Abstract
Objective
Cystic fibrosis (CF) is a common autosomal recessive genetic disease caused by a mutation in the CF transmembrane conductance regulatory (CFTR) gene. This study attempted to identify the most common CFTR mutations and any correlations between certain mutations and the clinical presentation of the disease in CF patients in southwestern Iran.
Methods
Twenty nine common CFTR gene mutations were examined in 45 CF patients.
Findings
Chronic cough, intestinal obstruction, dehydration, heat exhaustion and steatorrhea were the most common early clinical symptoms among our patients. The most common mutation was ΔF508, with an allele frequency of 21%. The homozygous ΔF508 mutation was observed in eight patients (18%), and three patients (7%) were ΔF508 carriers. The 2183AA > G mutation was observed in four patients, one of whom was also a ΔF508 carrier. The R1162X mutation was detected in two patients. The G542X, R334W and N1303K mutations were detected each in one patient, the first of whom was also a ΔF508 carrier.
Conclusion
Out of 45 patients, 27 (60%) had none of the CFTR gene mutations we tested for. The most frequent mutations in southwestern Iranian patients with CF should be identified by sequencing the entire CFTR gene in order to optimize the design of a diagnostic kit for common regional mutations.
Keywords: Cystic Fibrosis, CFTR Gene Mutations, Clinical Presentation
Introduction
Cystic fibrosis (CF) is a common autosomal recessive genetic disease caused by mutation in the CF transmembrane conductance regulatory (CFTR) gene. Missing or defective CFTR gene products in patients with CF lead to multiorgan dysfunctions such as severe lung disease, pancreas dysfunction and elevated sweat chloride[1]. Cystic fibrosis is less common among African, American and Asian people than among northern Europeans[2].
To date, 1932 mutations have been reported in the CFTR gene worldwide[3]. The most common CFTR gene mutation is ΔF508, which accounts for about 30% to 80% of all mutant alleles worldwide[1]. The frequency of this mutation is reportedly between 16% and 23% in different parts of Iran[4–8].
Because the type and distribution of these mutations vary widely among populations[3], this study attempted to identify the most common CFTR gene mutations in patients in southwestern Iran with CF and search for correlations between certain mutations and the clinical presentation of the disease.
Subjects and Methods
This cross-sectional study was conducted over a period of three years from 2009 to 2012 in southwestern Iran. The diagnosis of CF was based on the typical clinical features of the disease and two abnormal sweat chloride values (>60 mEq/L). Patients with CF were included in this study after written informed consent was obtained from the patients themselves (if ≥18 years old) or their parents (of <18 years old). The study protocol was approved by our University Ethics Committee.
Demographic data including sex, age of diagnosis, early clinical symptoms, family history of CF and consanguinity for parents were collected. The clinical presentation of CF was classified as respiratory symptoms (purulent sputum, chronic cough, bronchiolitis, recurrent pneumonia, recurrent wheeze, atelectasis, bronchiectasis, hemoptysis, recurrent sinusitis or cor pulmonale), gastrointestinal symptoms (steatorrhea, liver disease, intestinal obstruction, diarrhea, constipation, meconium ileus or growth failure) or other symptoms (dehydration, heat exhaustion, CF-related vasculitis or diabetes mellitus).
Genomic DNA was extracted from 200 µL of whole blood with the QiaAmp DNA Mini Kit (Qiagen, Valencia, CA, USA) and 29 common CFTR gene mutations (D1152H, 1717-1G > A, G542X, W1282X, N1303K, ΔF508, 3849 + 10kbC > T, 394delTT, 621 + 1G > T, S1251N, G551D, R117H, R1162X, R334W, A455E, 2183AA > G, 3659delC, 1078delT, I507, R347P, R553X, E60X, 3120 + 1G > A, 2789 + 5G > A, 1898 + 1G > A, 711 + 1G > T, G85E, 2184delA and R560T) were analyzed with the ELUCIGENE CF29 v. 2 kit using four multiplex PCR. The ΔF508 mutation was further characterized as heterozygous or homozygous with this kit. Direct frequency counts and descriptive statistics were used here to report the clinical and genetic features.
Findings
Forty-five patients with CF (27 males and 18 females) from southwestern Iran were included in this study. At diagnosis our patients ranged in age from one month to 19 years; 82% of them were under 2 years old at the time of diagnosis. Their early clinical symptoms are summarized in Table 1. As shown, most patients with CF had respiratory symptoms.
Table 1.
