset. Patients also demonstrated a lesion, <1.5 cm in diameter, in a perforating artery of the MCA on diffusion-weighted imaging. There could be no significant arterial stenosis in territories relevant to the infarction on computed tomography or magnetic resonance (MR) angiography. All patients satisfied the criteria of SVD subtype of infarction according to the Trial of ORG 10172 in Acute Stroke Treatment classification. Study exclusion criteria were: 1) age below 40 years; 2) significant vascular stenosis on CT- or MR-angiography; 3) presence of cardioembolic sources; 4) unobtainable demographic information; 5) unobtainable TCD data due to a poor temporal window; 6) vitamin supplementation; and 7) severe systemic disease (severe anemia, acute infection, hyperthyroidism) at the time of examination.
Vascular risk factors were assessed using patients' interview and laboratory data from hospitalization. The demographic data included age, gender, and previous history of hypertension, diabetes, hyperlipidemia, or cardiac disease. Laboratory data were obtained from the morning following admission, using a standard protocol, and included hematocrit, platelet count, serum creatinine, total cholesterol, low-density lipoprotein (LDL) cholesterol, fasting blood glucose, and plasma tHcyt. Based on the result of patients' interviews and blood tests, individual vascular risk factors were determined. Hypertension was diagnosed when a patient had a high baseline blood pressure, consisting of a systolic blood pressure (SBP) ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg on more than 1 occasion; treatment with an antihypertensive medication also qualified. Diabetes mellitus was diagnosed when a patient had a high fasting plasma glucose level (≥126 mg/dL) or had been treated with either oral hypoglycemic agents or insulin. Hyperlipidemia was defined as fasting serum total cholesterol of ≥240 mg/dL or a history of antihyperlipidemic medication. Ischemic heart disease was defined to include a history of myocardial infarction, unstable angina, coronary angioplasty by balloon or stent, or coronary bypass graft surgery. The measurement of plasma tHcyt levels were evaluated by fluorescence polarization immunoassay using a Hitachi D-2400 analyzer (Hitachi High-Technologies Corporation, Tokyo, Japan).
Transcranial Doppler study
TCD examination was performed within 3 days of stroke onset in all patients. Blood flow velocities at the BA and the ipsilateral and contralateral MCA were measured using the Power M mode transcranial Doppler technology (PMD-150, 2 channel, Spencer Technologies Inc., Seattle, WA, USA) with a 2-MHz probe. The parameters obtained from TCD examination were systolic flow velocity (SFV), diastolic flow velocity (DFV), and mean flow velocity (MFV); these were automatically calculated by TCD instrument. The PI was automatically calculated with the equation: PI=(SFV-DFV)/MFV.11 All SFV, DFV, and MFV values were measured along full segment of each MCA (45-60 mm) and BA (80-115 mm).12 In the present study, we selected the highest PI value detected from several measurements.
Statistical analysis
Demographic and laboratory data were represented as mean values for continuous variables, and percentages for categorical variables. For linear correlation analysis, we first conducted the Kolmogorov-Smirnov test to find out whether plasma tHcyt, PI, and MFV values in each examined artery have a normal standard distribution. The plasma tHcyt level was log-transformed (logHcyt), as this variable showed a skewed distribution. We evaluated the zero-order correlation coefficient (Pearson correlation coefficient) and the partial correlation coefficient (excluding the effects of age, gender, SBP, creatinine, hematocrit, glucose, LDL cholesterol, and platelet count) on the relationship between logHcyt and PI or MFV in each examined artery. Additionally we conducted multivariate linear regression analysis after adjusting for possible confounding factors (age, gender, SBP, creatinine, hematocrit, glucose, LDL cholesterol, and platelet count) to examine linear association between logHcyt and PI or MFV in each examined artery. To examine the presence of auto-correlation and multi-collinearity among variables, we included the Durbin-Watson test and the variance inflation factor (VIF) in the analysis. Statistical significance was given to p values of <0.05, and analyses were conducted using Statistical Package for the Social Science software (SPSS ver. 18.0, SPSS Inc., Chicago, IL, USA).
