Abstract
The current investigation evaluated nicotine withdrawal symptoms elicited by 12 hours of smoking deprivation on anxious and fearful responding to bodily sensations among daily smokers with and without Panic Disorder (PD). It was hypothesized that smokers with PD who were experiencing greater levels of nicotine withdrawal would experience the greatest levels of fearful responding to, and delayed recovery from, a 10% carbon dioxide-enriched air (CO2) biological challenge procedure. Participants were 58 adults who reported smoking 19.72 cigarettes daily (SD = 7.99). Results indicated that nicotine withdrawal and PD status interacted to predict greater post-challenge panic attack symptoms. Also, individuals with PD initially evidenced a quicker decrease in subjective anxiety following the challenge, but their rate of recovery decelerated over time as compared to those without PD. There was, however, no significant interaction for change in subjective anxiety pre- to post-challenge. Results are discussed in relation to the role of nicotine withdrawal in anxious and fearful responding for smokers with PD.
Keywords: Panic Disorder, Nicotine Withdrawal, Tobacco, Laboratory Challenge
Smoking rates have remained relatively stable in recent years (CDC, 2011). Some scholars have suggested that the stable rates of smoking may be at least partially explained by characteristics of 'todays' smokers, including those with mental disorders (Kalman, Morissette, & George, 2005; Lasser et al., 2000). Although smoking has historically been studied in relation to many co-occurring psychopathological conditions (e.g., schizophrenia, depressive disorders), comparatively less scholarly attention has been focused on anxiety and its disorders (Ziedonis et al., 2008). Yet, a growing corpus of work indicates that rates of smoking are higher among those with anxiety disorders relative to many other psychiatric conditions as well as those with no psychiatric illness (McCabe et al., 2004).
Some of the most robust relations thus far documented between smoking and anxiety disorders have been evident for panic psychopathology (Zvolensky, Feldner, Leen-Feldner, & McLeish, 2005). For example, epidemiological (Farrell et al., 2001), community (Hayward, Killen, & Taylor, 1989), and clinical (Pohl, Yeragani, Balon, Lycaki, & McBride, 1992) studies have found individuals with panic attacks are more apt to have a history of smoking compared to individuals without a panic attack history. Other investigations suggest smoking often precedes and increases the subsequent risk for developing panic attacks and panic disorder [PD] with and without agoraphobia (Bernstein, Zvolensky, Schmidt, & Sachs-Ericsson, 2007; Breslau & Klein, 1999; Isensee, Wittchen, Stein, Höfler, & Lieb, 2003; Johnson et al., 2000; Zvolensky et al., 2008). There also is evidence to suggest that panic attacks and PD can contribute to the maintenance of smoking (Zvolensky, Schmidt, & Stewart, 2003). For example, panic attacks are associated with more severe nicotine withdrawal (Marshall. Johnson, Bergman, Gibson, & Zvolensky, 2009), shorter durations of abstinence (Zvolensky, Lejuez, Kahler, & Brown, 2004), and overall lower success rates (Piper et al., 2010) following a smoking cessation attempt. Additionally, PD is related to increased motivation to smoke to reduce negative affect (Zvolensky et al., 2005). These data collectively indicate clinically and statistically significant bi-directional relations between smoking and panic.
Although there are a variety of factors, including genetic variables and mental and physical health vulnerability, that may impact the link between smoking and panic psychopathology, little is known about the mechainisms underlying their covariance (Zvolensky & Bernstein, 2005). Nicotine withdrawal may serve as one possible mechanism in the explanation of the observed relationships between smoking and panic psychopathology. Nicotine withdrawal reflects symptoms that emerge from a reduction of nicotine in the body via smoking deprivation (Gilbert, 1988). A diverse body of research indicates: (a) smoking deprivation among daily smokers results in a set of prototypical withdrawal symptoms (e.g., anxiety, depression, impatience, difficulty concentrating, and restlessness; Hughes, 2007; Hughes & Hatsukami, 1986; Hughes, Higgins, & Hatsukami, 1990); (b) negative affect is a central feature of such withdrawal (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Patten & Martin, 1996); and (c), withdrawal symptoms gradually emerge within minutes after a cigarette has been smoked (Jarvik et al., 2000; Schuh & Stitzer, 1995).
Acute and prolonged aversive interoceptive cues (e.g., bodily tension, anxiety) associated with nicotine withdrawal may have important implications for the onset and maintenance of panic psychopathology (Hogle, Kaye, & Curtin, 2010; Zvolensky, Schmidt, & McCreary, 2003). Namely, smokers with PD experiencing smoking deprivation may be more likely to attend to, and catastrophize about, unpleasant, acute nicotine withdrawal symptoms, presumably hastening elevations and delaying recovery from withdrawal states (Abrams, Zvolensky, Dorflinger, & Galatis, 2008). This type of perspective suggests PD may moderate a nicotine withdrawal-panic effect among daily smokers, as acute nicotine withdrawal symptoms may be more likely to be perceived as personally threatening among those with, compared to those without, PD (Zvolensky & Bernstein, 2005).
Although there has not been a direct evaluation of PD and nicotine withdrawal on anxious and fearful responding to bodily sensations, a number of indirect sources of evidence have been reported. For example, smokers with PD retrospectively report greater withdrawal symptom severity during past quit attempts compared to individuals without such a history (Marshall et al., 2009; Zvolensky et al., 2004b). There also is a limited body of work that suggests anxiety sensitivity (AS), a core cognitive component of panic psychopathology, may be related to the severity of nicotine withdrawal symptoms across laboratory and clinical studies (Johnson, Stewart, Rosenfield, Steeves, & Zvolensky, in press; Marshall et al., 2009; Mullane, Stewart, Rhyno, Steeves, Watt, & Eisner, 2008; Vujanovic & Zvolensky, 2009). Overall, empirical work suggests acute nicotine withdrawal may be related to increased risk of panic responding, yet direct empirical evidence is lacking for PD.
The overarching aim of the present study was to examine whether nicotine withdrawal symptoms elicited by a 12-hour smoking deprivation period (versus no deprivation) among daily smokers resulted in greater fearful responding to, and delayed recovery from, laboratory-induced bodily sensations among those with PD compared to those without PD. In the current investigation, PD was coded categorically, rather than indexed continuously, to facilitate comparability with existing panic psychopathology research, which has consistently employed a categorical classification. Alternatively, although participants were assigned to either smoking deprivation (SMD) or smoke as usual (SAU), this variable was indexed continuously as participants smoking rates, levels of nicotine dependence, and total hours of withdrawal were not standardized, thus, potentially resulting in a broader range of nicotine withdrawal.
It was hypothesized that smokers with PD who were experiencing greater levels of nicotine withdrawal, as a result of smoking deprivation, would experience the greatest levels of fearful responding to a 10% carbon dioxide-enriched air (CO2) laboratory procedure, as measured by: (1) intensity of post-challenge panic attack symptoms; (2) change in anxiety focused on bodily sensations from pre- to post-challenge; and (3) rate of recovery from the challenge in terms of anxiety focused on bodily sensations.
Method
Participant Selection
Study inclusion criteria included: (1) being a daily smoker for at least the past year (cigarettes per day ≥ 7); (2) having not decreased the number of cigarettes smoked per day by more than half in the past 6 months; (3) being 18 to 65 years old; and (4) reporting a willingness to abstain from smoking for a 12-hour period. Participants were excluded from the study based on evidence of: (1) a current medical condition that contraindicated CO2 administration (cardiovascular, endocrine, pulmonary, respiratory [including severe asthma], or gastrointestinal illness); (2) a past diagnosis of PD (assessed using the Structured Clinical Interview – Non-Patient Version for DSM-IV [SCID-N/P]; First, Spitzer, Gibson, & Williams, 1994); (3) limited mental competency (not oriented to person, place, or time) and the inability to give informed, voluntary, written consent to participate; (4) pregnancy or the possibility of being pregnant (by self-report); (5) current use of nicotine replacement therapy; (6) current or past history of psychotic-spectrum symptoms or disorders (7) current substance dependence; (8) prior experience with CO2 challenge; (9) suicidality (assessed using the SCID-NP; First et al.,1994) suicidality/depression section); and (10) any current use of psychotropic medication which could impact the effectiveness of the laboratory challenge1. Individuals in both the PD group and non-PD group were permitted to meet criteria for additional current and past diagnoses (please see Table 1).
