Abstract
Background
Maturity onset diabetes of the young type 2 (MODY) is an inherited disorder due to mutations in glucokinase (GCK) gene, which lead to mild fasting hyperglycemia.
Case Presentation
Herein an otherwise healthy 9-year old boy with hyperglycemia is presented in whom the diagnosis of MODY2 was suspected. Genetic studies showed heterozygous inactivating GCK gene mutation in exon 8 (c.1010delA) in this patient. The same mutation was found in his father as well. The patient received some dietary advices without any medication.
Conclusion
The identification of GCK mutation and diagnosis of MODY2 helps the clinicians to predict the disease course, prognosis and to exclude other types of diabetes.
Keywords: Maturity-onset Diabetes, GCK, Fasting Hyperglycemia, Diabetes
Introduction
Maturity onset diabetes of the young (MODY) is a heterogeneous group of disorders characterized by dysfunction of beta-cells, and usually referred to monogenic forms of diabetes mellitus to distinguish them from the common types of disease such as type 1 or type 2 diabetes[1]. Although making the definite diagnosis is a challenge because of similar clinical phenotype, it is crucial while optimal treatments are different[1].
Eleven different MODY types have been identified so far based on different gene mutations. Maturity onset diabetes of the young type 2 (MODY2, OMIM#125851) is a genetic form of diabetes, caused by heterozygous mutations in glucokinase (GCK) gene (OMIM*138079) which result in a reduction of the enzymatic activity[2]. This gene is located on the chromosome 7 (7p15.3-15.1) and contains 12 exons that encode the 465-amino-acid protein glucokinase[3]. Heterozygous inactivating mutations of the GCK gene lead to mild fasting hyperglycemia and diminished insulin secretion, along with decreased hepatic glucose uptake and glycogen synthesis[4].
In this report, an 8-year old boy with MODY2 is presented in whom a heterozygous inactivating GCK gene mutation was detected. To our best knowledge, this is the first report of GCK gene mutation in an Iranian child with MODY2.
Case Presentation
A 9-year-old boy from northern Iran was referred to the Children's Medical Center, the Pediatrics Center of Excellence in Tehran-Iran, for evaluation of high fasting plasma glucose. He was from unrelated parents, and his mild hyperglycemia has been accidentally discovered a few months before during routine laboratory tests. He did not have polydipsia and polyuria, neither any weight loss.
On examination, he was well and healthy, weight and height 43 kg and 148 cm respectively (>97th percentile of Iranian boys of corresponding age) with body mass index (BMI) of 24 kg/m2. He had no acanthosis nigricans. Fasting blood sugar (FBS) 114 mg/dL, HbA1c 6.5%. A standard oral glucose tolerance test (OGTT) with 75g of glucose equivalent was performed with a fasting glucose of 125 mg/dL and 2-hour glucose of 133 mg/dL. Fasting insulin concentration was 7.5 µIU/mL. No glucosuria or ketonuria were detected. Further investigations revealed normal liver function test, lipid profile, and abdominal ultrasound. In addition, the pancreatic islet cell autoantibodies (ICA) and glutamic acid decarboxylase autoantibodies (GADA) were negative. Both clinical presentation and laboratory data were highly suggestive of a GCK mutation.
Family history disclosed a diagnosis of diabetes mellitus in his paternal grandmother 10 years ago, at the age of 55 years, but she took no medication. Because there was no history of diabetes in his parents, evaluation was performed. The mother was healthy, with FBS of 88 mg/dL and blood glucose of 94 mg/dL at 120’ of OGTT. Instead, the 41-year-old father had an FBS of 115 mg/dL and his blood glucose was 162 mg/dL at 2-hours after GTT; his weight was 95 kg, his height 185 cm, with a resulting BMI of 27.9 kg/m2. These findings showed that diabetes or IFG/IGT was present in three consecutive generations in this family and reinforced the clinical diagnosis of MODY, type 2.
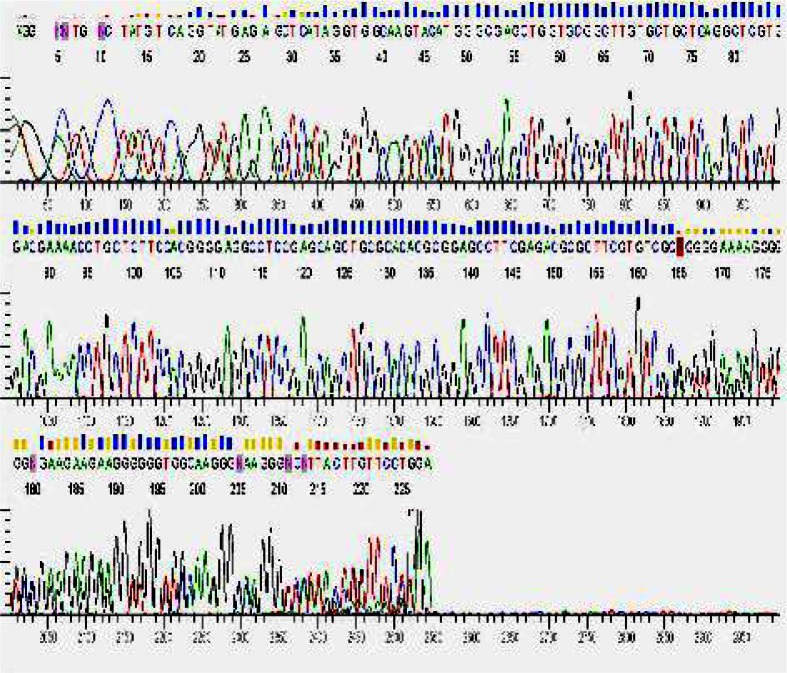
Consequently, after getting written informed consent, direct DNA sequencing of all exons and intron-exon bounders of the GCK gene was performed on patient's genomic lymphocytes DNA, which showed a heterozygous single nucleotide deletion in exon 8 (c.1010delA) causing a frame-shift with the formation of a premature stop codon (TGA) downstream at codon 352 (exon 9) (Fig. 1). The same mutation was found in his father as well. Therefore, the diagnosis of MODY2 was confirmed in the family.