RESULTS
In 94 patients with lacunar infarction, the mean age was 59 years and 31% were female. Left-sided lacunar infarctions were found in 57 (61%) patients. The demographic characteristics and laboratory findings of study subjects are summarized in Table 1. Detailed data on MFV and PI values in the ipsilateral MCA and contralateral MCA, and the BAs are summarized in Table 2. In pair-wise comparison between the ipsilateral MCA and contralateral MCA, no differences were found in SFV (p=0.48), DFV (p=0.86), MFV (p=0.93), and PI (p=0.52) between the two arteries. In pair-wise comparison between the ipsilateral or contralateral MCA and the BA, the SFV, DFV, and MFV values in either MCA were higher than those in the BA (all p<0.05). There were no differences in the PI values of the ipsilateral MCA (p=0.60) or the contralateral MCA (p=0.98) and those of the BA.
Table 1.
Demographic Characteristics of Patients with Lacunar Infarction
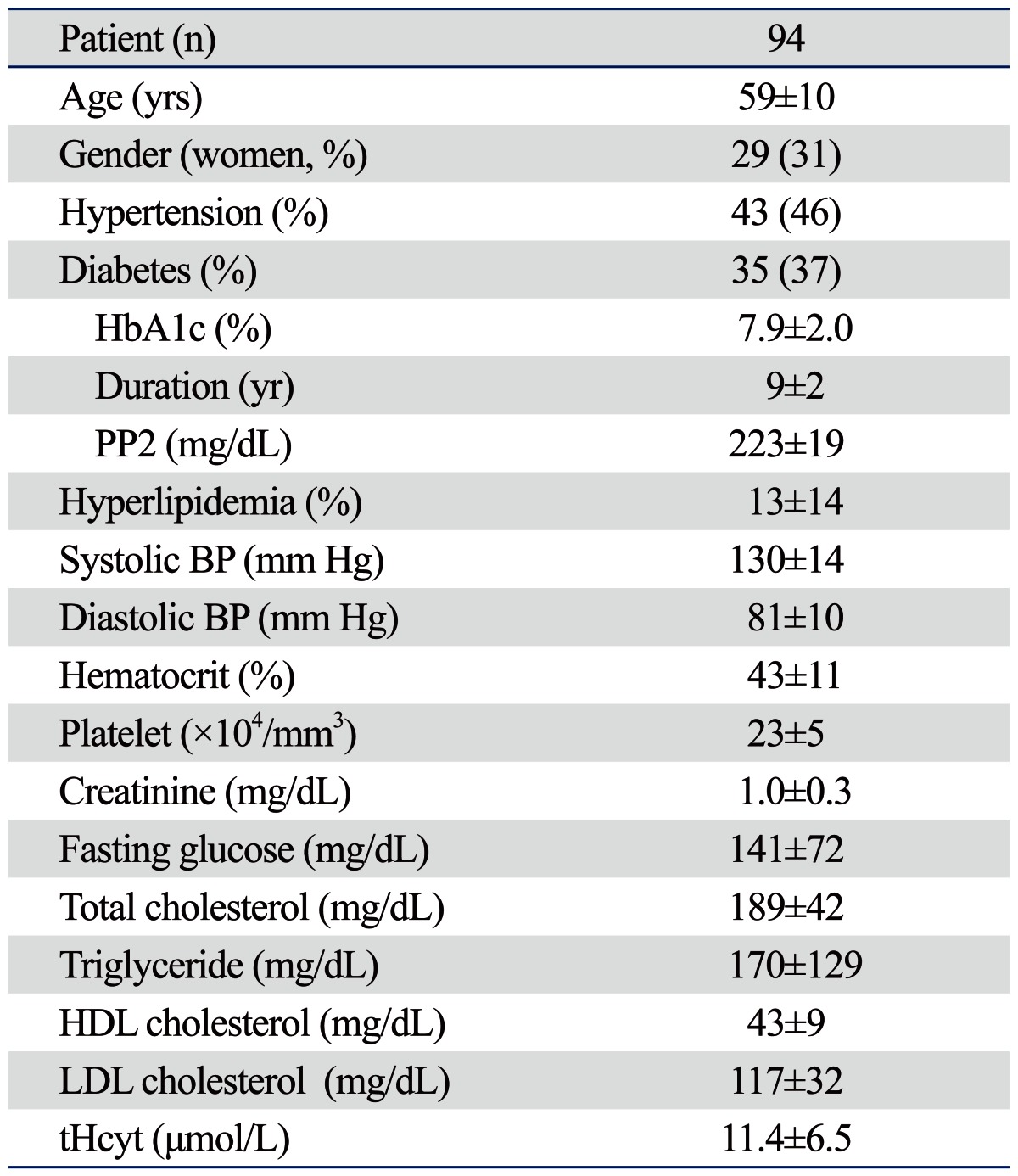
HbA1c, glycosylated hemoglobin; PP2, 2 hours post-prandial plasma glucose level; BP, blood pressure; LDL, low-density lipoprotein; tHcyt, total homocysteine; HDL, high-density lipoprotein.