Table 1.
Rates of Current and Past Psychopathology as a Function of Panic Disorder Group.
| Current Psychopathology | Past (Lifetime) Psychopathology | |||
|---|---|---|---|---|
| Positive Panic Disorder Group |
Negative Panic Disorder Group |
Positive Panic Disorder Group |
Negative Panic Disorder Group |
|
| Any Psychopathology (% yes) | 100% | 35.1% | 85.7% | 78.4% |
| Chi-Square Test | χ2 = 23.24, p < .001 | χ2 = .47, p = ns | ||
| Mean (SD) # Diagnosesa | 2.28 (1.39) | 0.49 (.80) | 2.38 (1.72) | 1.59 (1.38) |
| Independent Samples t-test | t(56) = −5.48, p < .01, d = 1.59 | t(56) = −1.17, p = .06 (trend), d =0.50 | ||
| Alcohol Abuse (% yes) | 4.8% | 10.8% | 23.8% | 29.7% |
| Alcohol Dependence | 0.0% | 0.0% | 38.1% | 18.9% |
| Amphetamine Abuse | 0.0% | 0.0% | 4.8% | 2.7% |
| Amphetamine Dependence | 0.0% | 0.0% | 4.8% | 2.7% |
| Bulimia Nervosa | 0.0% | 0.0% | 0.0% | 2.7% |
| Cocaine Abuse | 0.0% | 2.7% | 9.5% | 5.4% |
| Cocaine Dependence | 0.0% | 0.0% | 9.5% | 16.2% |
| Dysthymia | 23.8% | 0.0% | 0.0% | 2.7% |
| Generalized Anxiety Disorder | 23.8% | 0.0% | 0.0% | 2.7% |
| Hallucinogen/PCP Abuse | 0.0% | 0.0% | 23.8% | 0.0% |
| Major Depressive Disorder | 19.0% | 8.1% | 28.6% | 13.5% |
| Marijuana Abuse | 4.8% | 10.8% | 28.6% | 21.6% |
| Marijuana Dependence | 0.0% | 0.0% | 23.8% | 21.6% |
| Obsessive-Compulsive Disorder | 9.5% | 0.0% | 0.0% | 0.0% |
| Opiate Abuse | 0.0% | 0.0% | 0.0% | 2.7% |
| Opiate Dependence | 0.0% | 0.0% | 23.8% | 10.8% |
| Panic Disorder without Agoraphobia | 42.9% | 0.0% | 4.8% | 0.0% |
| Panic Disorder with Agoraphobia | 57.1% | 0.0% | 4.8% | 0.0% |
| Posttraumatic Stress Disorder | 9.5% | 2.7% | 9.5% | 0.0% |
| Social Anxiety Disorder | 28.6% | 10.8% | 0.0% | 0.0% |
| Specific Phobia | 5.0% | 2.7% | 0.0% | 0.0% |
Note: Percent of participants meeting criteria for current and past psychiatric diagnoses as determined using the Structured Clinical Interview for DSM-IV (SCID-NP; First, et al., 1995). d = Cohen’s d index of effect size.
Pre-Challenge Measures
Diagnostic inclusion and exclusion on the basis of PD or lifetime psychiatric history was determined using the SCID-N/P (First et al., 1994). Those with current PD, based on DSM-IV criteria, were assigned to the PD group. Those who endorsed previous symptoms of PD, but no longer meeting diagnostic criteria, were excluded from the study. Adequate reliability of the SCID-N/P has been demonstrated (First et al., 1994). The PI or trained, post-bachelor’s, research assistants administered the instrument. Interviews were audio-recorded and senior level graduate students cross-checked 18.96% of the interviews, chosen at random, with an inter-rater agreement of 98%, with no cases of disagreement with regard to PD diagnosis.
An expanded/adapted version of the validated Medical History Form (MHF; Scheftner & Endicott, 1984), a structured instrument administered by trained interviewers, was employed to assess lifetime medical history and the exclusionary criteria for the challenge portion of the study (Ormel, VonKorff, Ustun, & Pini, 1994). This interview has excellent psychometric properties and has been extensively used successfully in previous work for screening physical health problems and medication usage (Hays, Marshall, Wang, & Sherbourne, 1994).
Smoking history and pattern of use was assessed with the well-established SHQ (Brown et al., 2002) that includes items pertaining to smoking rate, age of onset, and years of regular smoking. As in past work, the SHQ was used as a descriptive measure of smoking history (Brown et al., 2002; Zvolensky et al., 2004c).
The Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) is a six-item scale designed to assess gradations in tobacco dependence, and is a revision of the Fagerström Tolerance Questionnaire (FTQ; Fagerström, 1978). Although the FTND typically exhibits low internal consistency (α = .61; Heatherton et al., 1991), it has shown positive relations with key smoking variables (e.g., saliva cotinine; Heatherton et al., 1991; Payne, Smith, McCracken, McSherry, & Antony, 1994), and high degrees of test-retest reliability (Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994). In the current investigation, this measure was employed to index nicotine dependence (α = .47 among the present sample).
The Panic Disorder Severity Scale (PDSS; Shear et al., 1997, 2001) is a semi-structured, seven-item clinical interview, designed to assess panic disorder symptom severity. The measure assesses both frequency and severity of panic attacks, anticipatory anxiety, panic-relevant avoidance, and related life impairment. The seven items are rated on five-point Likert-type scale from (0 = none to 4 = extreme; Shear et al., 1997). Past work indicates that the PDSS evidences good inter-rater and test-retest reliability (r = .71), as well as high internal consistency (α = .88; Shear et al., 2001). In the current sample, the PDSS total score for past-month symptom severity was utilized as a continuous, descriptive measure of current severity within the PD group. The PDSS evidenced strong internal consistency among the present sample (α = .95).
A noninvasive biochemical verification of smoking status was completed by CO analysis of breath samples at baseline as well as during participants’ second session, in order to verify smoking deprivation (SMD) and smoke as usual (SAU) group status (smoking deprivation ≤ 8–10 ppm cutoff ≤ regular smoker; Javors et al., 2005; Morabia et al., 2001). Expired CO levels were assessed using a CO Monitor (Bedfont Smokylizer, SN: A36688).
To assess marijuana smoking history and pattern, the Marijuana Smoking History Questionnaire was employed (MSHQ; Bonn-Miller & Zvolensky, 2009). The MSHQ is a self-report instrument that includes items pertaining to both lifetime and past 30 day marijuana smoking rate, age of onset at initiation, years of being a regular marijuana smoker, and other descriptive information (e.g., number of attempts to discontinue using marijuana).
The Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1992), developed by the World Health Organization, was employed to assess frequency of alcohol consumption and alcohol use problems. The AUDIT has evidenced excellent psychometric properties in past work (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993). The frequency and quantity items of the AUDIT were used as an index of weekly alcohol consumption, whereby a composite score was derived based on self-reported average weekly-based alcohol use frequency by quantity per occasion. In addition, an AUDIT total score was derived to assess alcohol use problems in the current sample. The AUDIT evidenced good internal consistency among the present sample (α = .83).
Baseline negative affectivity was assessed using the neuroticism scale of the well-established Big Five Inventory (BFI; John & Srivastava, 1999). The BFI is a 44-item self-report measure assessing the Big Five personality traits (John, 1989). Participants rate a series of phrases, which correspond to the adjectives considered to be markers of the five personality domains, on a five-point Likert scale (1 = disagree strongly to 5 =agree strongly). The BFI maintains good internal consistency (α = .75–.90) and good test-retest reliability (α = .80–.90; John & Srivastava, 1999). In the current investigation, the neuroticism subscale of the BFI (e.g., “is depressed, blue”) was used to index participants’ general tendency to experience negative mood states. Internal consistency of the BFI-Neuroticism subscale in the current sample was excellent (α = .93).
