Abstract
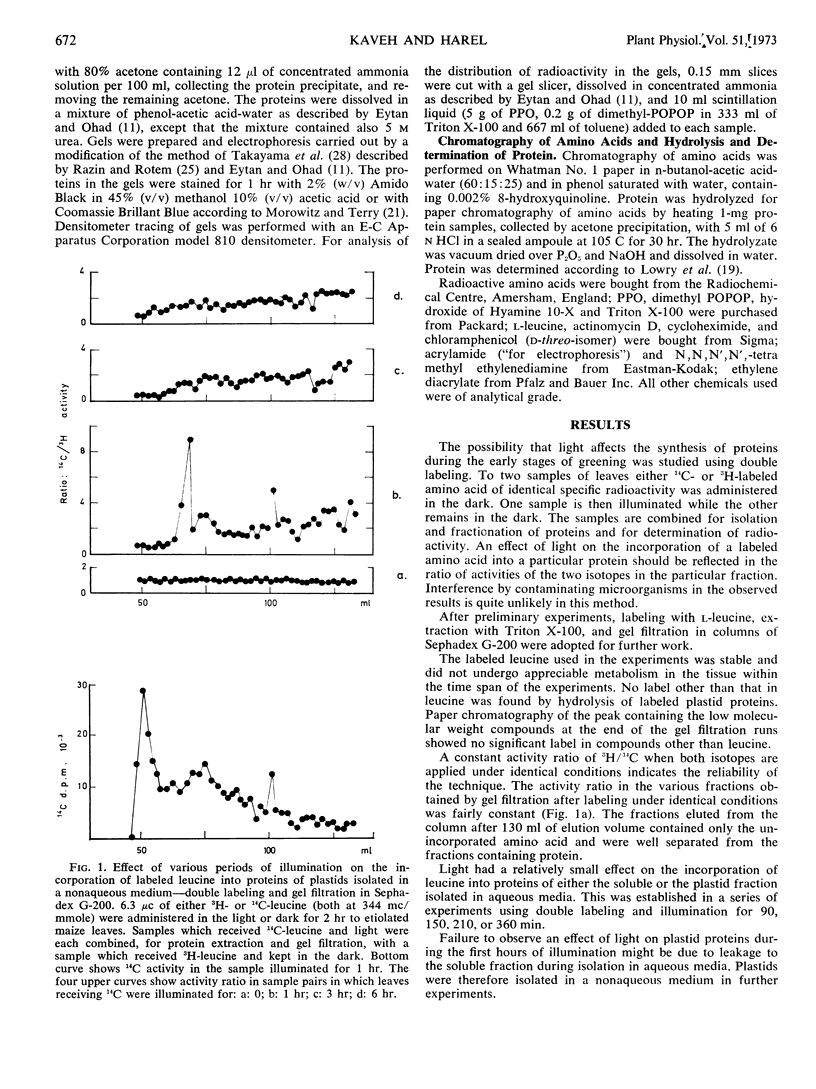
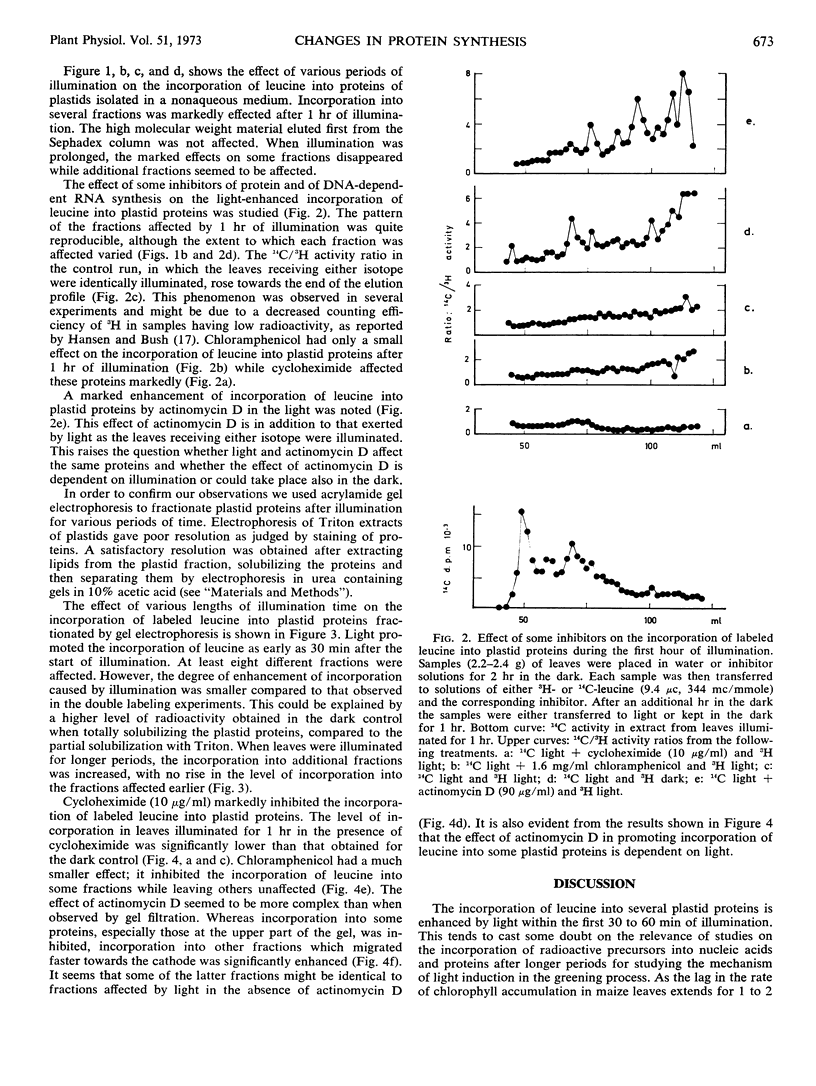
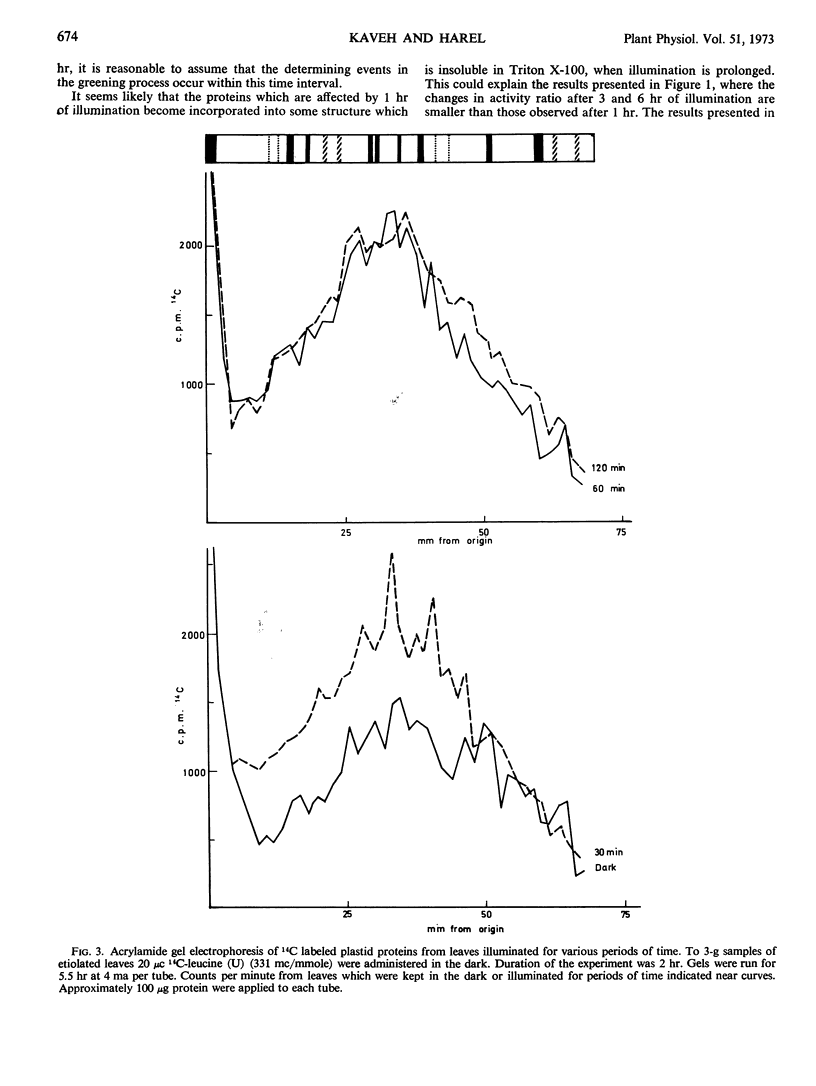
The effect of light on protein synthesis during the early stages of greening of etiolated maize (Zea mays) leaves was studied using double labeling with leucine and fractionation of proteins by gel filtration and acrylamide gel electrophoresis. The incorporation of labeled leucine into a relatively small number of plastid proteins is effected within the first 30 to 60 minutes of illumination. These proteins do not accumulate with time. When illumination is prolonged, additional proteins are effected.