Table 2.
Results of Transcranial Doppler (TCD) in Patients with Lacunar Infarction
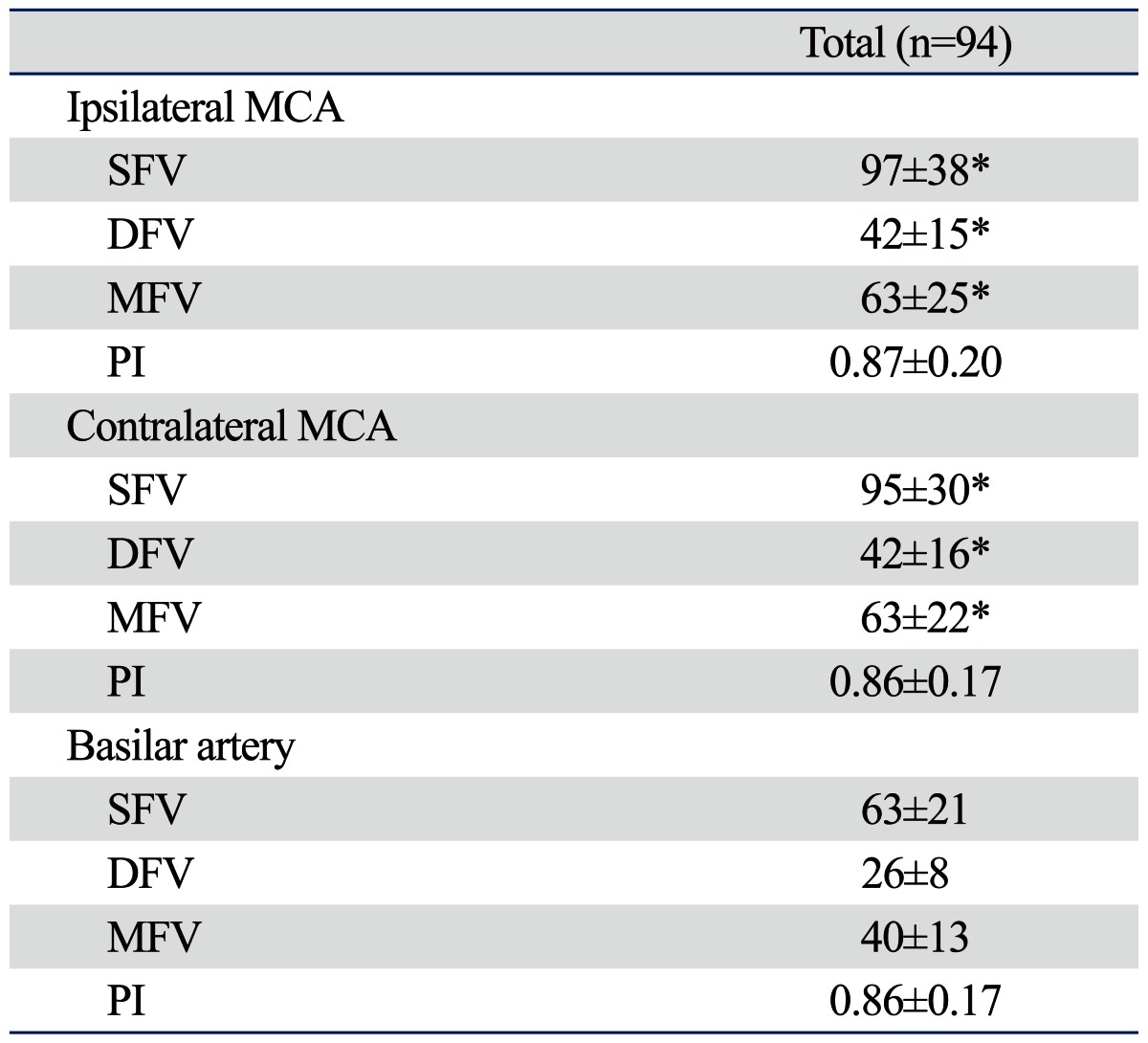
MCA, middle cerebral artery; SFV, systolic flow velocity; DFV, diastolic flow velocity; MFV, mean flow velocity; PI, pulsatility index.
