Abstract
Purpose
The association between Helicobacter pylori (H. pylori) and blood ammonia levels in cirrhotic patients is controversial. We aimed to clarify this controvercy by performing a meta-analysis of published studies.
Materials and Methods
We searched PubMed, EMBASE and Cochrane library for studies which explored the association between H. pylori and blood ammonia levels in cirrhotic patients before May 2012. Six cohort studies involved in 632 H. pylori positive and 396 H. pylori negative cirrhotic patients were eligible for our analysis. The summary estimates were presented as standard means differences (SMD) and 95% confidence intervals (CI) from individual studies.
Results
Overall, there was significant association between H. pylori infection and the elevated blood ammonia levels in cirrhotic patients (SMD=0.34, 95% CI=0.21-0.47, I2=42.1%). Sensitivity analysis further confirmed this association. Subgroup analysis showed that the association was found only in Asian ethnicity, but not in Caucasian ethnicity.
Conclusion
H. pylori infection is associated with elevated blood ammonia levels in cirrhotic patients, and more large scale studies and stratify analysis are warranted in order to further evaluate this association.
Keywords: Helicobacter pylori, blood, ammonia, meta-analysis
INTRODUCTION
Cirrhosis represents the final common histological pathway for a wide variety of chronic liver diseases. The blood ammonia levels of cirrhotic patients are usually higher than that of normal people. Generally, ammonia is produced by glutamine metabolism in the small bowel and bacterial flora in the large bowel.1 However, the stomach is an another source of ammonia when Helicobacter pylori (H. pylori) are present.2 Previous studies have showed that the ammonia produced in stomach contributes to the increase of blood ammonia levels in cirrhosis, and the eradication of H. pylori may reduce the blood ammonia levels.3,4
Several studies showed that ammonia levels both in gastric juice and blood were significantly higher in cirrhotic patients with H. pylori infection than those without.5-9 However, Vásconez, et al.10 and other studies11-13 failed to find that significant differences between cirrhotic patients with and without H. pylori infection in term of blood ammonia levels. In addition, Chakrabarti, et al.5 showed that the blood ammonia levels were not concordantly increased, although the gastric juice ammonia levels were increased when H. pylori infection was present simultaneously. Therefore, the current understanding of the association between H. pylori infection and blood ammonia levels in cirrhotic patients remains obscure. In an attempt to resolve this conflict, we conducted a meta-analysis of the data from all the published studies.
MATERIALS AND METHODS
Search strategy and study selection
In order to find all the studies which examined the association between H. pylori infection and blood ammonia levels in cirrhotic patients, we conducted meta-analysis according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis.14 We systematically searched the Cochrane clinical trials database, PubMed, EMBASE, Chinese Biomedical Database and Chinese National Knowledge Infrastructure prior to May 30, 2012. We used the following search terms: "Helicobacter pylori" or "H. pylori", "ammonia" and "liver cirrhosis". The search was not limited by language or publication status. We searched the references of all retrieved publications again to trace additional relevant studies. Moreover, relevant review articles and their references were checked. In cases of multiple publications of the same or overlapping cohort, only the studies with the largest sample size were included. Potentially relevant articles were then screened by at least two independent reviewers; disagreements were resolved by discussion or upon consensus from the third reviewer.
Inclusion and exclusion criteria
Studies that we identified should meet the following criteria: 1) study was observational design in human beings; 2) study investigated the association between H. pylori infection with blood ammonia levels in cirrhotic patients; 3) study must provide sufficient information about blood ammonia levels in cirrhotic patients with and without H. pylori infection. The exclusion criteria were: laboratory studies, review articles, animal studies, studies which provided no sufficient information of H. pylori infection and blood ammonia levels.
Data extraction and quality assessment
The following data were extracted: the first author's name, publication year, the country where the study was performed, sample size (numbers of cirrhotic patients with H. pylori infection and without), diagnosis methods of H. pylori infection, etiology of cirrhosis, Child-Pugh class, and means and SDs value of blood ammonia levels in each group. The review team used a standardized form adapted from the Cochrane Effective Practice and Organisation of Care (EPOC) Group's Risk of Bias criteria to systematically identify study quality.15 The instrument recorded 9 criteria, including whether studies used random and concealed allocation, documented similar baseline characteristics and outcomes between the intervention and control groups, and described a plan for missing data, as well as the likelihood of contamination between study groups, with maximum of 9 scores. Two authors independently conducted a literature search and extracted data. The discrepancy in data extraction was resolved by repeating the study review and discussion. Two blinded reviewers independently performed data extraction. Disagreements between the reviewers were resolved through discussion or by the third reviewer.
