Abstract
Objective
To evaluate mortality and short-term outcomes in very low birth weight infants admitted to the tertiary neonatal intensive care unit, Istanbul, Turkey.
Methods
Study data were recorded prospectively from January 1, 2010, to December 31, 2010. The clinical findings in neonates with birth weights <1000g were compared with infants with birth weights of between 1000g and 1499g.
Findings
In the present study, survival rates were 40% and 86.2% for infants weighing <1000g and 1000g to 1499g, respectively. There was no difference between males and females with respect to mortality (P>0.05). The mean (±standard deviation) birth weight was 985.6±150.15 g and mean gestational age was 27.5±2.04 weeks. The antenatal steroid rate was 37.2%, and the Cesarean section rate was 73%. Respiratory distress syndrome was diagnosed in 89% of the infants, with a 69% surfactant administration rate. Severe intracranial hemorrhage (IVH) (grade >II) was 14%. Grade 4 periventricular leukomalacia was 10%. Twelve (24%) infants had evidence of bronchopulmonary dysplasia (BPD). Retinopathy of prematurity (stage >II) was 4%. The correlation between ROP rate and need for ventilation therapy was present (r=0.52). Proven necrotizing enterocolitis (stage >2) was not observed. Patent ductus arteriosus (PDA) was diagnosed in 67% of the neonates. BPD, IVH, and PDA were statistically higher in neonates with a birth weight <1000g.
Conclusion
Survival rate of VLBW infants increased with increasing BW. Sex was not a risk factor for mortality. The need for ventilatory therapy may be an important risk factor for ROP in infants <1500g.
Keywords: Mortality, Morbidity, Neonate, Very Low Birth Weight
Introduction
Birth weight (BW) and gestational age (GA) are two of the most important factors that predict the short- and long-term quality of life of neonates. Low BW and GA are linked to morbidity and mortality during the newborn period[1–5]. Premature births result in newborn mortality and morbidity rates of 70% and 75%, respectively[6]. Very low birth weight (VLBW, <1500 g) infants are a unique group of patients in the neonatal intensive care unit (NICU). VLBW infants, especially those <1000 g (extremely low birth weight [ELBW]) are so physiologically immature that they are extremely sensitive to small changes in respiratory management, blood pressure, fluid administration, nutrition and virtually all other aspects of care. The immature biological and physiological characteristics of these premature infants often lead to pathologies such as respiratory distress syndrome (RDS), symptomatic patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL) and bronchopulmonary dysplasia (BPD), which affect prognosis. Moreover, these infants are also deemed to be a high-risk group for poor neurodevelopmental outcome[7].
Advanced technology in NICU facilities, the availability of sophisticated and experienced NICU staff, application of proper neonatal mechanical ventilation modes, surfactant therapy, and the advent of antenatal and postnatal corticosteroid treatments have increased the survival rates of neonates born with BW <1500 g up to 95%[8]. However, the elevated survival rates have also elicited an increase in morbidity rates.
In the present study, we aimed to determine the mortality and morbidity rates of VLBW infants admitted to a NICU, and to define associated maternal risk factors.
Subjects and Methods
The present study was performed at the Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey, between January 1, 2010, and December 31, 2010. This is a multidisciplinary tertiary care hospital with three delivery room Resuscitaires equipped with oxygen blenders and an Autobreath system, 26 level III NICU beds and two portable ventilators with transport incubator. Sixty-three VLBW infants were followed in the NICU during the study period (27 infants had a BW <1000 g and 36 infants were 1000 g to 1499 g), 49 of whom were included in the present study. The remainder (n=14) were excluded from the study due to incomplete clinical data. Infants with BW ≥1500g were not included in the study. Ethical approval for the study was obtained from the hospital's Ethics Committee.