*p<0.05 in comparison with individual TCD indices of the basilar artery.
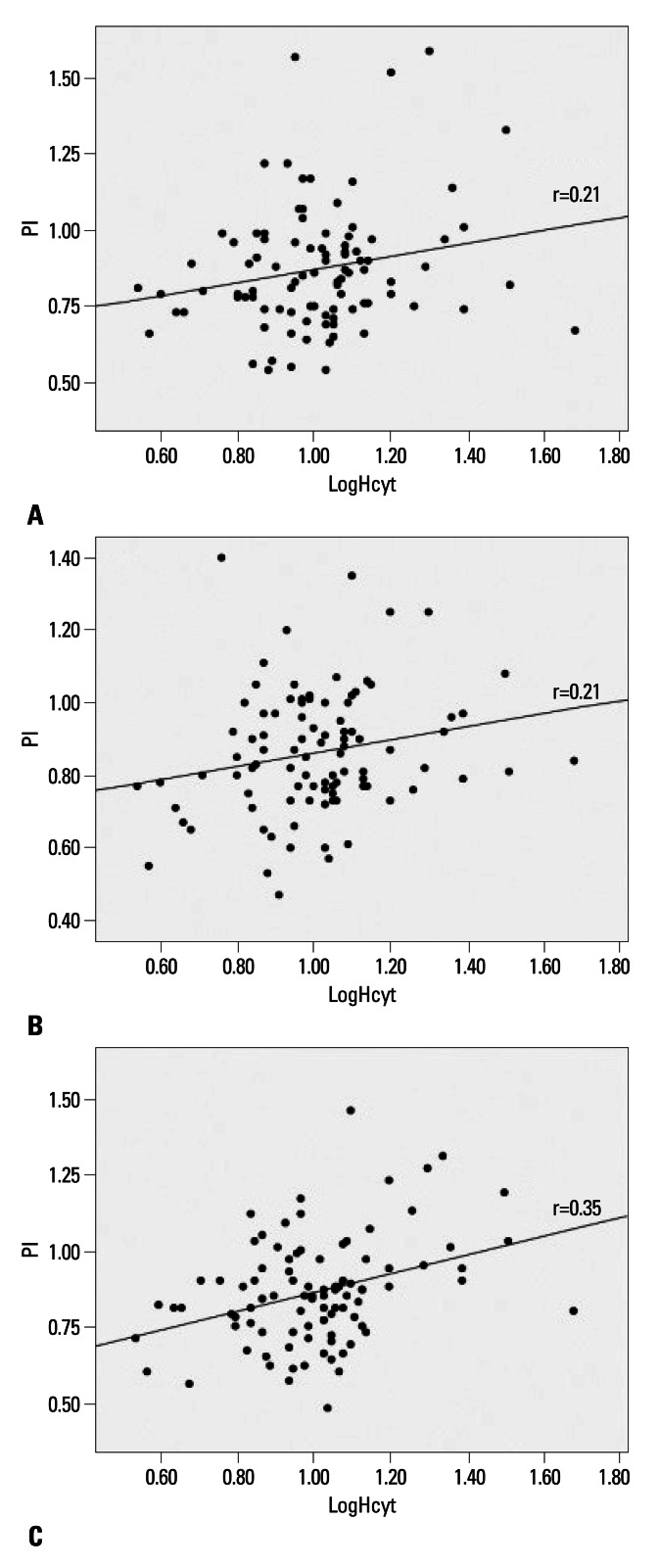
We calculated a Pearson's correlation coefficient between logHcyt, the PI and MFV of individual cerebral arteries. The logHcyt value was correlated with the PI of the ipsilateral MCA (r=0.21, p=0.03), the contralateral MCA (r=0.21, p=0.04), and the BA (r=0.35, p=0.01) (Fig. 1). This correlation was also observed when partial correlation analysis excluded the effect of other variables (age, gender, SBP, glucose, hematocrit, platelet count, creatinine, and LDL-cholesterol). There was no significant association between logHcyt and the MFV values of tested arteries (p>0.05).