Challenge Measures
The Minnesota Nicotine Withdrawal Scale (MWS; Hughes & Hatsukami, 1986) is a reliable and sensitive scale of nicotine withdrawal symptoms. Nicotine withdrawal symptoms were rated on a 4-point Likert-type scale (0 = not present to 3 = severe). As recommended by Hughes and Hatsukami (1998), we included DSM-IV withdrawal symptom items. The MWS was administered pre-challenge to assess variability in nicotine withdrawal as a function of their withdrawal assignment. Internal consistency for the current sample was strong (α = .85).
The Diagnostic Symptoms Questionnaire (DSQ; Sanderson, Rapee, & Barlow, 1988, 1989) was used to assess DSM-IV panic attack symptoms immediately post-challenge. This measure is frequently employed in challenge work (Zvolensky, Eifert, Lejuez, & McNeil, 1999). Ratings for the DSQ are made on a 9-point Likert-type scale (0 = not at all noticed to 8 = very strongly felt). The DSQ, specifically, lists DSM-IV panic symptoms and yields composite scores for mean intensity level for total, cognitive, and physical symptoms. Past biological challenge work has used these symptom composites (Forsyth, Eifert, & Canna, 2000). The current study sample evidenced strong internal consistency on the DSQ-total post-challenge (α = .94).
A Subjective Units of Distress Scale (SUDS; Wolpe, 1958) was used to index self-reported anxiety focused on bodily sensations. This Likert-type scale ranges from 0 (no anxiety right now) to 100 (extreme anxiety right now). Participants completed this scale (1) before the challenge procedure as an index of anticipatory anxiety, (2) after each minute of the challenge as an index of peri-challenge anxiety, (3) immediately after the challenge as an index of maximum anxiety focused on bodily sensations, and (4) after each minute of the recovery period following the challenge (see Procedure Section for details).
Physiological Assessment
Physiological data was collected via, a BCI Capnocheck II Handheld Capnograph/Oximeter (Model 8401) manufactured by Smiths Medical, interfaced with an infrared polygraph and data processing system. Data collected for manipulation verification included heart rate and respiration rate. For manipulation check analyses utilizing physiological data, raw data was used to calculate averaged minute values.
Procedure
Individuals who responded to study advertisements completed an initial baseline session in order to assess basic eligibility criteria. At the baseline session, participants underwent a diagnostic evaluation, questionnaire completion, and CO analysis. Regardless of eligibility, pparticipants were compensated $10 for the completion of the initial baseline session. If eligible, stratified random assignment procedures were utilized to assign participants to either the SMD or SAU groups. Specifically, computer randomization was used to stratify those in the PD group to SAU or SMD, and those in the non-PD group to SAU for SMD. Participants who self-reported using benzodiazepines on a PRN basis were then instructed to abstain for 12 hours prior to their second visit. All participants were asked to abstain from alcohol, caffeine, and other non-prescription/illicit substances for 12 hours prior. Their second visit was scheduled within 14 days of their laboratory visit. 24 hours prior to their laboratory appointment participants were contacted via telephone to be reminded of their appointment, group assignment, and abstinence from specified substances. Upon arrival to their second appointment, information on use of nicotine during the 12-hour withdrawal interval was obtained via self-report and CO analysis of breath samples was gathered for those assigned to the SMD group. For those in the SAU group, smoking behavior was standardized such that individuals in the SAU group were asked to smoke a final cigarette upon arrival to the laboratory for their second session. Following verification of their group status, participants were seated in an experimental room where electrodes and a C-PAP mask were attached by the experimenter.
At the second laboratory session, participants in both groups were questioned regarding the following: (1) substance use in the past 24 hours (including alcohol, caffeine, prescription medication, Over-the-Counter [OTC] medications, and NRT); (2) time of last cigarette; (3) what time they went to bed; (4) what time they awoke; and (5) level of alertness (Range: 0 = not at all; 1 = a little; 2 = somewhat; 3 = extremely).
Once the participant was adequately hooked up, the experimenter gave the following instructions (adapted from Harrington, Schmidt, & Telch, 1996):
“Please find a comfortable position. We are now ready to fit you with the capnograph tube and breathing mask, but before we do so, I would like to give you instructions for today’s procedure. We will begin with a ten-minute adaptation period during which I would like you to do your best to relax and sit still, but remain alert, while you get used to the sensors and the environment. Following the adaptation period, you will receive several inhalations of carbon-dioxide-enriched air. You may temporarily feel your heart racing, your palms may be sweaty, you might feel dizzy, and you might have some breathing problems, but remember, there will be no harmful long-term effects resulting from these inhalations and the symptoms are only short term . Please remember that you will be asked to make ratings about how you are feeling several times throughout the procedure, so please listen carefully. Please make your ratings immediately when prompted, based on how you feel right at that moment during the procedure. Do you have any questions before we begin? I am now going to attach the final electrodes and breathing [CPAP] mask.”
Before leaving the room, the experimenter made sure the participant was comfortable and said:
“I will now leave the room for the duration of the procedure. You will receive any instructions you need over the intercom.”
Participants then underwent a 10-minute adaptation period, followed by a 4-minute 10% CO2-enriched air administration (SUDS and DSQ completed immediately post-challenge), and a 10-minute recovery period. Participants were not given any information concerning the CO2 delivery (onset or offset time points). The experimenter remained behind a one-way mirror during the procedure and instructed participants to complete measures via an intercom. At the end of the challenge, the participants were unhooked, debriefed, and compensated $30 for their participation.
Data Analytic Approach
The main and interactive relations between PD and nicotine withdrawal were evaluated with regard to CO2-responding using a hierarchical multiple regression procedure (Cohen & Cohen, 1983). For these analyses, predictor variables were divided into three levels in the hierarchy: (1) negative affectivity (BFI-N: Neuroticism; mean-centered), cigarettes per day (mean-centered), and sex were entered as control variables at level one; (2) PD (no, yes; [variables originally dummy coded as 0, 1, and then transformed via weighted-effect coding to account for unequal group sizes]), and nicotine withdrawal (MWS total scores obtained pre-challenge [upon arrival to lab], mean-centered) were simultaneously entered into the model as main effects at level two; and (3) the interaction term for PD status and nicotine withdrawal was entered at level three. The criterion variable for the first hierarchical multiple regression analysis was intensity of post-challenge panic attack symptoms (DSQ-total composite score), and the criterion variable for the second regression analysis was level of anxiety focused on bodily sensations (post-challenge SUDS ratings). With regard to the prediction of anxiety focused on bodily sensations, in addition to controlling for negative affectivity, we entered pre-challenge SUDS rating (mean-centered) at level one, which allowed examination of the dependent variable as an index of reactivity (i.e., change).
For the recovery hypothesis, an individual growth curve model estimated in R (R Development Core Team, 2010) was employed to examine the trajectory of change in SUDS responding to the challenge procedure over time. First, intercept and time (linear and quadratic) parameters were entered as random effects, followed by each of the main effects (PD and nicotine withdrawal [entered as a multi-level variable]), and finally the interactions among PD, nicotine withdrawal, and time (both linear and quadratic). This approach allowed for the examination of main and interactive effects of PD and nicotine withdrawal on SUDS rating directly in response to the challenge, and throughout the recovery period, via repeated measurements. In addition to the baseline SUDS value, criterion variables for the response through recovery periods were comprised of twelve repeated assessments of SUDS.