Study design
The neonates were categorized into two groups: BW <1000 g (group A) and BW 1000 g to 1499 g (group B). The following data were acquired from their medical records and recorded electronically: sex, GA, BW, height, head circumference, antenatal steroid therapy, Apgar scores at 1 min and 5 min of life, need of active delivery room resuscitation, clinical diagnoses such as RDS, culture-positive sepsis, meningitis, intrapulmonary hemorrhage, indirect hyperbilirubinemia, ventilatory support (durations of mechanical ventilation, nasal continuous positive airway pressure (CPAP) and free oxygen support) and surfactant therapy, morbidities such as symptomatic PDA, NEC, IVH, PVL, BPD and ROP, duration of total parenteral nutrition, length of hospital stay, discharge status, and maternal demographic and clinical information (mother's age, pregnancy records, presence of any obstetrical disease). GA was based on the last menstrual period and/or obstetrical ultrasound performed before 22 weeks of gestation, and confirmed by the two neonatologists using the New Ballard Score[9].
Study protocols
Based on the need for oxygen at postnatal day 28, plus oxygen requirement at postnatal day 36 for neonates with a GA <32 weeks, and oxygen requirement at postnatal day 56 for neonates with a GA ≥32 weeks, BPD was classified as mild (room air), moderate (<30% oxygen), or severe (≥30% oxygen with need for mechanical ventilation)[10]. ROP diagnosis was established following examination of the fundus, based on the International Classification of Retinopathy[11]. The same ophthalmologist performed all examinations. NEC diagnosis was achieved by the modified Bell classification, and based on the clinical and radiographic findings as well as the laboratory results[12]. PVL (necrosis of white matter in a characteristic distribution) was diagnosed by examining the postnatal transient periventricular echodensities persisting for ≥7 days (Grade 1), transient periventricular echodensity evolving into small, localised fronto-parietal cysts (Grade 2), periventricular echodensities evolving into extensive periventricular cystic lesions (Grade 3), and densities extending into the deep white matter evolving into extensive cystic lesions (Grade 4) in the cerebral white matter on cranial ultrasonography, and confirmed by cranial magnetic resonance imaging[13, 14].
Symptomatic PDA diagnosis was reached based on serial echocardiographic examinations performed by the same cardiologist at 24 to 96 postnatal hours of life and during the first two weeks of life, as needed. Germinal matrix hemorrage (GMH)/IVH was diagnosed based on the Papile staging of the transfontanelle ultrasonographic assessments performed by the same radiologist at 2 and 7 days as well as during discharge, and by computed tomography in some cases[15]. Grade I consists of subependymal germinal matrix hemorrhage with no or minimal IVH. Grade II is defined as subependymal and intraventricular hemorrhage without ventricular dilatation. Grade III is the combination of subependymal and intraventricular hemorrhage with ventricular dilatation by clot. Grade IV is the periventricular hemorrhagic infarction (PVHI), as a complication of the germinal matrix hemorrhage [14, 16].
Statistical analysis
The data were analyzed by descriptive statistical methods (mean, standard deviation, frequency distribution), whereas the groups were compared by the two-sample t test and chi-squared test. Number Cruncher Statistical System 2007 software (Kaysville, Utah, USA) was used for the statitical analyses. Survival analyses were performed using the Kaplan-Meier (log-rank) survival test. Statistical significance was set at P<0.05.
Findings
Forty-nine infants were included in the present study. Twenty (40%) of the infants weighed <1000 g and 29 weighed between 1000 g and 1499 g. Demographic and clinical characteristics of the groups are summarized in Table 1. Statistically significant differences were found between the groups with regard to GA, BW, birth length, head circumference, mode of delivery, history of active delivery room resuscitation at birth, Apgar scores at 1 min and 5 min, application of nasal CPAP, ventilatory therapy and need for surfactant therapy (P<0.05). While there was a significant difference between the groups with regard to RDS, there was no difference in terms of sepsis, hyperbilirubinemia, or pulmonary hemorrhage. GA was significantly lower in group A, but there was no significant difference relative to antenatal steroid therapy, sex, referral point to NICU, nasal CPAP duration, duration of total parenteral nutrition, age at NICU admission, and length of NICU stay. The Cesarean section (CS) rate was 73% (36 of 49 infants) and it was observed to be more prevalent in group B. Surfactant therapy was given to 95% of group A and 51.7% of group B.
Table 1.