Fig. 1.
Correlation analysis between log-transformed homocysteine (logHcyt) and pulsatility index (PI) of ipsilateral MCA (A), contralateral MCA (B), and basilar artery (C). r, Pearson's correlation coefficient; MCA, middle cerebral artery.
In multivariate linear regression analysis, logHcyt showed a significant linear correlation with the PI of the ipsilateral MCA (β=0.26, p=0.01), the contralateral MCA (β=0.21, p=0.04), and the BA (β=0.39, p=0.001), after adjusting for cardiovascular risk factors (age, SBP, glucose, and LDL-cholesterol), gender, hematocrit, platelet count, and creatinine (Table 3). There was no linear association between logHcyt and the MFVs of tested arteries. Neither auto-correlation (Durbin-Watson statistics: 1.8-2.2) nor multi-collinearity (VIF <3) was found on individual regression analysis.
Table 3.
Results of Linear Regression Model of Log-Transformed tHcyt Levels (LogHcyt) and TCD Indices in the Ipsilateral and Contralateral Middle Cerebral Arteries and the Basilar Artery
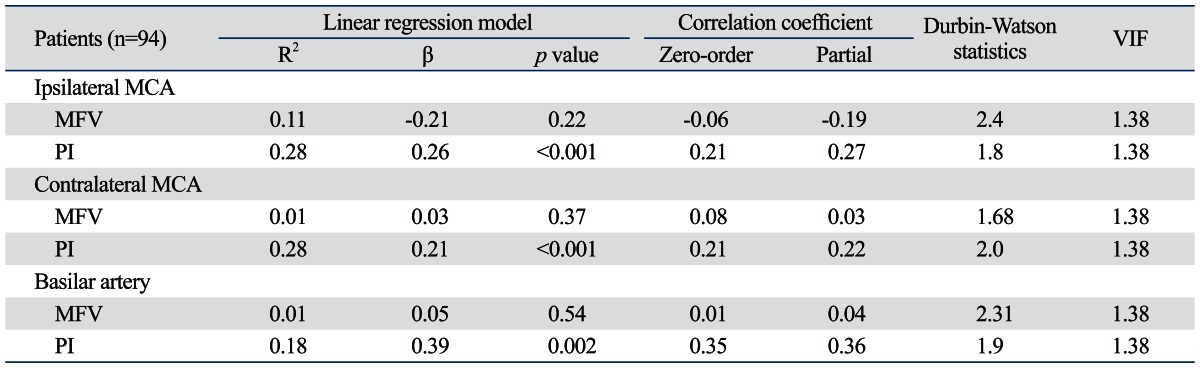
VIF, variance inflation factor; MCA, middle cerebral artery; MFV, mean flow velocity; PI, pulsatility index; TCD, transcranial Doppler; BP, blood pressure.
R2, adjusted R square; β, standardized coefficients.
Adjusted for age, gender (dummy variable), systolic BP, hematocrit, platelet count, fasting glucose, creatinine, and low-density lipoprotein cholesterol.
DISCUSSION
The present study demonstrated that plasma tHcyt levels show a significant association with the PI of major cerebral arteries in patients with lacunar infarction. This association remains significant after adjusting for possible confounders, including age, gender, SBP, creatinine, hematocrit, glucose, LDL cholesterol, and platelet count. This finding indicates that plasma tHcyt plays a role in the increase of distal arterial resistance of major cerebral arteries in lacunar infarction.