Results
A total of 77 persons were eligible for the experimental session (46.8% women; Mage= 29.66, SD = 12.42, range = 18–62). Of the 77 eligible persons, based upon the aforementioned guidelines, 58 participants (46.6% female; Mage = 29.12, SD = 11.79) were retained in the study, with usable data2. Generally consistent with the State of Vermont (State of Vermont, 2008), 91.4% of the final sample identified as Caucasian, 3.4% Black/Non-Hispanic, 3.4% ‘other,’ and 1.7% as Asian.
Of the 58 participants, 21 (36.2%) met criteria for current PD with or without agoraphobia, as determined by the Structured Clinical Interview – Non-Patient Version for DSM-IV (SCID-N/P; First et al., 1994). These individuals scored an average of 11.2 (SD = 3.7) on the Panic Disorder Severity Scale (PDSS; Shear et al., 1997), a score typical of one who is “moderately ill” (i.e., PDSS score 10–13 without agoraphobia and 11–15 with agoraphobia; Furakawa & Shear, 2009).
Approximately 58.6% (n = 34) of the total sample met criteria for current psychopathology, with the most common disorders after PD being social anxiety disorder (17.2%), major depressive disorder (12.1%), generalized anxiety disorder, alcohol abuse, and dysthymia (each 8.6%) (Table 1).
In terms of smoking characteristics, participants reported being a daily smoker for 11.96 years (SD = 10.98) (Smoking History Questionnaire [SHQ]; Brown, Lejuez, Kahler, & Strong, 2002) and reported smoking an average of 19.72 daily (SD = 7.99) upon study entry with moderate levels of nicotine dependence (M = 3.93, SD = 1.71). Approximately 45% of the sample reported drinking 2–3 times weekly or more, and scored an average of 8.88 (SD = 5.10) on the Alcohol Use Disorders Identification Test (AUDIT: Babor, de la Fuente, Saanders, & Grant, 1992), indicating hazardous or harmful drinking behavior (Babor et al., 1992). As indexed by the Marijuana Smoking History Questionnaire (MSHQ: Bonn-Miller & Zvolensky, 2009), approximately 93.9% of the sample reported having used marijuana at least once in their lifetime, with 55.3% having used at least once in the past month (Table 2).
Table 2.
Descriptive Statistics and Group Differences in Key Baseline Variables as a Function of Panic Disorder Status.
| Mean (SD) or % | Observed Range |
Test for Group Difference | |
|---|---|---|---|
| Sex (% female) | 46.6% | χ2 = .946, p = .42 | |
| Positive Panic Disorder Group | 36.2% | ||
| Negative Panic Disorder Group | 63.8% | ||
| Age | 29.12 (11.79) | 18 – 62 | t(56) = −.26, p = .79 |
| Positive Panic Disorder Group | 29.67 (11.41) | 18 – 54 | |
| Negative Panic Disorder Group | 28.81 (12.15) | 18 – 62 | |
| Group Assignment (% SMD) | χ2 = .18, p = .79 | ||
| Positive Panic Disorder Group | 42.9% | ||
| Negative Panic Disorder Group | 48.6% | ||
| Cigarettes Smoked Daily | 19.72 (7.99) | 7–50 | t(56) = 1.39, p = .17 |
| Positive Panic Disorder Group | 17.81 (5.57) | 9–30 | |
| Negative Panic Disorder Group | 20.8 (12.15) | 7–50 | |
| Nicotine Dependence | 3.93 (1.72) | 0–8 | t(56) = .09, p = .93 |
| Positive Panic Disorder Group | 3.90 (1.67) | 1–8 | |
| Negative Panic Disorder Group | 3.95 (1.76) | 0–7 | |
| Age First Smoked | 14.25 (3.97) | 8–39 | t(55) = 1.55, p = .13 |
| Positive Panic Disorder Group | 13.19 (2.29) | 8–18 | |
| Negative Panic Disorder Group | 14.86 (4.59) | 9–39 | |
| Age Regular Smoker | 16.40 (3.61) | 10–39 | t(55) = 1.03, p = .31 |
| Positive Panic Disorder Group | 15.76 (2.28) | 12–22 | |
| Negative Panic Disorder Group | 16.78 (4.18) | 10–39 | |
| Years Regular Smoker | 11.96 (10.98) | 1–48 | t(55) = −.67, p = .51 |
| Positive Panic Disorder Group | 13.24 (11.28) | 2–39 | |
| Negative Panic Disorder Group | 11.22 (10.89) | 1–48 | |
| PDSS Total Score | |||
| Positive Panic Disorder Group | 11.20 (3.84) | 4–17 | t(54) = −9.07, p < .001, d = 2.55 |
| Negative Panic Disorder Group | 1.64 (3.67) | 0–16 | |
| AUDIT Total Score | 8.88 (5.10) | 2–22 | t(38) = −2.31, p < .05, d = .71 |
| Positive Panic Disorder Group | 11.29 (5.98) | 3–22 | |
| Negative Panic Disorder Group | 7.58 (4.13) | 2–18 | |
| Marijuana Use (Past 30 days – %Yes) | 55.3% | χ2 = .74, p = .54 | |
| Positive Panic Disorder Group | 47.1% | ||
| Negative Panic Disorder Group | 60.0% | ||
| Negative Affectivity | 23.78 (8.71) | 8–39 | t(56) = −5.22, p < .001, d = 1.45 |
| Positive Panic Disorder Group | 30.33 (6.70) | 16–39 | |
| Negative Panic Disorder Group | 20.05 (7.47) | 8–36 | |
Note: SMD = Smoking Deprivation Group; Cigarettes Smoked Daily = Number reported smoking upon study entry; CO PPM = Parts Per Million of Carbon Monoxide in Lungs at Baseline Indexed with a Carbon Monoxide Monitor (Bedfont Smokylizer, SN: A36688); Nicotine Dependence = Fagerstöm Test for Nicotine Dependence (FTND; Heatherton et al.,1991); Age First Smoked, Age Regular Smoker and Years Regular Smoker (Smoking History Questionnaire; Brown et al., 2002), PDSS Total = Panic Disorder Severity Scale (PDSS; Shear et al., 1997, 2001); AUDIT Total = Alcohol Use Disorders Identification Test Total Score (Babor et al., 1992); Marijuana Use (Past 30 days - % Yes) (The Marijuana Smoking History Questionnaire; Bonn-Miller & Zvolensky, 2005); Negative Affectivity (BFI-Neuroticism = Big Five Inventory – Neuroticism Subscale; John & Srivastava, 1999).d = Cohen’s d index of effect size.
The PD and non-PD groups were first compared via independent samples t-tests and chi-square analyses on descriptive and baseline variables. Those in the PD group met criteria for a significantly greater number of diagnoses than those in the non-PD group (M = 2.33, SD = 1.39 and M = 0.49, SD = .80, respectively; t(56) = −6.43, p < .001, d = 1.62) and evidenced significantly higher AUDIT scores than the non-PD group (M = 11.28, SD = 5.98 and M = 7.58, SD = 4.13; t(38) = −2.31, p < .05, d = .72) as well as greater levels of negative affectivity (M = 30.33, SD = 6.70 and M = 19.92, SD = 7.53; t(55) = −5.24, p < .001, d = 1.45). No other significant differences were evident (Table 2).
Manipulation Checks for Laboratory Protocol
All participants assigned to the SMD group self-reported at least 12-hours (M = 14.69, SD = 6.56) of smoking deprivation prior to the CO2 biological challenge. CO analysis of breath sample indicated a significant decrease in ppm from Session 1 to Session 2 for those in the SMD group (Mses1 = 18.11, SD: 6.33; Mses2 = 4.56, SD = 3.38, t(26) = 14.88, p < .001).
Post-challenge heart and respiration rates, as well as post-challenge SUDS ratings, were significantly greater than pre-challenge levels (Heart rate: t(45) = 4.94, p < .001; Respiration Rate: t(52) = 8.15, p < .001; SUDS: t(57) = 8.81, p < .001), indicating the CO2 biological challenge effectively elicited both physiological and subjective symptoms of anxiety .