Demographic and clinical characteristics of the study population
| Parameter | Weight <1000 g (n=20) | Weight 1000-1499 g (n=29) | P value | |
|---|---|---|---|---|
| Gestational age (wks) [mean (SD)] | 25.0 (2.2) | 30.1 (1.9) | <0.001 | |
| Sex [n (%)] | Male | 11 (55) | (44.8) | 0.4 |
| Female | 9 (45) | 16 (55.2) | ||
| Birth weight (g) [mean (SD)] | 750.5 (165.6) | 1220.7 (134.7) | <0.001 | |
| Length (cm) [mean (SD)] | 32.6 (2.1) | 37.3 (1.94) | <0.001 | |
| Head circumference (cm) [mean (SD)] | 23.8 (2.5) | 27.1 (1.26) | <0.001 | |
| Delivery mode [n (%)] | Vaginally | 10 (50) | 3 (10.3) | 0.002 |
| C-section | 10 (50) | 26 (89.7) | ||
| Antenatal steroid [n (%)] | 4 (20) | 5 (17.2) | 0.8 | |
| Resuscitation [n (%)] | 19 (95) | 18 (62.1) | 0.008 | |
| Apgar scores at 1 min [mean (SD)] | 3.8 (1.36) | 5.8 (2.2) | 0.001 | |
| Apgar scores at 5 min [mean (SD)] | 7.5 (1.1) | 8.4 (1.2) | 0.01 | |
| Nasal Continuous Positive Airway Pressure [n (%)] | Post-extubation | 5 (25) | 13.0 (44.8) | 0.02 |
| Delivery-room | 0 (0) | 5.0 (17.2) | ||
| Ventilatory therapy, days [mean (SD)] | 28.7 (5.4) | 10.1 (2.8) | 0.01 | |
| Surfactant therapy [n (%)] | 19 (95) | 15 (51.7) | 0.001 | |
| Referral point to NICU [n (%)] | Delivery room | 20 (100) | 27 (93.1) | 0.2 |
| Other hospital | 0 (0) | 2 (6.9) | ||
| Age at NICU admission h [mean (SD)] | 0(0) | 1.3 (6.7) | 0.4 | |
| NCPAP (days) [mean (SD)] | 6.0 (5.7) | 4.94 (2.9) | 0.6 | |
| Respiratory Distress Syndrome [n (%)] | 20 (100) | 24 (82.8) | 0.04 | |
| Sepsis [n (%)] | 12 (60) | 24 (82.8) | 0.07 | |
| Indirect hyperbilirubinemia [n (%)] | 13 (65) | 22 (75.9) | 0.4 | |
| Pulmonary hemorrhage [n (%)] | 1 (5) | 1 (3.4) | 0.8 | |
| TPN (days) [mean (SD)] | 23.9 (26.2) | 17.7 (9.5) | 0.2 | |
| NICU stay (days) [mean (SD)] | 56.4 (53.0) | 47.6 (23.8) | 0.5 | |
C-section: Cesarean section; NICU: Neonatal Intensive Care Unit; TPN: Total Parenteral Nutrition
Maternal risk factors (maternal age, antenatal follow-up, systemic maternal disease, maternal disease in pregnancy such as preeclampsia, eclampsia, HELLP syndrome, abruptio placentae and urinary tract infection) are presented in Table 2. There was no significant difference between the groups with respect to maternal risk factors.
Table 2.
Maternal factors influencing neonatal outcome
| Parameter | Weight <1000 g [n (%)] (n=20) | Weight 1000-1499g [n (%)] (n=29) | P value |
|---|---|---|---|
| Maternal age, years [mean (SD)] | 28.7 (6.0) | 29.2 (6.4) | 0.7 |
| Antenatal follow-up | 18 (90) | 28 (96.5) | 0.7 |
| Systemic disease | 5 (25) | 8 (27.6) | 0.8 |
| Maternal disease in pregnancy | 13 (65) | 20 (69) | 0.8 |
| Preeclampsia | 2 (10) | 10 (34.5) | 0.05 |
| Eclampsia | 0 (0) | 1 (3.4) | 0.4 |
| HELLP syndrome | 4 (20) | 1 (3.4) | 0.06 |
| Abruptio placentae | 1 (5) | 2 (6.9) | 0.8 |
| Urinary tract infection | 5 (25) | 8 (27.6) | 0.8 |
| Oligohydramnios | 1 (5) | 6 (20.7) | 0.1 |
| Preterm premature rupture of membranes | 3 (15) | 4 (13.8) | 0.9 |
HELLP: Hemolytic anemia, Elevated Liver enzymes and Low Platelet count
Neonatal morbidities of the study groups admitted to the NICU are presented in Table 3. While BPD and IVH were diagnosed significantly more often in the group A, PDA was diagnosed significantly more often in the group B (P<0.05). There were no differences in the need for oxygen therapy at the 28th day, ROP, laser therapy, NEC and PVL (P>0.05).