Hyperhomocysteinemia induces endothelial dysfunction due to the reduced bioavailability of eNOS, brought by increased oxygen free radical species.4,5 One experimental study reported that hyperhomocysteinemia produces endothelial dysfunction in the cerebral arterioles, at the concentration that is significantly lower than that is necessary to produce the same effect in the aorta.13 This result provides evidence that cerebral arterioles are more sensitive to the vascular effects of hyperhomocysteinemia than are larger vessels. Clinical studies also have shown that the association between plasma tHcyt and the SVD subtype of ischemic stroke is stronger than that between tHcyt and any other subtype of stroke.6-8 In a healthy population, an increased tHcyt level is closely linked to the development of silent lacunar lesion14,15 and cerebral white matter hyperintensity lesions,15,16 both of which are radiological indices of SVD in the brain.
PI reflects distal vascular resistance of cerebral vessels, and is useful in predicting the risk of ischemic cerebrovascular disease.10,17-19 In lacunar infarction, it is expected that distal arterial resistance increases due to occlusion or insufficient blood flow in a perforating artery, originated from a major cerebral artery. Therefore, the PI of a major cerebral artery is a good candidate for a marker to evaluate local hemodynamic change in the branch artery or microvessel. Several studies support this finding, having demonstrated that the PI is increased in pathologic conditions of cerebral microangiopathy,20,21 including lacunar infarction and SVD.10,22 There is, however, a paucity of data regarding the relationship between plasma tHcyt and PI in lacunar infarction. Jeong, et al.9 reported that, in patients with lacunar infarction and hyperhomocysteinemia, the PI values are increased: they examined MFV and PI values of the right and left MCA and the BA in patients with lacunar stroke, and found that the PI in all tested arteries increased as homocysteine levels rose. Our study was designed to test the PI of the ipsilateral and contralateral MCA relative to the infarction, in order to examine whether acute hemodynamic change occurs differently between the relevant MCA and the opposite MCA. However, no differences were found in MFV and PI values of the ipsilateral MCA and contralateral MCA, and correlation with plasma tHcyt was found in all tested arteries, regardless of lesion locality. Consistent with Jeong's study,9 we found a strong correlation between plasma tHcyt and the PI of the BA, a part of the posterior circulation. Based on the previous TCD study and our own results, an increased PI represents general pathological status of SVD rather than local hemodynamic changes following acute lacunar stroke.
One strength of the present study is the inclusion of cases with lacunar infarction only in the distribution of the anterior cerebral circulation, enabling us to examine the effect of tHcyt on the PI of arteries which are both relevant and irrelevant to the infarction site. In addition, we conducted TCD examinations and collected the plasma tHcyt levels in the narrow time window (within 72 hours after stroke) compared with previous studies, which may reduce the impact of temporal variations in cerebral hemodynamics following stroke.
The limitations of the present study should be addressed. First, our sample size was relatively small. Second, the time point of blood sampling and TCD examination was not exactly the same. There might have been some effect occurring in the intervening time span. Plasma tHcyt has been reported to fluctuate after vascular events,23 and TCD results are also variable after 24 hours of vascular accidents.24 Third, we did not include control subjects. It is difficult to include sufficient number of healthy subjects who agreed to perform blood test and TCD. In previous study, Jeong, et al.9 showed linear trend between cerebral PIs and tHcyt tertiles in lacunar infarction, whereas this trend was not observed in control subjects. In patients with lacunar infarction, our results are similar to those of previous study.9 Based on the biological role of plasma Hcyt on endothelial dysfunction, reduced vascular elastic compliance and vascular blood flows, we believe that plasma tHcyt level is surrogate marker for increased resistance of small cerebral arteries or arterioles, which can be a pathological substrate for lacunar infarction, such as lipohyalonosis and/or arteriosclerosis. Fourth, a causal relationship between plasma tHcyt and increased PI could not be defined due to the lack of a serial measurement. Therefore, our findings require future validation.