Statistical analysis
We used standard mean differences (SMD) and corresponding 95% confidence intervals (CI) to evaluate the estimates of the association between blood ammonia levels and H. pylori infection. Because the studies were done with populations of varying effect sizes, thus, the study weights of in-study and between-study variances were considered with heterogeneity, we, therefore, used the random-effects method of DerSimonian and Laird16 to calculate the summary results. When the distributions of value of ammonia is skewed and the data are presented in the form of median and inter-quartile range (IQR) in the study, we converted these data into the form of means±SD by using the method recommend by the Cochrane reviewers. The estimator SD=IQR/1.35 was used to estimate SD from the IQR.17
We assessed the heterogeneity between studies in meta-analysis by the Cochran Q test, and considered p values lower than 0.10 as an indicator of significant heterogeneity because of the low statistical power. We also calculated the inconsistency index I2 to quantify heterogeneity. I2 was documented for the percentage of the observed variation between studies which was caused by heterogeneity rather by chance.18
In addition to exploring sources of heterogeneity, we performed sensitivity analysis and subgroup analysis. Sensitivity analysis was performed to assess robustness and examine the results for possible bias. Subgroup analysis was carried out to look at more narrowly drawn subsets of the studies.
In order to evaluate the effect of Child-Pugh class on the blood ammonia levels, a multivariate meta-regression analysis was performed by including other possibly confounding factors that may be relevant.
To investigate whether publication bias might affect the validity of the estimates, funnel plots were constructed. Funnel plot asymmetry was assessed by using Begg's test and Egger test.19,20 All statistical tests were two-sided and we considered a p value <0.05 as an indicator of significance except where specifically noted. Software STATA version 11.0 (Stata Corporation, College Station, TX, USA) was used for all statistical analysis.
RESULTS
Literature search
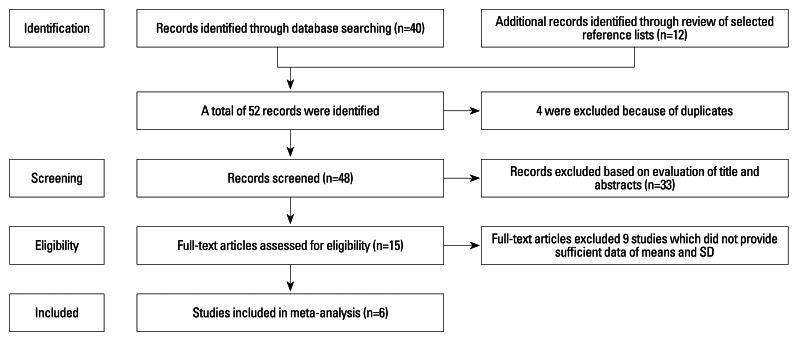
Primary literature search retrieved 48 records. After screening the titles and abstracts, 33 studies were excluded because they were either laboratory studies, review articles, or irrelevant to the current analysis. The remaining 15 studies were selected for detailed evaluation. Of them, 7 studies were excluded because they did not provide sufficient data of means and SD after contacting original authors, 2 studies were excluded because the data of ammonia provided was from gastric juice instead of blood levels. Therefore, 6 cohort studies5,8,10,13,21,22 involved in 632 patients with H. pylori infection and 396 cirrhotic patients without H. pylori infection were finally included in the present meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of the study selection process.
Characteristics of included studies and quality assessment
Among the 6 studies, 3 were Caucasian ethnicity,5,10,13 the other 3 were Asian ethnicity.8,21,22 Two studies used only one method to detect H. pylori infection.10,13 One study provided blood ammonia value in the form of median and IQR.21 The blood sample of one study was arterial blood,5 the other 5 studies were venous blood sample. Two studies showed significant association between H. pylori infection and blood ammonia levels in cirrhotic patients;8,22 the remaining 4 studies did not find the association.5,10,13,21 The quality of included studies was assessed by EPOC criteria, with the scores ranging from 5-7 (Table 1).
Table 1.