Table 3.
Neonatal morbidities in infants with a birth weight <1000g and 1000-1499g
| Parameter | Weight <1000 g n (%) (n=20) | Weight 1000-1499 g n (%) (n=29) | P value | |
|---|---|---|---|---|
| Need for oxygen at 28 th day | 7 (35) | 6 (20) | 0.4 | |
| Bronchopulmonary dysplasia | Mild | 1 (5) | 5 (17.2) | 0.04 |
| Moderate | 2 (10) | 0 (0) | ||
| Severe | 4 (20) | 1 (3.4) | ||
| Retinopathy of prematurity | Grade 1 | 5 (25) | 10 (34.5) | 0.9 |
| Grade 2 | 2 (10) | 2 (6.9) | ||
| Grade 3 | 1 (5) | 1 (3.4) | ||
| Laser therapy | 3 (15) | 4 (13.8) | 0.9 | |
| Intraventricular hemorrhage | Total | 11 (55) | 4 (13.8) | 0.002 |
| Grade 1 | 4 (20) | 3 (10.3) | ||
| Grade 2 | 1 (5) | 0 (0) | ||
| Grade 3 | 1 (5) | 1 (3.4) | 0.01 | |
| PVHI | 5 (25) | 0 (0) | ||
| Necrotising enterocolitis | (Grade 1) | 6 (30) | 3 (10.3) | 0.08 |
| Patent ductus arteriosus | 10 (50) | 23 (79.3) | 0.03 | |
| Periventricular leukomalacia | Grade 1 | 1 (5) | 27 (93.1) | 0.09 |
| Grade 4 | 3 (15) | 2 (6.9) | ||
PVHI: Periventricular hemorrhagic infarction
The correlation between ROP rate and need for ventilation therapy was present (r=0.52), whereas the correlations between ROP rate and low APGAR score, symptomatic PDA, sepsis, and red blood cell transfusions were absent.
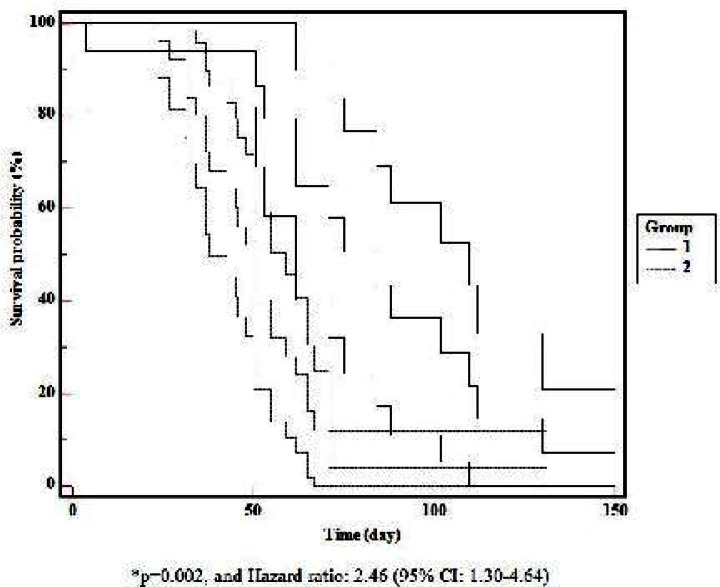
When mortality rate was evaluated relative to the BW in the entire study population, it was found to be 60% (12 of 20) in group A, 13.8% (4 of 29) in group B. Mortality-related neonatal morbidities are presented in Table 4. There was a significant difference between the BWs relative to mortality (P=0.002, and Hazard ratio: 2.46, 95% CI: 1.30-4.64) (Fig. 1). Mortality was found to have a negative correlation with BW (r=-0.48). The mortality rate was significantly higher in the group A (P<0.05). Although the mortality rate among male infants was 41% in the present study, there was no difference between males and females with respect to mortality (P>0.05).