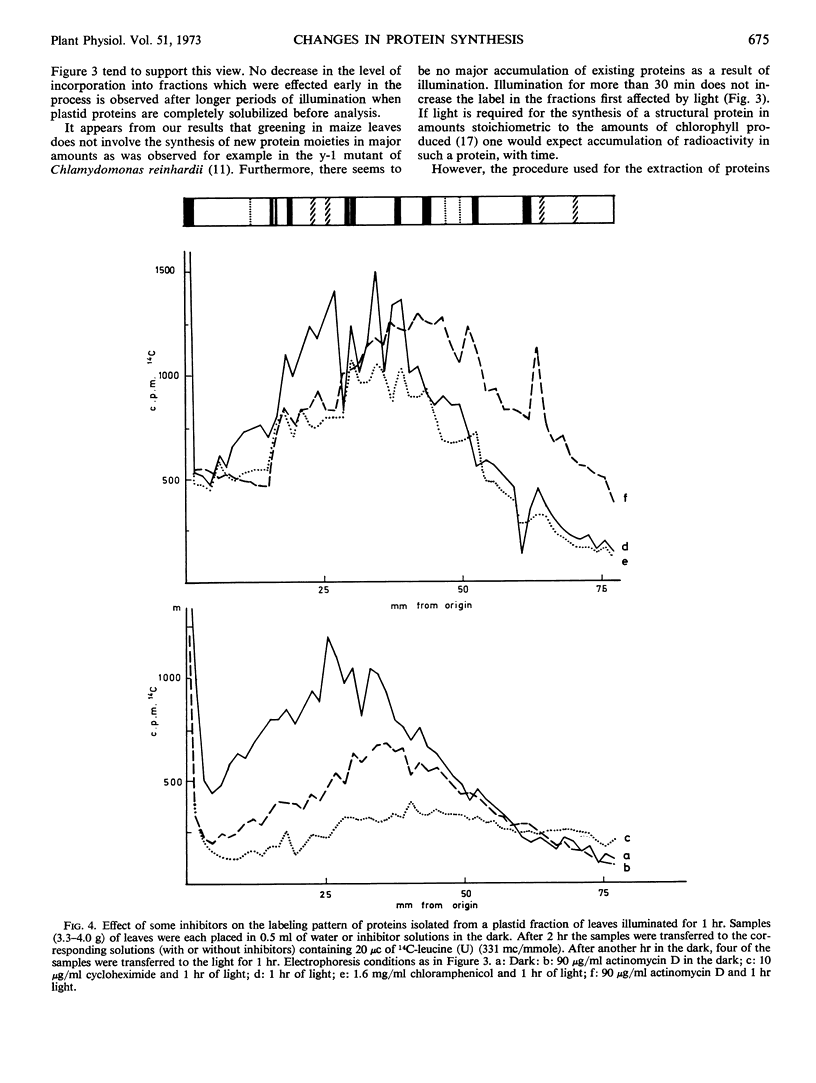
Experiments using inhibitors of protein synthesis suggest that at least some of the proteins effected by 1 hour of illumination might be synthesized in the cytoplasm and not in plastids. Actinomycin D inhibits the incorporation of labeled leucine into some of the protein fractions, but enhances the incorporation into other fractions far above the effect exerted by light.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. A., Smillie R. M. Binding of chloramphenicol by ribosomes from chloroplasts. Biochem Biophys Res Commun. 1966 May 25;23(4):535–539. doi: 10.1016/0006-291x(66)90762-5. [DOI] [PubMed] [Google Scholar]
- CLARK M. F. POLYRIBOSOMES FROM CHLOROPLASTS. Biochim Biophys Acta. 1964 Dec 16;91:671–674. doi: 10.1016/0926-6550(64)90025-8. [DOI] [PubMed] [Google Scholar]
- Chen S., McMahon D., Bogorad L. Early effects of illumination on the activity of some photosynthetic enzymes. Plant Physiol. 1967 Jan;42(1):1–5. doi: 10.1104/pp.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Generation of cellulase activity during protein synthesis by pea microsomes in vitro. Arch Biochem Biophys. 1969 Feb;129(2):581–587. doi: 10.1016/0003-9861(69)90217-3. [DOI] [PubMed] [Google Scholar]
- Drumm H. E., Margulies M. M. In vitro protein synthesis by plastids of Phaseolus vulgaris. IV. Amino acid incorporation by etioplasts and effect of illumination of leaves on incorporation by plastids. Plant Physiol. 1970 Apr;45(4):435–442. doi: 10.1104/pp.45.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. [Chloroplast ribosomes: stereospecificity of inhibition by chloramphenicol]. Science. 1969 Jan 31;163(3866):477–478. doi: 10.1126/science.163.3866.477. [DOI] [PubMed] [Google Scholar]
- Eytan G., Ohad I. Biogenesis of chloroplast membranes. VI. Cooperation between cytoplasmic and chloroplast ribosomes in the synthesis of photosynthetic lamellar proteins during the greening process in a mutant of Chlamydomonas reinhardi y-1. J Biol Chem. 1970 Sep 10;245(17):4297–4307. [PubMed] [Google Scholar]