Correlations among Key Study Variables
See Table 3 for zero-order correlations among baseline study variables. In line with prediction, PD status was significantly and positively correlated with post-challenge SUDS (r = .29, p < .05) and DSQ total (r = .34, p < .01). However, it was not correlated with pre-challenge SUDS. As hypothesized, nicotine withdrawal was positively correlated post-challenge SUDS (r = .46, p < .01), DSQ total (r = .31, p < .05), and pre-challenge SUDS (r = .70, p < .01).
Table 3.
Zero-Order (or Bivariate, as applicable) Correlations among Challenge Study Variables.
| Variable | 1 | 2 | 3 | 5 | 6 | 7 | 8 | 9 | M (SD) |
|---|---|---|---|---|---|---|---|---|---|
| 1. Negative Affectivity | 1 | −.20 | .13 | .52** | .57** | .48** | .51** | .58** | 23.78 (8.71) |
| 2. Cigarettes Per Day | 1 | .01 | .01 | −.18 | −.05 | .02 | −.27* | 19.72 (7.99) | |
| 3. Sex (female) | 1 | .24* | −.13 | .14 | .20 | .14 | 46.6% | ||
| 5. Baseline SUDS | 1 | .25 | .70** | .70** | .46** | 33.24 (29.26) | |||
| 6. PD Status (yes) | 1 | .14 | .29* | .34** | 36.2% | ||||
| 7. MWS | 1 | .46** | .32* | 7.62 (5.97) | |||||
| 8. Post-Challenge SUDS | 1 | .68** | 62.07 (33.36) | ||||||
| 9. DSQ Total | 1 | 2.86 (1.91) |
Note:
p < .05,
p < .01;
Negative Affectivity (BFI-Neuroticism = Big Five Inventory – Neuroticism Subscale (John & Srivastava, 1999); Sex (0 = Male, 1 = Female); Baseline SUDS (Subjective Units of Distress Scale rating [Wolpe, 1959] upon arrival to Session 2); PD Status (0 = No, 1 = Yes); Post-Challenge SUDS (Subjective Units of Distress Scale rating [Wolpe, 1959] post-challenge); DSQ Total = Diagnostic Sensations Questionnaire composite score (Sanderson et al., 1988, 1989).
Prediction of Panic Attack Symptoms and Post-Challenge Anxiety (SUDS)
With regard to DSQ total symptom composite, results indicated that the proposed model significantly predicted 42.4% of variance (F(6, 51) = 6.26, p < .01). Level one of the model accounted for a significant 37.1% of variance (p < .01), with negative affectivity being a significant predictor (t = 4.86, β = .54, sr2 = .27, p < .001). Level two of the model was not significant (ps > .05). Level three of the model significantly predicted an additional 5.1% of variance (p < .05), with the interaction term being a significant predictor (t = 2.13, β = .24, sr2 = .08, p < .05).
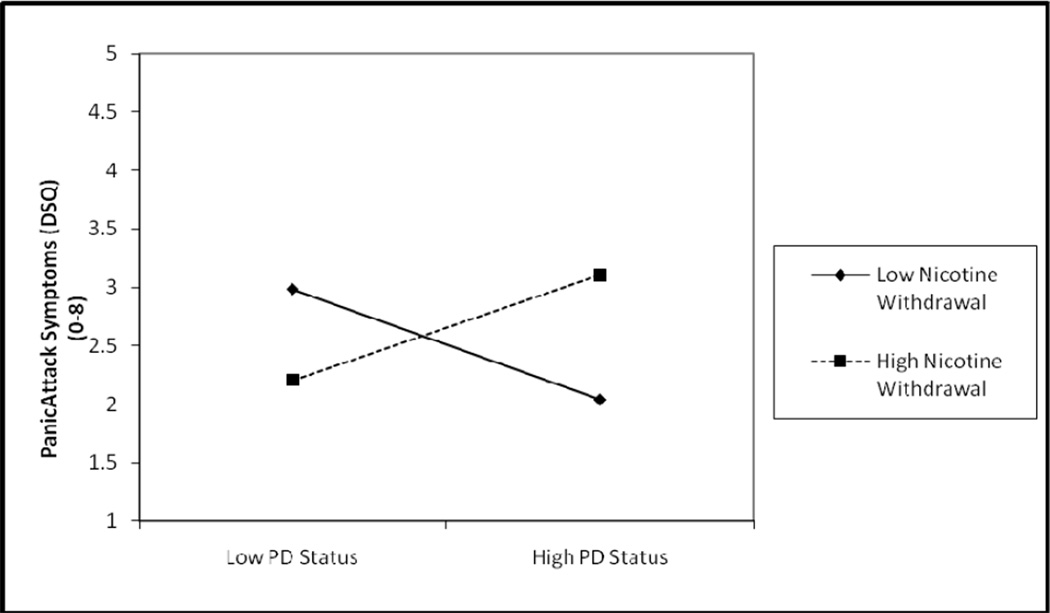
The form of the significant interaction was then examined (Dawson & Richter, 2006. Here, unstandardized regression coefficients for the weighted-effect coded PD term, mean-centered nicotine withdrawal term, and their interaction term were entered, as well as the intercept value, based upon the predictor and moderator term mean and standard deviation values (Dawson & Richter, 2006). As can be seen in Figure 1, individuals with PD and higher levels of nicotine withdrawal evidenced the highest mean level of DSM-IV panic attack symptoms post-challenge.
Figure 1.
Graphical Depiction of Nicotine Withdrawal Moderating the Relationship between PD Status and Panic Attack Symptoms.
Note. DSQ = Diagnostic Symptoms Questionnaire (Sanderson, Rapee, & Barlow, 1988, 1989)
The proposed model significantly accounted for 52.6% of variance in post-challenge SUDS ratings (F(7, 50) = 7.92, p < .01). Specifically, level one of the model accounted for a significant 51.1% of variance (p < .001), with baseline SUDS being a significant predictor (t = 4.89, β = .57, sr2 = .22, p < .001) and negative affectivity evidencing a non-significant statistical trend (t = 1.89, β = .22, sr2 = .03, p = .06). In contrast to expectation, levels two and three of the model were not significant predictors of post-challenge SUDS (ps > .05). See Table 4.
Table 4.
Multiple Regression Analyses for the Dependent Variable of Post-Challenge Anxiety (SUDS).
| R2 | T | B | sr2 | p | ||
|---|---|---|---|---|---|---|
| DSQ Mean | .42 | < .01 | ||||
| Level 1 | .37 | < .01 | ||||
| Negative Affectivity | 4.86 | .54 | .30 | < .001 | ||
| Cigs/day | −1.49 | −.16 | .04 | .14 | ||
| Sex | .66 | .07 | .00 | .51 | ||
| Level 2 | .00 | .92 | ||||
| PD Status | .20 | .03 | .00 | .84 | ||
| MWS | .40 | .05 | .00 | .70 | ||
| Level 3 | .05 | < .05 | ||||
| PD Status × MWS | 2.13 | .24 | .08 | < .05 | ||
| Post-Challenge SUDS | .53 | < .01 | ||||
| Level 1 | .51 | .< .001 | ||||
| Negative Affectivity | 1.89 | .21 | .03 | .06 | ||
| Baseline SUDS | 4.89 | .57 | .22 | < .001 | ||
| Cigs/day | .55 | .06 | .00 | .59 | ||
| Sex | .37 | .04 | .00 | .71 | ||
| Level 2 | .01 | .77 | ||||
| PD Status | .31 | .04 | .00 | .76 | ||
| MWS | −.58 | −.08 | .00 | .57 | ||
| Level 3 | .01 | .32 | ||||
| PD Status × MWS | 1.00 | −.10 | .01 | .32 | ||
Note: DSQ Mean = Diagnostic Sensations Questionnaire composite score (Sanderson, Rapee, & Barlow, 1988, 1989); Post-Challenge SUDS (Subjective Units of Distress Scale rating [Wolpe, 1959] post-challenge); Negative Affectivity (BFI-Neuroticism = Big Five Inventory – Neuroticism Subscale (John & Srivastava, 1999); Baseline SUDS (Subjective Units of Distress Scale rating [Wolpe, 1959] upon arrival to Session 2); MWS (Minnesota Withdrawal Scale; Hughes & Hatsukami, 1986); PD Status × MWS = Interaction between PD status and Nicotine Withdrawal.