Table 4.
Mortality-related morbidities in neonates
| Parameter | Weight <1000 g (n=12) | Weight 1000-1499 g (n=4) |
|---|---|---|
| Respiratory distress syndrome | 12 (100) | 4 (100) |
| Necrotising enterocolitis | 2 (16.7) | none |
| Patent ductus arteriosus | 5 (41.7) | 1 (25) |
| Intraventricular hemorrhage | 7 (58) | none |
| Periventricular leukomalacia | 2 (16.7) | none |
| Sepsis | 4 (33.3) | 4 (100) |
| Pulmonary Hemorrhage | 1(8.3) | 1 (25) |
| Bronchopulmonary dysplasia | 1 (8.3) | none |
| Retinopathy of prematurity | none | none |
Data presented n (%) unless otherwise indicated
Fig. 1.
Comparison of Kaplan-Meier survival curves (long-rank test) in infants with birth weight of <1000g and 1000-1499g. Mortality was found to have a negative correlation with birth weight.
Discussion
Increasing numbers of very preterm and VLBW infants are surviving because of advances in both perinatal and neonatal care over the past two decades[17]. The present study compared survival to hospital discharge and short-term morbidity for all VLBW infants who werelive born and admitted for neonatal intensive care in a tertiary care hospital in 2010. In many cases, obstetrician-gynecologists teamed with neonatologists and contributed to saving ELBW infants, by delivering them via CS, thereby favorably influencing the outcome for the newborn[18]. Minguez-Milio et al[19] reported that delivery mode did not affect survival; however, delivery via CS was associated with lower morbidity and better prognosis for neurodevelopmental long-term outcome in ELBW infants. Jakuskiene et al[20] showed that delivery via CS increased the survival rate. In our study, the CS rate was 73% and was observed to be used more frequently in infants with a BW of 1000 g to1499 g. Improvements in current obstetrical care contribute to the improved outcome of neonates, with a larger number of infants receiving antenatal corticosteroids and a significantly reduced proportion of infants with a low Apgar score at 5 min of life[21]. Warner et al[22] reported an antenatal steroid therapy rate of 53% in infants with a BW of 400 g to 1500 g. In our study, the rate of antenatal steroid therapy was too low in infants <1000 g and 1000 g to 1499 g (20% versus 17.2%, respectively). Apgar scores at 1 min and 5 min were lower in infants <1000 g than in infants 1000 g to 1499g, and delivery room resuscitation requirements were higher in infants <1000 g than in infants 1000 g to 1499 g (95% versus 62.1%, respectively). Despite the higher rates of antenatal follow-up observed in the present study, the low rate of antenatal steroid use can be explained by the advanced labor of these pregnant women at their admission to the hospital. In addition, more than one-half of the pregnant women had a pregnancy-related disease such as preeclampsia, eclampsia, HELLP syndrome, abruptio placentae, urinary tract infection, oligohydramnios and preterm premature rupture of membranes. High rates of RDS and surfactant administration were more prevalent in <1000 g infants, and the longer durations of mechanical ventilation observed in the present study emphasized the clinical importance of antenatal steroid administration.
The American Academy of Pediatrics[23] reported the BPD rate among infants <1500 g to be 23%. Zhang et al[24] reported the incidence of BPD to be 48.3% in infants with BW of ≤1500 g. In our study, the incidence of BPD was 35% in the <1000 g group and 20.6% in 1000 g to 1499 g group, similar to results reported in other studies. Our NICU practices, such as appropriate respiratory support with a policy for safe administration of oxygen, optimal fluid and nutrition management, and infection controls, might be associated with the incidence of BPD found in the present study.