Early clinical symptoms in patients from southwestern Iran with cystic fibrosis (n = 45)
| Clinical symptoms | Frequency | |
|---|---|---|
| Respiratory symptoms | Chronic cough | 38 (84%) |
| Bronchiolitis | 37 (82%) | |
| Recurrent pneumonia | 35 (78%) | |
| Purulent sputum | 34 (76%) | |
| Recurrent wheeze | 31(69%) | |
| Atelectasias | 18 (40%) | |
| Bronchiectasis | 6 (13%) | |
| Recurrent sinusitis | 4 (9%) | |
| Hemoptysis | 2 (4%) | |
| Cor pulmonale | 1(2%) | |
| Gasterointestinal symptoms | Steatorrhea | 37 (82%) |
| Growth failure | 24 (53%) | |
| Liver disease | 9 (20%) | |
| Distal intestinal obstruction | 6 (13%) | |
| Diarrhea | 3 (7%) | |
| Constipation | 2 (4%) | |
| Meconium ileus | 2 (4%) | |
| Other symptoms | Dehydration | 2 (4%) |
| Heat exhaustion | 2 (4%) | |
| Vasculitis | --- | |
| Diabetes | --- |
Parental consanguinity was found in 40 patients (89%) and a family history of CF was reported by 15 patients (37%) in this group. A CFTR gene mutation was detected in 18 patients (40%). The frequencies of the mutations we detected are summarized in Table 2. Fig. 1 shows the results of molecular analysis of one of the patients. The most common mutation was ΔF508, with an allele frequency of 21%. The homozygous ΔF508 mutation was observed in eight patients (18%), and three patients (7%) were ΔF508 carriers. Compound heterozygous mutations were found in two ΔF508 carriers.
Table 2.
Frequencies of CFTR gene mutations in a sample of patients in southwestern Iran with cystic fibrosis
| CFTR gene mutations | n = 45 |
|---|---|
| ΔF508 (M)/ΔF508 (M) | 8 (18%) |
| ΔF508 (N)/ 2183AA > G | 3 (7%) |
| ΔF508 (N)/ R1162X | 2 (4%) |
| ΔF508 (N)/ R334W | 1 (2%) |
| ΔF508 (N)/ N1303K | 1 (2%) |
| ΔF508 (M)/ G542X | 1 (2%) |
| ΔF508 (M)/ 2183AA > G | 1 (2%) |
| ΔF508 (M)/ΔF508 (N) | 1 (2%) |
| Undefined | 27 (60%) |
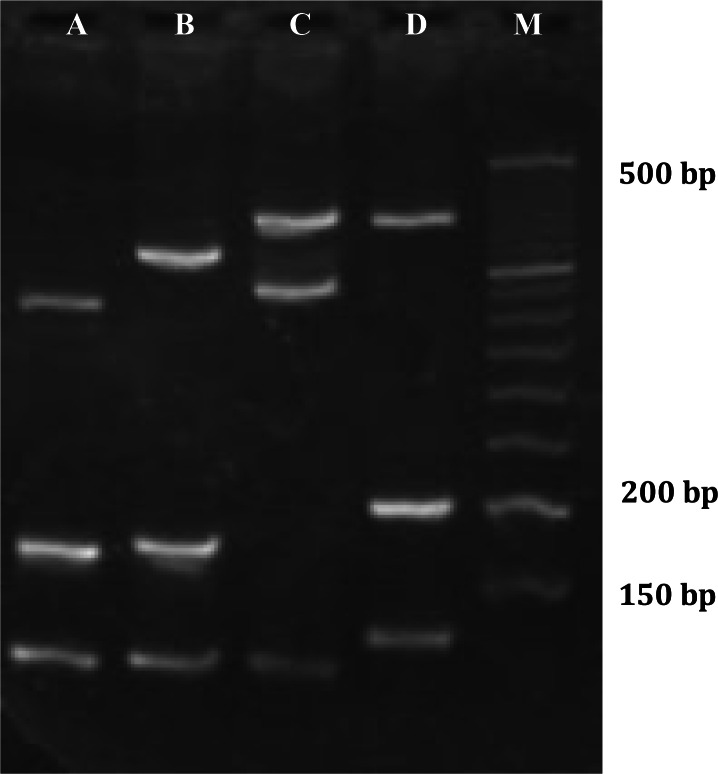
Fig. 1.
The results of CFTR gene analysis with the ELUCIGENE CF29 v.2 kit. This patient was compound heterozygous for the mutant ΔF508 allele (lane A) and the 2183AA > G mutation (lanes C and D). Due to 2184delA primer cross-reactivity with the 2183AA > G mutant DNA sequence, this sample with the 2183AA > G mutation resulted in diagnostic bands of 425 bp and 169 bp in lanes C and D, respectively. A copy of the mutant ΔF508 allele (160 bp) is seen in lane A, and a copy of the normal ΔF508 allele (also 160 bp) is observed in lane B. The top and bottom bands in each lane are internal positive controls, and lane M shows the 1-kb ladder marker (http://www.gen-probe.com/pdfs/downloads/cfcf029v2_ifugb010.pdf).
Discussion
Defects in the CFTR gene are the main cause of CF, and molecular analysis of this gene is important for the differential diagnosis. However, the type of CFTR gene mutation has little effect on the age of onset and clinical presentation of the disease[9].
The results of this study showed no correlation between the mutations we detected and the clinical presentations in our patients. Therefore, clinical signs of CF are still the best guide for diagnosing the disease, and should be taken into account by pediatricians. Our results document a 1- to 2-year delay between the first clinical presentation and the diagnosis of CF. Delayed diagnosis remains a problem which usually results in progressive disease and even irreversible changes[10].