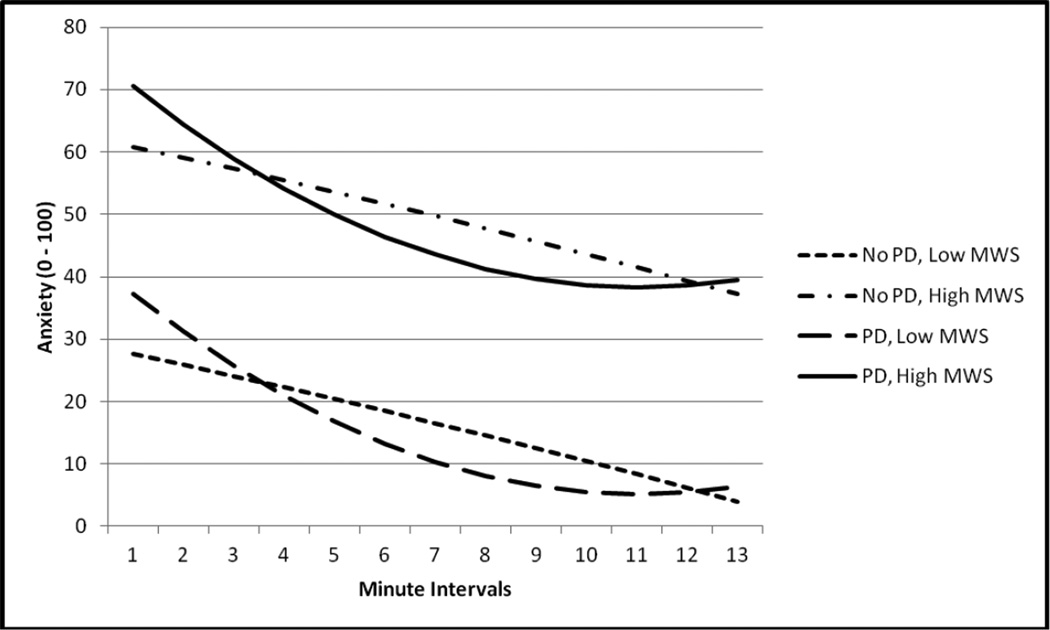
With regard to recovery from the challenge as indexed by SUDS, there was a significant effect for intercept (t(1, 688) = 11.54, p < .01), which indicates a 'true baseline SUDS' value of 44.37. There also was a fixed effect for MWS (t(1, 54) = 4.67, p < .01), indicating a 3.00 increase in SUDS for a one unit increase in nicotine withdrawal across participants. In addition, there was both a significant linear (Time × PD Status; t(1, 688) = −2.27, p < .05) and quadratic effect for PD status (Time2 × PD Status; t(1, 688) = 2.53, p < .05) across time. A reduced model was then fit to the data, in which non-significant factors were removed, to better understand the role of PD on SUDS across time (Table 5). This reduced model was then depicted graphically (see Figure 2) in order to better understand the relationships between PD status across time. SUDS trajectory for those with PD evidenced an initial instantaneous decrease of 4.59 in SUDS, however, because PD status also interacted with the quadratic time term (which w as positive), the magnitude of the instantaneous negative effect of time on SUDS decelerated at each successive time point among those with PD.
Table 5.
Prediction of Response to, and Recovery from, Challenge
| Dependent Variable: SUDS | B | df | t | p |
|---|---|---|---|---|
| Intercept | 44.37 | 688 | 11.54 | < .01 |
| PD Status | 9.18 | 54 | 1.42 | .16 |
| MWS | 3.00 | 54 | 4.67 | < .01 |
| PD Status × MWS | 0.13 | 54 | .12 | .91 |
| Time | −1.74 | 54 | 1.21 | .15 |
| Time × PD Status | −4.59 | 688 | −2.27 | < .05 |
| Time × MWS | −.01 | 688 | −.06 | .95 |
| Time × PD Status × MWS | −.07 | 688 | −.21 | .83 |
| Time2 | −.01 | 688 | −.27 | .79 |
| Time2 × PD Status | .34 | 688 | 2.53 | < .05 |
| Time2 × MWS | .00 | 688 | −.44 | .66 |
| Time2 × PD Status × MWS | .01 | 688 | .51 | .62 |
|
Prediction of Response to, and Recovery from, Challenge, Reduced SUDS Model | ||||
| Dependent Variable: SUDS | B | df | t | p |
| Intercept | 44.24 | 692 | 11.65 | < .01 |
| PD Status | 9.70 | 55 | 1.53 | .13 |
| MWS | 2.78 | 55 | 6.65 | < .01 |
| Time | −1.73 | 55 | 1.21 | .14 |
| Time × PD Status | −4.69 | 692 | −2.39 | < .05 |
| Time2 | −.02 | 692 | −.23 | .81 |
| Time2 × PD Status | .34 | 692 | 2.63 | < .01 |
Note: SUDS - Subjective Units of Distress Scale (Wolpe, 1958); MWS (Minnesota Withdrawal Scale; Hughes & Hatsukami, 1986); Time = Linear effect of time; Time2 = Quadratic effect of time.
Figure 2.
Graphical Depiction of Two-Way Interactions between PD Status and Time Predicting SUDS.
Note. MWS = Nicotine withdrawal Score (Minnesota Withdrawal Scale; Hughes & Hatsukami,1986).
Discussion
The present investigation examined whether nicotine withdrawal symptoms elicited by a 12-hour smoking deprivation period (versus no deprivation) among daily smokers resulted in greater fearful responding to, and delayed recovery from, laboratory-induced bodily sensations among those with PD compared to those without PD. With regard to post-challenge panic attack symptoms, the interaction between PD and nicotine withdrawal significantly predicted total panic attack symptoms post-challenge (5% unique variance). The form of the interaction inidcated that individuals experiencing high, rather than low, levels of nicotine withdrawal, evidenced the greatest panic attack symptoms post-challenge. This finding is generally line with integrative theoretical models of smoking-panic comorbidity that suggest that the combination of PD and elevated levels of nicotine withdrawal symptoms may place such daily smokers at an increased risk for panic-relevant responding to bodily sensations (Zvolensky & Bernstein, 2005). Namely, these persons may tend to catastrophize about and experience panic-relevant sensations as more intense, potentially contributing to the maintenance of panic among this group of smokers. Interestingly, when looking at participants without PD, the form of the interaction indicates that post-challenge panic attack symptoms were greater among the low withdrawal group. One possible explanation of this finding is that those in the non-PD group may be relatively naïve to the experience of panic. Without experiencing withdrawal, which may have provided an explanation for some of their symptoms, these participants may have interpreted the sensations as particularly threatening or uncomfortable. It also may also be possible that those assigned to the withdrawal group, and theoretically experiencing the most intense levels of withdrawal, may have attributed some of their uncomfortable sensations to nicotine withdrawal, rather than panic.