Characteristics of Six Included Studies
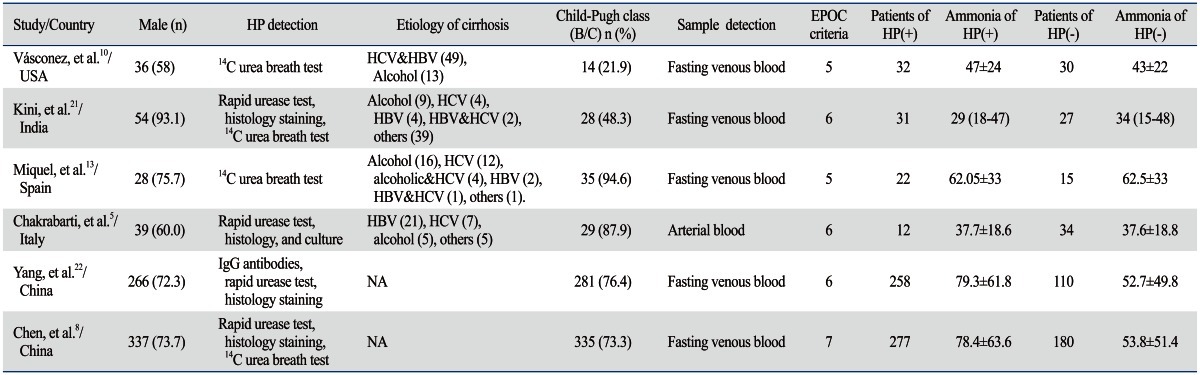
HP, Helicobacter pylori; NA, not available; EPOC, Effective Practice and Organisation of Care.
Overall analysis
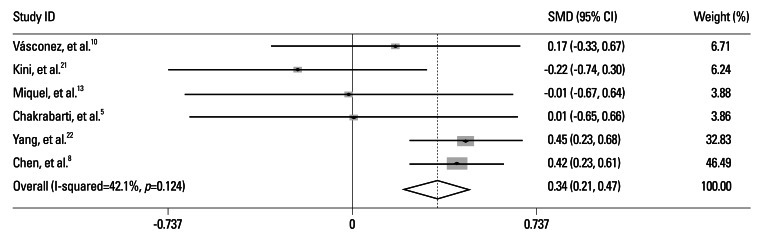
The overall analysis of 6 studies showed significant association between H. pylori infection and elevated blood ammonia levels in cirrhotic patients [standard means differences (SMD)=0.34, 95% CI=0.21-0.47, p=0.000]. There was no significant heterogeneity across the studies (I2=42.1%, p=0.124) (Fig. 2).
Fig. 2.
Meta-analysis of nine included studies. SMD, standard means differences; CI, confidence intervals.
Sensitivity analysis
After omitting one study which used arterial blood as a detection sample, the results were similar to the overall analysis (SMD=0.28, 95% CI=0.06-0.49, p=0.011) and without significant heterogeneity (p=0.107, I2=47.4%). After excluding one study which provided the data in the form of median and IQR, the association became more prominent (SMD=0.38, 95% CI=0.24-0.51, p=0.000) and without significant heterogeneity (p=0.427, I2=0.0%).
Meta-regression analysis
In order to evaluate the effect of Child-Pugh class on the blood ammonia levels, we performed a multivariate meta-regression by including patients' age, gender and ethnicity. However, the results suggested that Child-Pugh B/C (coefficient=-0.503, p=0.300) did not significantly affect the association between H. pylori infection and blood ammonia levels.
Subgroup analysis
We preformed subgroup analysis between Caucasian and Asian ethnicity, and the results showed significant association in Asian ethnicity (SMD=0.38, 95% CI=0.24-0.52) without significant heterogeneity (p=0.057), however, this association was not found in Caucasian ethnicity (SMD=0.08, 95% CI=-0.26-0.42) and without significant heterogeneity (p=0.877).