- Harel E., Bogorad L. Effect of light on ribonucleic Acid metabolism in greening maize leaves. Plant Physiol. 1973 Jan;51(1):10–16. doi: 10.1104/pp.51.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Blobel G. Characterization of the chloroplastic and cytoplasmic ribosomes of Chlamydomonas reinhardi. J Mol Biol. 1969 Apr 14;41(1):121–138. doi: 10.1016/0022-2836(69)90130-2. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morowitz H. J., Terry T. M. Characterization of the plasma membrane of Mycoplasma laidlawii. V. Effects of selective removal of protein and lipid. Biochim Biophys Acta. 1969 Jul 15;183(2):276–294. doi: 10.1016/0005-2736(69)90084-4. [DOI] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B. D., Trewavas A. J. Changes in the pattern of protein synthesis induced by 3-indolylacetic Acid. Plant Physiol. 1967 Aug;42(8):1081–1086. doi: 10.1104/pp.42.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Beevers L. Nucleic Acid Metabolism during Greening and Unrolling of Barley Leaf Segments. Plant Physiol. 1970 Aug;46(2):315–319. doi: 10.1104/pp.46.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Rottem S. Identification of Mycoplasma and other microorganisms by polyacrylamide-gel electrophoresis of cell proteins. J Bacteriol. 1967 Dec;94(6):1807–1810. doi: 10.1128/jb.94.6.1807-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger B. J., Fairfield S. A., Epler J. L., Barnett W. E. Identification and origin of some chloroplast aminoacyl-tRNA synthetases and tRNAs. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1207–1213. doi: 10.1073/pnas.67.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER D., WILDMAN S. G. THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY CELL-FREE EXTRACTS FROM TOBACCO LEAVES. Biochemistry. 1964 Jul;3:954–959. doi: 10.1021/bi00895a019. [DOI] [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]