Inconsistent with prediction, the main effects of PD status and nicotine withdrawal did not significantly predict post-challenge panic attack symptoms or anxiety reactivity. Likewise, there was no significant interaction for self-reported anxiety reactivity. These findings were surprising given past empirical work suggesting (a) persons with PD respond with greater subjective anxiety in response to a biological challenge compared to other nonclinical and other psychiatric, populations (Zvolensky & Eifert, 2000); and (b) acute nicotine withdrawal (e.g., withdrawal symptoms elicited by 2 hours or less of smoking deprivation) significantly predict increased risk for greater subjective anxiety to bodily sensations in the laboratory (Marshall et al., 2009; Zvolensky et al., 2005). There are two key factors that may help explain the present results. In regard to PD, the current study is a methodological departure from this past work in that it expressly sampled daily smokers with and without PD whereas past studies did not. Thus, it is possible that by ‘equating’ for tobacco use across the current sample, some of the subjective anxiety variably typically attributed to PD status may have been unintentionally minimized (Ziedonis et al., 2008). Additionally, in terms of nicotine withdrawal, the present study utilized a longer period of smoking deprivation than past work (e.g., 12 hours versus 2 hours; Marshall et al., 2009). Some work suggests longer compared to shorter periods of smoking deprivation may not be related to as dramatic increases in risk for anxious and fearful responding (Abrams et al., 2008; Piper & Curtin, 2006; Vujanovic, Marshall-Berenz, Beckham, Bernstein, & Zvolensky, 2010; Vujanovic & Zvolensky, 2009). Based upon these results, it may be useful to complete a dose-response study of smoking deprivation (e.g., 2 hours versus 4 hours versus 12 hours of smoking deprivation) in terms of anxious and fearful responding to bodily sensations and other anxiety-provoking stressors.
With regard to recovery from the challenge, there was a significant main effect for nicotine withdrawal predicting an increase in SUDS. This finding suggests that those with elevated levels of nicotine withdrawal evidenced significantly greater levels of anxiety upon initiation of the laboratory session. Thus, even though it is possible that shorter durations of smoking deprivation may trigger greater anxiety reactivity (Abrams et al., 2008), the 12-hour period of deprivation in the present study did, in fact, elicit significant elevations in anxiety. No significant effect was evident for PD status, suggesting the singular presence of this clinical condition was not related to elevations in anxiety in the context of smoking deprivation. Notably, there was, as expected, a significant interaction between PD and both linear and quadratic time terms. As seen in Figure 2, these interactive relations suggest an immediate decrease in anxiety for all four groups following challenge termination, with a significantly more dramatic decrease observed among individuals with PD. However, this trajectory decelerated throughout recovery. It is possible that the expected termination of the CO2 among persons with PD may have served to decrease anxiety upon challenge termination (i.e., these persons were relieved); whereas these same individuals later evidenced slower emotional recovery (Zvolenskyet al., 2004a). Thus, the combination of greater nicotine withdrawal and PD may contribute to a lesser ability to effectively recover from bodily stress over the long-term. Alternatively, it is possible that those with PD simply recovered more quickly from the challenge than the non-PD counterparts. Similar to the explanation stated above, it is possible that the novelty of the sensations among those in the non-PD group may have resulted in slower recovery. Although not a primary focus of the study, it is noteworthy that the current sample evidenced high levels of concurrent substance use (past 30 day marijuana use = 55.3%; AUDIT score [Babor et al., 1992], M = 8.88, SD = 5.10). These findings are in line with observations that substance use multicomorbidity may be a common phenomenon among certain high-risk samples (e.g., those with psychiatric disorders; Ziedonis et al., 2008). Furture work could usefully begin to isolate the unique and general effects of polysubstance use on anxiety and its disorders, and in contrast, the role of anxiety psychopathology in regard to the etiology of polysubstance use.
There are several limitations of the present investigation worth comment. First, the nature of the sample recruited resulted in a variety of factors, including substance use, which may have directly (e.g., via proximity of use) or indirectly (e.g., via association between propensity for substance use and general negative affectivity) impacted challenge responding. Second, participants reported smoking a range of seven to 50 cigarettes on average per day (M = 19.72, SD = 7.99) as well as variable, and on average relatively low, levels of nicotine dependence (M = 3.93, SD = 1.72). Such variability may have impacted the effectiveness of the 12-hour smoking deprivation period employed. Future work could be improved by investigating the present research questions among a less variable sample of heavy smokers. Finally, the permission of comorbid psychopathology in the non-PD group may have resulted in less variability, with regard to a variety of characteristics, between the PD and non-PD groups, than initially planned. Specifically, although the two groups differed significantly with regard to current psychopathology, they did not significantly differ in regard to history of past diagnoses. Thus, there may have been less differentiation between these groups with regard to general mental health.
Overall, the current investigation provides a novel empirical perspective on the main and interactive effects of nicotine withdrawal and PD on panic responsivity among daily smokers. Findings from this study help elucidate the role of nicotine withdrawal in terms of exacerbating anxious and fearful responding to bodily cues among daily smokers with and without PD. This line of inquiry can shed light on the etiology of panic psychopathology among smokers and ultimately inform the development of novel specialized interventions for this difficult-to-treat population.
Acknowledgments
This research was supported by a grant (1 F31 DA024919-03) awarded to Ms. Leyro (1 F31 MH080453-01A1).
Footnotes
Participants prescribed benzodiazepines on a PRN (pro re nata: “as the circumstances arise”/as needed) basis were included in the study upon agreement not to use for at least twelve hours prior to both study visits, allowing adequate time for their effects to wear off. Per self-report, these participants were not using benzodiasepines daily and reported using them no more frequently than several times a month. The instruction to not use within 12 hours of study participation was given because benzodiazepines may reduce both physiological and psychological reactivity to distressing events (Bailey, Papadopoulos, Seddon, & Nutt, 2009), and may therefore interfere with the main study hypotheses.
Of the 77 participants eligible for the CO2 appointment, three did not attend. Of the remaining 74, 29 met criteria for PD, with 36 randomized to SAU and 38 to SMD. Of the 36 assigned to SAU, 5 were excluded from analyses due to equipment failure indicating the CO2 manipulation was unsuccessful (n = 3), failure to retain SAU status (n = 1), and use of a contraindicated substance prior to participation (n =1). Of those assigned to SMD, data for 11 was excluded due to equipment failure resulting in CO2 manipulation was unsuccessful (n = 6), failure to maintain SMD condition (n = 3), and use of a contraindicated substance prior to participation (n = 2). This resulted in a total of 58 participants with usable data for the current analyses.