Publication bias
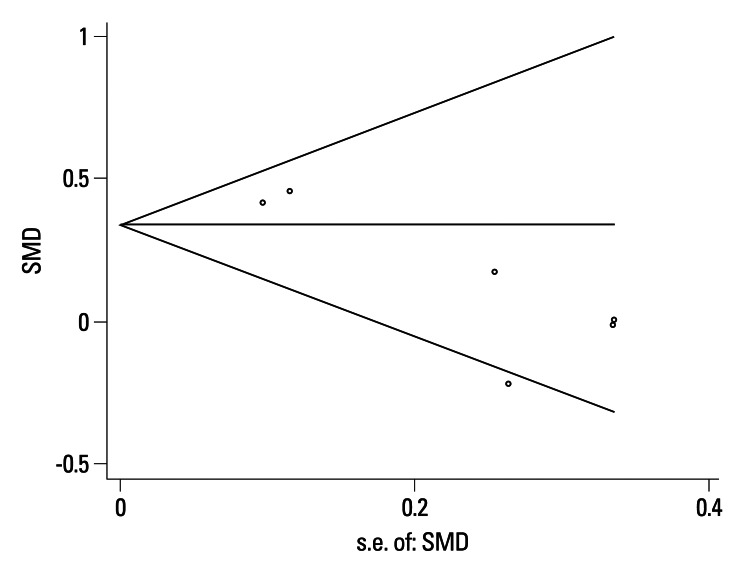
No publication bias was detected (Egger's test: p=0.106, Begg's test: p=0.260) (Fig. 3).
Fig. 3.
Funnel Plot of the Meta-Analysis (Begg's test). SMD, standard means differences.
DISCUSSION
H. pylori, a bacterium which commonly infects human stomach, has urease activity which is many times more potent than that of enterobacteria.23,24 The H. pylori urease in the gastric juice breaks down urea into ammonia and carbon dioxide, and the ammonia is then rapidly absorbed into the blood.25 Previous studies showed that ingestion of ammonia leads to a rise in arterial levels in half of normal subjects and in virtually all cirrhotic patients, the latter demonstrating a considerable increment with peak levels at 15 min.26,27 The different pattern of change of blood ammonia between normal subjects and cirrhotic patients may due to severe dysfunction of cirrhosis; the hepatic capacity of urea synthesis is significantly reduced.28 However, there are also several studies to show that blood ammonia levels are not significantly different between cirrhotic patients with and without H. pylori infection. Furthermore, although H. pylori infection is able to generate ammonia in the stomach, the amount appears to be too small to affect blood ammonia levels.11,29-31 Generally, the inconsistent results among the studies may be due to inadequate statistical power of small sample size, ethnic differences, publication bias or others reasons. Meta-analysis is a powerful means to estimate the true effect size as opposed to a less precise effect size derived in a single study under a given single set of assumptions and conditions.18
In the present meta-analysis, the number of patients with or without H. pylori infection was 632 and 396, respectively, which provided more reliable estimates. Meanwhile, the negligibility of heterogeneity suggested the uniformity of the studies included in regard with patients selection, diagnosis and design, etc. Furthermore, we performed sensitivity analysis to further test the robust of the overall results, and confirmed significant association of the overall results. However, SMD expresses the size of the intervention effect by standardized the variability of each study but it is not indicate the real differences among study, therefore, the vaule 0.34 of SMD in the present study is not indicate the absolute difference of blood ammonia levels and cannot directly be used for clinical application.
Child-Pugh class has been widely used as a descriptive indicator of chronic liver disease. At the stage of Child-Pugh class B/C, the capacity of urea synthesis is reduced significantly, and hyperammonemia will occur when the maximal rate of urea synthesis becomes less than 30%.28 Because cirrhotic patients with Child-Pugh class B/C are prone to develop hyperammonemia compared to patients with Child-Pugh class A, we hypothesized that cirrhotic patients with Child-Pugh class B/C are likely to affect the association between H. pylori infection and blood ammonia levels. In the present studies, however, the multivariate meta-regression analysis did not indicate that Child-Pugh class B/C can affect the association. Of note, due to limited original data available, we could not directly compare blood ammonia levels between patients with Child-Pugh class B/C and Child-Pugh class A. The effect of Child-Pugh class on the association between H. pylori infection and blood ammonia levels needs to be investigated in future studies. The prevalence of H. pylori infection is higher in developing countries than in developed countries.32 In the subgroup analysis, the results showed that the association of H. pylori infection and blood ammonia levels was significant in Asian ethnicity, but not in Caucasian ethnicity. Such discrepancy is very likely due to different prevalence of H. pylori infection in Asian and Caucasian ethnicity, and the high infection of H. pylori in Asian ethnicity leads to a relatively higher ammonia production in the stomach, especially in cirrhotic patients. However, this conclusion should be interpreted with caution because of limited study number.