Jakuskiene et al[20] found the incidence of ROP requiring laser/cryocoagulation to be 9% for infants with a GA between 22 and 27 weeks and 2% for infants with a GA between 28 and 32 weeks. In our study, ROP incidence was 40% in the <1000 g group and 44.8% in the 1000 g to 1499 g group. Laser treatment was applied in 15% of infants <1000 g and 13.8% of infants 1000 g to 1499 g. The most important risk factor for ROP is a 1 min Apgar score of below 5 and the presence of symptomatic PDA[20]. Other, less frequent, risk factors for ROP are sepsis, IVH (grade III and PVHI), pneumonia, BW <1000 g, GA <28 weeks, the need for ventilation therapy and anemia requiring transfusion[20]. In our study, the correlation between ROP rate and the need for ventilator therapy was present, whereas the correlations between ROP rate and low Apgar score, symptomatic PDA, sepsis, and requiring transfusion were absent.
Cole et al[25] reported the NEC incidence in VLBW infants to be 10%. Wilson et al[26] reported the highest NEC incidence to be 42% in infants with a BW <1000 g. In our study, stage I NEC incidence was 30% in the <1000 g group, and no NEC ≥ stage II was detected. Early minimal enteral nutrition with human milk seems to be a factor in this low incidence of NEC.
GMH/IVH incidence is known to be 30% in developed countries[27]. Jakuskiene et al[20] reported an IVH incidence rate of 39.1%. In our study, GMH/IVH incidence was 55% for the <1000g group and 13.8% for the 1000 g to 1499 g group, similar to the above-mentioned studies. PVL is common in cases with a GA between 26 and 29 weeks, and it is known to peak in infants with a GA of 27 weeks[20]. Jakuskiene et al[20] found the PVL incidence to be 13% in infants with a GA of 22 to 27 weeks, and 6% in those with a GA of 28 to 32 weeks. In our study, PVL incidence was 20% in the <1000 g group and 6.9% in the 1000 g to 1499 g group.
In developed countries, PDA incidence is known to be 30%[11]. Jakuskiene et al[20] found that PDA incidence was 22% to 42% for infants with a GA of 22 to 27 weeks and 19% for infants with a GA of 28 to 32 weeks[20]. In our study, PDA incidence was 50% in the <1000 g group and 20.7% in the 1000 g to 1499 g group. In total, it was found to be 32.6% in neonates <1500 g. The results of our study were consistent with those reported in the literature.
In the literature, the length of hospital stay is reported to be 97 days for infants with a BW <1000 g and 59 days for infants with a BW of 1000 g to 1500 g[27]. In our study, the hospital stay was 56 days for the <1000 g group and 47 days for the 1000 g to 1499 g group.
The mortality rate was found to be increased when BW was decreased. Medlock et al[28] reported that GA and BW are the best predictors of survival in very preterm infants, and female sex frequently predicts survival. According to the results of our study, sex was not a predictor of mortality in infants with a BW <1500 g. Mortality rates in infants <1000 g was higher than in infants 1000 g to 1499 g.
RDS was the sole diagnosed respiratory risk factor for mortality. Symptomatic PDA, IVH and sepsis in <1000g neonates were the most frequent morbidities. ROP was not observed in VLBW infants who died in the NICU. BPD was diagnosed in only one VLBW infant.
The major limitation of this study is the low number of the study population.
Conclusion
We found that the survival rate of VLBW infants increased with increasing BW. Infant sex was not a risk factor for mortality. Sepsis, hyperbilirubinemia, pulmonary hemorrhage, need for oxygen therapy at the 28th day, ROP, NEC, and PVL did not occur more often in infants <1000 g compared with infants 1000 g to 1499 g. The need for ventilatory therapy may be an important risk factor for ROP in infants <1500 g.
Acknowledgment
Permission for human studies from authors’ Institutional Review Board is taken. All the contributors meet the criteria for authorship, and there is not a list of contributors to be disclosed and acknowledged.