In conclusion, we found a positive correlation between plasma tHcyt levels and PI, and suggests that plasma tHcyt induces a circulatory disturbance in the perforating arteries or microvessels originating from major cerebral arteries, leading to the development of cerebral SVD.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090057).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008;39:1327–1332. doi: 10.1161/STROKEAHA.107.500124. [DOI] [PubMed] [Google Scholar]
- 3.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 4.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103:2624–2629. doi: 10.1182/blood-2003-09-3078. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Pacher P, Rischák K, Szollár L, Koller A. Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 1999;19:1899–1904. doi: 10.1161/01.atv.19.8.1899. [DOI] [PubMed] [Google Scholar]
- 6.Perini F, Galloni E, Bolgan I, Bader G, Ruffini R, Arzenton E, et al. Elevated plasma homocysteine in acute stroke was not associated with severity and outcome: stronger association with small artery disease. Neurol Sci. 2005;26:310–318. doi: 10.1007/s10072-005-0505-7. [DOI] [PubMed] [Google Scholar]
- 7.Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109:2766–2772. doi: 10.1161/01.CIR.0000131942.77635.2D. [DOI] [PubMed] [Google Scholar]
- 8.Khan U, Crossley C, Kalra L, Rudd A, Wolfe CD, Collinson P, et al. Homocysteine and its relationship to stroke subtypes in a UK black population: the south London ethnicity and stroke study. Stroke. 2008;39:2943–2949. doi: 10.1161/STROKEAHA.107.513416. [DOI] [PubMed] [Google Scholar]
- 9.Jeong SK, Kim DH, Cho YI. Homocysteine and pulsatility index in lacunar infarction. Clin Neurol Neurosurg. 2011;113:459–463. doi: 10.1016/j.clineuro.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging. 2001;11:229–235. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 11.Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med. 1974;67(6 Pt 1):447–449. doi: 10.1177/00359157740676P113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011;196:221–237. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Dayal S, Arning E, Bottiglieri T, Böger RH, Sigmund CD, Faraci FM, et al. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke. 2004;35:1957–1962. doi: 10.1161/01.STR.0000131749.81508.18. [DOI] [PubMed] [Google Scholar]
- 14.Kohara K, Fujisawa M, Ando F, Tabara Y, Niino N, Miki T, et al. MTHFR gene polymorphism as a risk factor for silent brain infarcts and white matter lesions in the Japanese general population: the NILS-LSA Study. Stroke. 2003;34:1130–1135. doi: 10.1161/01.STR.0000069163.02611.B0. [DOI] [PubMed] [Google Scholar]
- 15.Vermeer SE, van Dijk EJ, Koudstaal PJ, Oudkerk M, Hofman A, Clarke R, et al. Homocysteine, silent brain infarcts, and white matter lesions: the Rotterdam Scan Study. Ann Neurol. 2002;51:285–289. doi: 10.1002/ana.10111. [DOI] [PubMed] [Google Scholar]
- 16.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg. 1988;68:745–751. [PubMed] [Google Scholar]
- 18.Lee KY, Sohn YH, Baik JS, Kim GW, Kim JS. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke. 2000;31:1111–1115. doi: 10.1161/01.str.31.5.1111. [DOI] [PubMed] [Google Scholar]
- 19.Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg Neurol. 2004;62:45–51. doi: 10.1016/j.surneu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Cho MH, Lee KY, Kim CS, Kim HJ, Nam JS, et al. Cerebral arterial pulsatility and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2008;79:237–242. doi: 10.1016/j.diabres.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Wijnhoud AD, Koudstaal PJ, Dippel DW. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J Clin Ultrasound. 2006;34:70–76. doi: 10.1002/jcu.20193. [DOI] [PubMed] [Google Scholar]
- 22.Lee KO, Lee KY, Lee SY, Ahn CW, Park JS. Lacunar infarction in type 2 diabetes is associated with an elevated intracranial arterial pulsatility index. Yonsei Med J. 2007;48:802–806. doi: 10.3349/ymj.2007.48.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard VJ, Sides EG, Newman GC, Cohen SN, Howard G, Malinow MR, et al. Changes in plasma homocyst(e)ine in the acute phase after stroke. Stroke. 2002;33:473–478. doi: 10.1161/hs0202.103069. [DOI] [PubMed] [Google Scholar]
- 24.Kaps M, Teschendorf U, Dorndorf W. Haemodynamic studies in early stroke. J Neurol. 1992;239:138–142. doi: 10.1007/BF00833913. [DOI] [PubMed] [Google Scholar]