There are several study limitations. First, for accurate detection of H. pylori infection, at least two methods should be used.33,34 However, two of the six studies included used only one method to detect the status of H. pylori infection, even though we omitted them in sensitivity analysis to eliminate the potential bias. Second, a few published studies are available, thus affecting the statistical power to detect significant findings. Third, the etiology of cirrhosis in the included studies was diverse, possibly undermining the robustness of the results and also making it uncertain whether these results can be extrapolated to cirrhotic patients with single etiology. Fourth, due to limited information available, the etiology of cirrhosis, the number of H. pylori and their distribution in the stomach, gastric pH, gastric membrane permeability to ammonia, and portal vein branch circulation were not included in our analysis. These factors are considered to be a major threat to the validity of inferences made on the cause and the effect.35 Finally, converting non-normally distributed statistics (median and range) to normally distributed statistics (means and SD) in our analysis could be a cause of bias.
In summary, our results suggested that H. pylori infection is associated with elevated blood ammonia levels in cirrhotic patients. And this association seems to be more prominent in Asian ethnicity than Caucasian ethnicity. However, because of the limited number of published studies and methodological flaws of the studies included, more large randomized studies are warranted in order to further confirm this association.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Souba WW. Interorgan ammonia metabolism in health and disease: a surgeon's view. JPEN J Parenter Enteral Nutr. 1987;11:569–579. doi: 10.1177/0148607187011006569. [DOI] [PubMed] [Google Scholar]
- 2.LeVeen HH, LeVeen EG, LeVeen RF. Awakenings to the pathogenicity of urease and the requirement for continuous long term therapy. Biomed Pharmacother. 1994;48:157–166. doi: 10.1016/0753-3322(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 3.Demirtürk L, Yazgan Y, zci O, Ozel M, Toğrol E, Gültepe M, et al. The effect of Helicobacter pylori eradication on gastric juice and blood ammonia concentrations and on visual evoked potentials in cirrhotics. Helicobacter. 2001;6:325–330. doi: 10.1046/j.1083-4389.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- 4.Queiroz DM, Rocha AM, Rocha GA, Cinque SM, Oliveira AG, Godoy A, et al. Association between Helicobacter pylori infection and cirrhosis in patients with chronic hepatitis C virus. Dig Dis Sci. 2006;51:370–373. doi: 10.1007/s10620-006-3150-y. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti P, Zullo A, Hassan C, Pandit A, Chowdhury A, Santra A, et al. Helicobacter pylori, gastric juice, and arterial ammonia levels in patients with cirrhosis. J Clin Gastroenterol. 2002;34:578–581. doi: 10.1097/00004836-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Dasani BM, Sigal SH, Lieber CS. Analysis of risk factors for chronic hepatic encephalopathy: the role of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:726–731. doi: 10.1111/j.1572-0241.1998.214_a.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyaji H, Ito S, Azuma T, Ito Y, Yamazaki Y, Ohtaki Y, et al. Effects of Helicobacter pylori eradication therapy on hyperammonaemia in patients with liver cirrhosis. Gut. 1997;40:726–730. doi: 10.1136/gut.40.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SJ, Wang LJ, Zhu Q, Cai JT, Chen T, Si JM. Effect of H pylori infection and its eradication on hyperammo-nemia and hepatic encephalopathy in cirrhotic patients. World J Gastroenterol. 2008;14:1914–1918. doi: 10.3748/wjg.14.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Hady H, Zaki A, Badra G, Lotfy M, Selmi C, Giorgini A, et al. Helicobacter pylori infection in hepatic encephalopathy: relationship to plasma endotoxins and blood ammonia. Hepatol Res. 2007;37:1026–1033. doi: 10.1111/j.1872-034X.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 10.Vásconez C, Elizalde JI, Llach J, Ginès A, de la Rosa C, Fernández RM, et al. Helicobacter pylori, hyperammonemia and subclinical portosystemic encephalopathy: effects of eradication. J Hepatol. 1999;30:260–264. doi: 10.1016/s0168-8278(99)80072-5. [DOI] [PubMed] [Google Scholar]
- 11.Zullo A, Hassan C, Morini S. Helicobacter pylori infection in patients with liver cirrhosis: facts and fictions. Dig Liver Dis. 2003;35:197–205. doi: 10.1016/s1590-8658(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 12.Zullo A, Rinaldi V, Efrati C, Hassan C, Caroli S, Riggio O, et al. Zinc, ammonia, and Helicobacter pylori infection in liver cirrhosis. Dig Liver Dis. 2000;32:836–838. doi: 10.1016/s1590-8658(00)80366-7. [DOI] [PubMed] [Google Scholar]
- 13.Miquel J, Barcena R, Boixeda D, Fernández J, SanRoman AL, Martín-de-Argila C, et al. Role of Helicobacter pylori infection and its eradication in patients with subclinical hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2001;13:1067–1072. doi: 10.1097/00042737-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group CEPaOoC. EPOC resources for review authors. [accessed on 2011 July 5]. Available at: http://epoc.cochrane.org/epoc-resources.