Conflict of Interest
None
References
- 1.Kierans WJ, Kendall PRW, Foster LT, et al. New birth weight and gestational age charts for the British Columbia population. BC Med J. 2006;48(1):28–32. [Google Scholar]
- 2.Walsh MC, Fanaroff AA. Epidemiology (Part 1) In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin's Neonatal-Perinatal Medicine, Disease of the Fetus and Infant. 9th ed. St. Louis, Missouri: Mosby Elsevier; 2011. pp. 19–23. [Google Scholar]
- 3.Boulet SL, Alexander GR, Salihu HM, et al. Fetal growth risk curves: Defining levels of fetal growth restriction by neonatal death risk. Am J Obstet Gynecol. 2006;195(6):1571–77. doi: 10.1016/j.ajog.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 4.Kernaghan D, Ola B, Fraser RB, et al. Fetal size and growth velocity in the prediction of the large for gestational age (LGA) infant in a glucose impaired population. Eur J Obstet Gynecol and Reprod Biol. 2007;132(2):189–92. doi: 10.1016/j.ejogrb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Valero De Bernabé J, Soriano T, Albaladejo R, et al. Eur J Obstet Gynecol Reprod Bio. 2004;116(1):3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Semin Fetal Neonatal Med. 2004;9(6):429–35. doi: 10.1016/j.siny.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Escobar GJ, Littenberg B, Petitti DB. Outcome among surviving very low birth weight infants: A meta-analysis. Arch Dis Child. 1991;66(2):204–11. doi: 10.1136/adc.66.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlo WA. The high-risk infant. In: Kliegman RM, Stanton BF, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 552–64. [Google Scholar]
- 9.Ballard JL, Khoury JC, Wang L, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatrics. 1991;119(3):417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Insitutes of Health Consensus Definition of Bronchopulmonary Dysplasia. Pediatrics. 2005;116(6):1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Committee members: an international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127–33. [PubMed] [Google Scholar]
- 12.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol. 2003;24(8):1661–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Wezel-Meijler G. Classification of Per- and Intra-ventricular Haemorrhage, Periventricular Leukomalacia, and White Matter Echogenicity. In: Wezel-Meijler G, editor. Neonatal Cranial Ultrasonography. Berlin, Heidelberg: Springer; 2007. pp. 69–83. [Google Scholar]
- 15.Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103(2):273–7. doi: 10.1016/s0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 16.Bassan H, Feldman HA, Limperopoulos C, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35(2):85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5(2):89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 18.Bozhinova S, Penkov V, Rosmanova R, et al. Sectio caesarea in extremely low birth weight newborn babies--modern tendency or objective necessity. Akush Ginekol (Sofiia) 2005;44(Suppl 3):28–32. [PubMed] [Google Scholar]
- 19.Minguez-Milio JA, Alcázar JL, Aubá M, et al. Perinatal outcome and long-term follow-up of extremely low birth weight infants depending on the mode of delivery. J Matern Fetal Neonatal Med. 2011;24(10):1235–8. doi: 10.3109/14767058.2011.552990. [DOI] [PubMed] [Google Scholar]
- 20.Jakuskiene R, Vollmer B, Saferis V, et al. Neonatal outcomes of very preterm infants admitted to a tertiary center in Lithuania between the years 2003 and 2005. Eur J Pediatr. 2011;170(10):1293–303. doi: 10.1007/s00431-011-1431-8. [DOI] [PubMed] [Google Scholar]
- 21.Darlow BA, Cust AE, Donoghue DA. Improved outcomes for very low birthweight infants: evidence from New Zealand national population based data. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F23–8. doi: 10.1136/fn.88.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner B, Musial MJ, Chenier T, Donovan E. The effect of birth hospital type on the outcome of very low birth weight infants. Pediatrics. 2004;113(1 Pt 1):35–41. doi: 10.1542/peds.113.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Committee on Fetus and Newborn. American Academy of Pediatrics and Canadian Paediatric Society. Postnatal cotricosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109(2):330–8. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Fang J, Su H, Chen M. Risk Factors for Bronchopulmonary Dysplasia of Neonates Born at ≤1500g of Birth Weight (1999-2009) Pediatr Int. 2011;53(6):915–20. doi: 10.1111/j.1442-200X.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 25.Cole CR, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122(3):573–82. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson R, Kanto WP, Jr, McCarthy BJ, et al. Epidemiologic characterisitcs of necrotizing enterokolitis a population-based study. Am J Epidemiol. 1981;114(6):880–7. doi: 10.1093/oxfordjournals.aje.a113258. [DOI] [PubMed] [Google Scholar]
- 27.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 28.Medlock S, Ravelli AC, Tamminga P, et al. Prediction of mortality in very premature infants: a systematic review of prediction models. PLoS One. 2011;6(9):e23441. doi: 10.1371/journal.pone.0023441. [DOI] [PMC free article] [PubMed] [Google Scholar]