Contributor Information
Teresa M. Leyro, University of California, San Francisco
Michael J. Zvolensky, University of Houston
References
- Abrams K, Zvolensky MJ, Dorflinger L, Galatis A. Fear reactivity to bodily sensations among heavy smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2008;16:230–239. doi: 10.1037/1064-1297.16.3.230. [DOI] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT- Alcohol Use Disorders Identification test: Guidelines for use in primary health care. Geneva: World Health Organization; 1992. [Google Scholar]
- Bailey JE, Papadopoulos AA, Seddon KK, Nutt DJ. A comparison of the effects of a subtype selective and non-selective benzodiazepine receptor agonist in two CO2 models of experimental human anxiety. Journal of Psychopharmacology. 2009;23:117–122. doi: 10.1177/0269881108089603. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychology Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Schmidt NB, Sachs-Ericcson N. Developmental course(s) of lifetime cigarette use and panic attack comorbidity: An equifinal phenomenon? Behavior Modification. 2007;31:117–135. doi: 10.1177/0145445506295056. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ. An evaluation of the nature of marijuana use and its motives among young adult active users. American Journal on Addictions. 2009;18:409–416. doi: 10.3109/10550490903077705. [DOI] [PubMed] [Google Scholar]
- Breslau N, Klein DF. Smoking and panic attacks: An epidemiologic investigation. Archives of General Psychiatry. 1999;56:1141–1147. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2011 http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Dawson JF, Richter AW. Interpreting interaction effects. 2006 Retrieved from http://www.jeremydawson.co.uk/slopes.htm. [Google Scholar]
- Farrell M, Howes S, Bebbington P, et al. Nicotine, alcohol and drug dependence and psychiatric comorbidity: results of a national household survey. British Journal of Psychiatry. 2001;179:432–437. doi: 10.1192/bjp.179.5.432. [DOI] [PubMed] [Google Scholar]
- Fagerström K. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV non-patient edition (SCID-N/P, Version 2.0) New York: Biometrics Research Department; 1994. [Google Scholar]
- Forsyth JP, Eifert GH, Canna MA. Evoking analogue subtypes of panic attacks in a nonclinical population using carbon dioxide-enriched air. Behaviour Research and Therapy. 2000;38:559–572. doi: 10.1016/s0005-7967(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Jensen RA, Meliska CJ. A system for administering quantified doses of tobacco smoke to human subjects: Plasma nicotine and filter pad validation. Pharmacology, Biochemistry and Behavior. 1988;31(4):905–908. doi: 10.1016/0091-3057(88)90403-0. [DOI] [PubMed] [Google Scholar]
- Harrington P, Schmidt NB, Telch MJ. Prospective evaluation of panic potentiation following 35% CO2 challenge in a non-clinical sample. American Journal of Psychiatry. 1996;153:823–825. doi: 10.1176/ajp.153.6.823. [DOI] [PubMed] [Google Scholar]
- Hays R, Marshall G, Wang E, Sherbourne C. Four-year cross-lagged associations between physical and mental health in the medical outcomes study. Journal of Consulting and Clinical Psychology. 1994;62:441–449. doi: 10.1037//0022-006x.62.3.441. [DOI] [PubMed] [Google Scholar]
- Hayward C, Killen JD, Taylor C. Panic attacks in young adolescents. The American Journal of Psychiatry. 1989;146:1061–1062. doi: 10.1176/ajp.146.8.1061. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Kalant FB, Sellers, Vingilis EMER, editors. Research advances in alcohol and drug problems. Vol. 10. Plenum publishing; 1990. [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biological Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee B, Wittchen H, Stein MB, Hofler M, Lieb R. Smoking increases the risk of panic: Findings from a prospective community study. Archives of General Psychiatry. 2003;60(7):692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacological Biochemistry and Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cutoff levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- John OP. Towards a taxonomy of personality descriptors. In: Buss DM, Cantor N, editors. Personality psychology: Recent trends and emerging directions. New York: Springer-Verlag; 1989. pp. 261–271. [Google Scholar]
- John OP, Srivastava S. The Big Five trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of Personality: Theory and Research. 2nd ed. New York: Guilford; 1999. pp. 102–138. [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA: Journal of the American Medical Association. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychology of Addictive Behaviors. doi: 10.1037/a0024133. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal of Addiction. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Marshall EC, Johnson K, Bergman J, Gibson LE, Zvolensky MJ. Anxiety sensitivity and panic reactivity to bodily sensations: Relation to quit-day (acute) nicotine withdrawal symptom severity among daily smokers making a self-guided quit attempt. Experimental and Clinical Psychopharmacology. 2009;17:356–364. doi: 10.1037/a0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zvolensky MJ. Smoking behaviors across anxiety disorders. Journal of Anxiety Disorders. 2004;18:7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Morabia A, Bernstein MS, Curtin F, Berode M. Measurement of carbon monoxide and salivary thiocyanate. Preventive Medicine. 2001;32:82–88. doi: 10.1006/pmed.2000.0779. [DOI] [PubMed] [Google Scholar]
- Mullane JC, Stewart SH, Rhyno E, Steeves D, Watt M, Eisner A. Anxiety, sensitivity and difficulties with smoking cessation. In: Columbus AM, Columbus AM, editors. Advances in psychology research. Vol 54. Hauppauge, NY: Nova Science Publishers; 2008. pp. 141–155. [Google Scholar]
- Ormel J, VonKorff M, Ustun T, Pini S. Common mental disorders and disability across cultures: Results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA: Journal of the American Medical Association. 1994;272:1741–1748. doi: 10.1001/jama.272.22.1741. [DOI] [PubMed] [Google Scholar]
- Patten C, Martin J. Measuring tobacco withdrawal: A review of self-report questionnaires. Journal of Substance Abuse. 1996;8:93–113. doi: 10.1016/s0899-3289(96)90115-7. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerstrom Tolerance Questionnaire (FTQ) with the Fagerstrom Test for Nicotine Dependence (FTND) in a clinical sample. Addictive Behaviors. 1994;19:307–317. doi: 10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: An analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115:96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl R, Yeragani VK, Balon R, Lycaki H, McBride R. Smoking in patients with panic disorder. Psychiatry Research. 1992;43:253–262. doi: 10.1016/0165-1781(92)90058-b. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström Tolerance Questionnaire and the Fagerström Test for Nicotine Dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statisticalcomputing. Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.r-project.org/ [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. Panic induction via inhalation of 5.5% CO2 enriched air: A single subject analysis of psychological and physiological effects. Behaviour Research and Therapy. 1988;26:333–335. doi: 10.1016/0005-7967(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Scheftner W, Endicott J. Luke’s Medical Center. Vol. 1753. Chicago, IL: West Congress Parkway; 1984. Medical history form II. Available from Dr. Scheftener at Rush-Presbyterian-St; p. 60612. [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Papp LA. Multicenter collaborative Panic Disorder Severity Scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Wang T. Reliability and validity of the panic disorder severity scale: Replication and extension. Journal of Pyschiatric Research. 2001;35:293–296. doi: 10.1016/s0022-3956(01)00028-0. [DOI] [PubMed] [Google Scholar]
- State of Vermont Department of Health. 2008 Vermont population estimates. 2008 Retrieved January 30, 2011 from http://healthvermont.gov/research/documents/RACENOTE08.pdf.
- Vujanovic AA, Marshall-Berenz EC, Beckham JC, Bernstein A, Zvolensky MJ. Posttraumatic stress symptoms and cigarette deprivation in the prediction of anxious responding among trauma- exposed smokers: A laboratory test. Nicotine & Tobacco Research. 2010;12:1080–1088. doi: 10.1093/ntr/ntq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Zvolensky MJ. Anxiety sensitivity, acute nicotine withdrawal symptoms, and anxious and fearful responding to bodily sensations: A laboratory test. Experimental and Clinical Psychopharmacology. 2009;17:181–190. doi: 10.1037/a0016266. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Stanford, CA: Stanford University Press; 1958. [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Riley WT. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Baker KM, Leen-Feldner EW, Bonn-Miller MO, Feldner MT, Brown RA. Anxiety sensitivity: Association with intensity of retrospectively-rated smoking-related withdrawal symptoms and motivation to quit. Cognitive Behaviour Therapy. 2004c;33:114–125. doi: 10.1080/16506070310016969. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bernstein A. Cigarette smoking and panic psychopathology. Current Directions in Psychological Science. 2005;14:301–305. [Google Scholar]
- Zvolensky MJ, Eifert GH. A review of psychological factors/processes affecting anxious responding during voluntary hyperventilation and inhalations of carbon dioxide-enriched air. Clinical Psychology Review. 2000;21:375–400. doi: 10.1016/s0272-7358(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH, Lejuez CW, McNeil DW. The effects of offset control over 20% CO2-enriched air on anxious responding. Journal of Abnormal Psychology. 1999;108:624–632. doi: 10.1037//0021-843x.108.4.624. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, McLeish AC. Smoking and panic attacks, panic disorder, and agoraphobia: A review of the empirical literature. Clinical Psychology Review. 2005;25:761–789. doi: 10.1016/j.cpr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Leen-Feldner EW, Feldner MT, Bonn-Miller WO, Lejuez CW, Kahler C, Stuart G. Emotional responding to biological challenge as a function of panic disorder and smoking. Journal of Anxiety Disorders. 2004b;18:19–32. doi: 10.1016/j.janxdis.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Panic attack history and smoking cessation: An initial examination. Addictive Behaviors. 2004a;29:825. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, McCreary BT. The impact of smoking on panic disorder: An initial investigation of a pathoplastic relationship. Journal of Anxiety Disorders. 2003;17:447–460. doi: 10.1016/s0887-6185(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Stewart SH. Panic disorder and smoking. Clinical Psychology: Science and Practice. 2003b;10:29–51. [Google Scholar]