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. 2008. The Cochrane Callaboration. Available at: http://www.cochrane-handbook.org.
- 18.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 21.Kini D, Aggarwal R, Saraswat VA, Naik SR. Role of Helicobacter pylori infection in hyperammonemia and subclinical hepatic encephalopathy in cirrhosis of liver. Indian J Gastroenterol. 2001;20:237–240. [PubMed] [Google Scholar]
- 22.Yang CS, Cao SY, He XJ, Wang YX, Zhang YL. [Study of correlation between Helicobacter pylori infection and hyperammonemia and hepatic encephalopathy in cirrhotic patients] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:422–424. [PubMed] [Google Scholar]
- 23.Dunn BE, Campbell GP, Perez-Perez GI, Blaser MJ. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 24.Turbett GR, Nandapalan N, Campbell IG, Nikoletti SM, Mee BJ. Characterisation of the urease from Helicobacter pylori and comparison with the ureases from related spiral gastric bacteria. FEMS Microbiol Immunol. 1991;3:19–24. doi: 10.1111/j.1574-6968.1991.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 25.Neithercut WD, Rowe PA, el Nujumi AM, Dahill S, McColl KE. Effect of Helicobacter pylori infection on intragastric urea and ammonium concentrations in patients with chronic renal failure. J Clin Pathol. 1993;46:544–547. doi: 10.1136/jcp.46.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans WB, Aoyagi T, Summerskill WH. Gastrointestinal urease in man. II. Urea hydrolysis and ammonia absorption in upper and lower gut lumen and the effect of neomycin. Gut. 1966;7:635–639. doi: 10.1136/gut.7.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conn HO. Studies of the source and significance of blood ammonia. IV. Early ammonia peaks after ingestion of ammonium salts. Yale J Biol Med. 1972;45:543–549. [PMC free article] [PubMed] [Google Scholar]
- 28.Rudman D, DiFulco TJ, Galambos JT, Smith RB, 3rd, Salam AA, Warren WD. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. J Clin Invest. 1973;52:2241–2249. doi: 10.1172/JCI107410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber M, Rössle M, Siegerstetter V, Ochs A, Haag K, Kist M, et al. Helicobacter pylori infection does not correlate with plasma ammonia concentration and hepatic encephalopathy in patients with cirrhosis. Hepatogastroenterology. 2001;48:541–544. [PubMed] [Google Scholar]
- 30.DuBois S, Eng S, Bhattacharya R, Rulyak S, Hubbard T, Putnam D, et al. Breath ammonia testing for diagnosis of hepatic encephalopathy. Dig Dis Sci. 2005;50:1780–1784. doi: 10.1007/s10620-005-2937-6. [DOI] [PubMed] [Google Scholar]
- 31.Calvet X, Nogueras C, Roqué M, Sanfeliu I. Helicobacter pylori is not a risk factor for hepatic encephalopathy. Dig Liver Dis. 2001;33:414–419. doi: 10.1016/s1590-8658(01)80013-x. [DOI] [PubMed] [Google Scholar]
- 32.Webberley MJ, Webberley JM, Newell DG, Lowe P, Melikian V. Seroepidemiology of Helicobacter pylori infection in vegans and meat-eaters. Epidemiol Infect. 1992;108:457–462. doi: 10.1017/s0950268800049967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardone G, Coscione P, D'Armiento FP, Del Pezzo M, Pontillo M, Mossetti G, et al. Cirrhosis negatively affects the efficiency of serologic diagnosis of Helicobacter pylori infection. Ital J Gastroenterol. 1996;28:332–336. [PubMed] [Google Scholar]
- 34.Taylor-Robinson SD, Jackson N, Buckley C. Helicobacter pylori, ammonia and the brain. Gut. 1997;40:805–806. doi: 10.1136/gut.40.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston SC. Identifying confounding by indication through blinded prospective review. Am J Epidemiol. 2001;154:276–284. doi: 10.1093/aje/154.3.276. [DOI] [PubMed] [Google Scholar]