Cystic fibrosis, with a prevalence of 1 in 2500, is considered a common disease among Caucasians. The carrier rate of CF is reported to be about 1 in 25 among Caucasians with no family history[11].
Because consanguinity is known to be the main cause of genetic disorders[12], a high carrier rate implies greater risk in populations with high levels of consanguineous marriage, including some ethnic groups in Iran. The overall rate of consanguineous marriage among Iranians is reportedly 38.6%[13], and ranges from 30% to 85% in different parts of the country[14]. In our sample, 89% of the patients belonged to consanguineous families.
Unlike European countries and in agreement with other reports from Iran, the frequency of ΔF508 was around 24% in our sample of patients with CF. We found no new CFTR gene mutations in this study, and all the mutations reported here have been previously reported in different parts of Iran[4–8].
Although no accurate data are available regarding the prevalence of CF in different parts of Iran, the disease appears not to be rare in this country[5]. To reduce the frequency of new cases of CF, genetic counseling before marriage and prenatal diagnosis for families with one or more affected children are necessary. Considering the high number of mutations in the CFTR gene and the heterogenous distribution of these mutations among different populations, determining the most common mutations in each area is necessary in order to design effective local diagnostic kits. Our results showed that a commercial kit designed to detect 29 different mutations failed to identify any CFTR gene mutations in 60% of our patients with CF.
Conclusion
To design an effective domestic kit, the entire CFTR gene should be sequenced and all CFTR gene rearrangements should be identified to detect prevalent and specific local mutations[15]. Such a kit would be helpful not only for the reliable diagnosis of CF but also for premarital genetic counseling and prenatal diagnosis.
Acknowledgment
This work was supported by a grant from Shiraz University of Medical Sciences (Grant No.85-3156). We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the use of English in the manuscript.
Conflict of Interest
None
References
- 1.Moskowitz SM, Chmiel JF, Sternen DL, et al. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet Med. 2008;10(12):851–68. doi: 10.1097/GIM.0b013e31818e55a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132(4):589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Mutation Database (CFMDB) Available at: http://www.genet.sickkids.on.ca/cftr/StatisticsPage.html, Access date: Sep 17, 2012.
- 4.Elahi E, Khodadad A, Kupershmidt I, et al. A haplotype framework for cystic fibrosis mutations in Iran. J Mol Diagn. 2006;8(1):119–27. doi: 10.2353/jmoldx.2006.050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jalalirad M, Houshmand M, Mirfakhraie R, et al. First study of CF mutations in the CFTR gene of Iranian patients: detection of DeltaF508, G542X, W1282X, A120T, R117H, and R347H mutations. J Trop Pediatr. 2004;50(6):359–61. doi: 10.1093/tropej/50.6.359. [DOI] [PubMed] [Google Scholar]
- 6.Alibakhshi R, Zamani M. Mutation analysis of CFTR gene in 70 Iranian cystic fibrosis patients. Iran J Allergy Asthma Immunol. 2006;5(1):3–8. [PubMed] [Google Scholar]
- 7.Alibakhshi R, Kianishirazi R, Cassiman JJ, et al. Analysis of the CFTR gene in Iranian cystic fibrosis patients: identification of eight novel mutations. J Cyst Fibros. 2008;7(2):102–9. doi: 10.1016/j.jcf.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Bonyadi M, Omrani O, Rafeey M, Bilan N. Spectrum of CFTR gene mutations in Iranian Azeri Turkish patients with cystic fibrosis. Genet Test Mol Biomarkers. 2011;15(1-2):89–92. doi: 10.1089/gtmb.2010.0091. [DOI] [PubMed] [Google Scholar]
- 9.Vanscoy LL, Blackman SM, Collaco JM, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(10):1036–43. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell PM, Li Z, Kosorok MR, et al. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med. 2003;168(9):1100–8. doi: 10.1164/rccm.200303-434OC. [DOI] [PubMed] [Google Scholar]
- 11.Rohlfs EM, Zhou Z, Heim RA, et al. Cystic fibrosis carrier testing in an ethnically diverse US population. Clin Chem. 2011;57(6):841–8. doi: 10.1373/clinchem.2010.159285. [DOI] [PubMed] [Google Scholar]
- 12.Bittles AH, Black ML. Evolution in health and medicine Sackler colloquium: Consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1779–86. doi: 10.1073/pnas.0906079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saadat M, Ansari-Lari M, Farhud DD. Consanguineous marriage in Iran. Ann Hum Biol. 2004;31(2):263–9. doi: 10.1080/03014460310001652211. [DOI] [PubMed] [Google Scholar]
- 14.Kushki AM, Zeyghami B. The effect of consanguineous marriages on congenital malformation. J Res Med Sci. 2005;10(5):298–301. [Google Scholar]
- 15.Audrézet MP, Chen JM, Raguénès O, et al. Genomic rearrangements in the CFTR gene: extensive allelic heterogeneity and diverse mutational mechanisms. Hum Mutat. 2004;23(4):343–57. doi: 10.1002/humu.20009. [DOI] [PubMed] [Google Scholar]