Fig. 1.
GCK gene mutation analysis of the patient showing a heterozygous single nucleotide deletion in exon 8 (c.1010delA).
Consequently, after getting written informed consent, direct DNA sequencing of all exons and intron-exon bounders of the GCK gene was performed on patient's genomic lymphocytes DNA, which showed a heterozygous single nucleotide deletion in exon 8 (c.1010delA) causing a frame-shift with the formation of a premature stop codon (TGA) downstream at codon 352 (exon 9) (Fig. 1). The same mutation was found in his father as well. Therefore, the diagnosis of MODY2 was confirmed in the family.
Serial blood glucose measurements in the patient obtained by glucometer at home showed variable fasting readings ranging between 115 mg/dL and 155 mg/dL; fasting insulin concentration was 6.4 µIU/mL, HbA1c was 6.6%. A second OGTT showed blood glucose of 117, 134, 128, and 118 mg/dL at 0, 60, 120, 180 minutes, respectively, confirming a non-progressive, mild alteration of glucose metabolism. The patient received some dietary advices and no medication and a follow-up appointment in the diabetes clinic. His last biochemical tests after 4 months showed FBS of 127 mg/dL. His latest HbA1c was 6.4%. We recommended further dietary restriction and regular follow-up.
Discussion
Patients with MODY2 usually have low rise in blood glucose after GTT, while their HbA1c is almost invariably below 7.5%[5, 6] similar to what is observed in our patient. MODY2 is inherited in an autosomal dominant pattern; however, because patients are usually asymptomatic, family history of diabetes is not always present. Molecular evaluation for MODY2 in children is important in order to make a definite diagnosis and recommend appropriate treatment protocol[7].
In our patient, the diagnosis of MODY2 was suspected based on clinical history, and mutation in the GCK gene confirmed the diagnosis. It has previously been suggested that children with incidental hyperglycemia and negative autoantibody, having a parent with the same condition are good candidates for molecular screening of the GCK gene[8]. Although it has been calculated that MODY may be responsible for up to 1-2% of all diabetes cases[9], the prevalence of MODY due to GCK mutation might be much higher, considering that this condition is probably under- diagnosed because of mild or no symptoms, and hyperglycemia is incidentally detected in medical check-up and routine screening in most cases. Patients with MODY2 are expected to have impaired fasting glucose or impaired glucose tolerance test, while less than half of affected individuals have diabetes according to ADA parameters[10]. As insulin or oral hypoglycemic agents do not improve glycemic control, pharmacologic treatment in MODY2 patients is not recommended[11], while a proper diet is sufficient in most cases[10].
Conclusion
The identification of GCK mutation and diagnosis of MODY2 helps the clinicians to predict the disease course and prognosis and to exclude other types of diabetes.
Acknowledgment
This study was supported by a grant from Tehran University of Medical Sciences and Health Services (89-04-80-11945).
References
- 1.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343:d6044. doi: 10.1136/bmj.d6044. [DOI] [PubMed] [Google Scholar]
- 2.Galan M, Vincent O, Roncero I, et al. Effects of novel maturity-onset diabetes of the young (MODY)-associated mutations on glucokinase activity and protein stability. Biochem J. 2006;393(Pt 1):389–96. doi: 10.1042/BJ20051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffel M, Patel P, Lo YM, et al. Missense glucokinase mutation in maturity-onset diabetes of the young and mutation screening in late-onset diabetes. Nat Genet. 1992;2(2):153–6. doi: 10.1038/ng1092-153. [DOI] [PubMed] [Google Scholar]
- 4.Byrne MM, Sturis J, Clement K, et al. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Invest. 1994;93(3):1120–30. doi: 10.1172/JCI117064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stride A, Vaxillaire M, Tuomi T, et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45(3):427–35. doi: 10.1007/s00125-001-0770-9. [DOI] [PubMed] [Google Scholar]
- 6.Martin D, Bellanne-Chantelot C, Deschamps I, et al. Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2) Diabetes Care. 2008;31(7):1321–3. doi: 10.2337/dc07-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timsit J, Bellanne-Chantelot C, Dubois-Laforgue D, et al. Diagnosis and management of maturity-onset diabetes of the young. Treat Endocrinol. 2005;4(1):9–18. doi: 10.2165/00024677-200504010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lorini R, Klersy C, d'Annunzio G, et al. Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care. 2009;32(10):1864–6. doi: 10.2337/dc08-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001;345(13):971–80. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 10.Velho G, Robert JJ. Maturity-onset diabetes of the young (MODY): genetic and clinical characteristics. Horm Res. 2002;57(Suppl 1):29–33. doi: 10.1159/000053309. [DOI] [PubMed] [Google Scholar]
- 11.Hattersley A, Bruining J, Shield J, et al. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):33–42. doi: 10.1111/j.1399-5448.2009.00571.x. [DOI] [PubMed] [Google Scholar]