Abstract
Background
Intracranial arteriovenous malformation rarely causes pulmonary hypertension and congestive heart failure in the newborn. Its diagnosis is challenging because cardiomegaly may suggest an intra-cardiac structural lesion.
Case Presentation
We present two newborns, one 2-day-old male and the other 11-day-old female, with intracranial arteriovenous malformation and misdiagnosis of congenital heart disease.
Conclusion
Precise echocardiography revealed the secondary signs of cranial arteriovenous malformation and had the major role in early diagnosis.
Keywords: Dilated brachiocephalic arteries, Congestive heart failure, Doppler echocardiography
Introduction
Congenital vascular malformations are structural abnormalities that result from arrests in normal morphogenetic processes. Arterial malformations have two vascular patterns: arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs). AVMs (microfistulas) are multiple arterial feeders joined via a nidus to draining veins. AVFs (macrofistulas) are direct shunts between large arterial and venous channels[1]. They do not have the normal intervening capillary bed[2].
Most lesions affect both sexes equally, except cerebral AVMs, which affect males more frequently than females. Arterial malformations rarely result in hemodynamic symptoms. Systemic AVMs with profound hemodynamic effects occur in the brain, liver, thorax, and extremities. Central nervous system AVMs manifest symptoms according to their hemodynamic effects. Infants presenting with congestive heart failure (CHF) typically have large AVFs[1].
AVF is a relatively rare cause of severe congestive heart failure in the newborn[3]. If flow is increased because of shunting from a deep AVM or by direct arterial communication, the vein of Galen may become massively dilated[4].
An aneurysm of the vein of Galen consists of an AVM involving the carotid and or vertebrobasilar circulation in direct communication with the great cerebral vein.
An arterial branch may enter the vein directly, or there may be a complex racemose network of non-capillary vessels interposed between the artery and vein[5].
They may present later in infancy or early childhood with hydrocephalus, seizures, or focal or generalized neurologic signs and symptoms. Smaller AVMs within the brain parenchyma frequently present in childhood with signs of cerebral or subarachnoid hemorrhage, occurring from the AVM itself or from a coexisting arterial aneurysm[1].
Case Presentation
A 2-day–old male newborn was referred to our hospital for cardiac surgery with diagnosis of Total Anomalous Pulmonary Venous Connection (TAPVC). His medical history revealed that he was born after an uneventful normal vaginal delivery with normal Apgar score. Gradually during the first 24 hours he became cyanotic, his reflexes decreased and the level of consciousness reduced and he experienced one episode of seizure.
On physical examination the remarkable findings consisted of tachypnea (78/min), tachycardia (137/min), decreased oxygen saturation (80% with oxygen 100%), reduced consciousness, reduced neonatal reflexes, weak peripheral pulses, widened carotid pulse pressure, systolic and diastolic cardiac murmur, loud second heart sound and continuous murmur over skull. Chest X-ray revealed cardiomegaly and ECG showed right axis deviation.
Transthoracic echocardiography showed enlarged right heart chambers including right atrium, right ventricle and pulmonary artery. Superior vena cava was enlarged and brachiocephalic arteries were tortuous and dilated.
Evaluation via color Doppler showed diastolic flow reversal (diastolic run off) in descending and abdominal aorta without aortic valvular regurgitation, coronary artery fistula, aorticopulmonary window or patent ductus arteriosus. This diastolic flow reversal was confirmed by pulsed Doppler study (Figs. 1 and 2).
Fig. 1.
Color Doppler echocardiogram from suprasternal view showing reverse flow in descending aorta
Fig. 2.
Pulsed Doppler tracing in abdominal aorta showing reverse flow throughout diastole
Patient had moderate tricuspid valve regurgitation with 50 mmHg pressure gradient (pulmonary hypertension) and right to left shunt at foramen ovale and ductal level. Pulmonary veins had normal connection and drainage to posterior left atrial wall and there was no cardiac anomaly.
According to echocardiographic findings and high suspicion for cranial AVF, brain sonography was performed which showed echolucsent cystic lesion within brain. Brain CT scan showed linear calcification and atrophic changes of right side cerebral hemisphere.
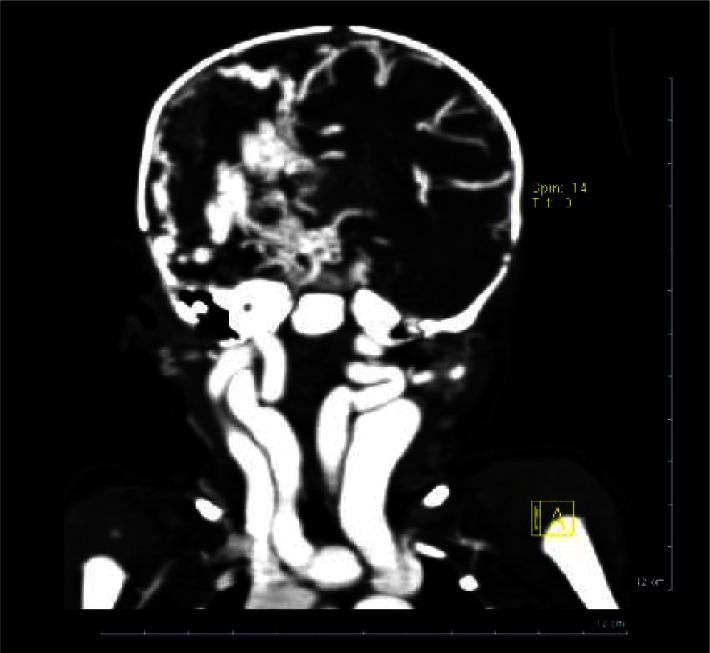
Administration of contrast material demons-trated marked dilation of the vein of Galen associated with dilation of transverse sinus and the straight sinus (Fig. 3).
Fig. 3.
Multislice CT scan with contrast. Coronal view showing dilated neck arteries and veins and atrophy of right hemisphere
After confirmation of no congenital heart disease and presence of cereberal AV malformation as the cause of congestive heart failure by these modalities, we consulted a neurologist but unfortunately by awareness of poor prognosis the parents refused any more treatment and discharged the baby from hospital.
Case 2
An 11-day-old female newborn was referred to us with the diagnosis of cardiomyopathy. She was born by cesarian section and discharged from nursery in good condition. Four days later she was visited by pediatrician because of jaundice, he noticed cardiac murmur and severe cardiomegaly on chest x-ray and referred her to our hospital for more cardiac evaluation by initial diagnosis of cardiomyopathy at the age of 11 days (Fig. 4).
Fig. 4.
CXR on age of 11 days showing severe cardiomegaly
On physical examination she had tachypnea (60/min), tachycardia (160/min), and grade 3/6 systolic cardiac murmur, loud second heart sound and continuous murmur over skull. Precise echocardiography revealed right to left shunt via PFO and small PDA and reduced cardiac ejection fraction. Brachiocephalic arteries were dilated and there was retrograde aortic runoff in descending thoracic aorta in diastole. There was no PDA, AP window and no aortic regurgitation so she was highly suspicious of cranial AV fistula. More diagnostic evaluation by brain sonography and CT scanning confirmed the diagnosis. Transcatheter embolization of afferent arteries for this patient was successful. Now, after 6 months follow up baby is in good condition with normal cardiac function and no neurologic complication (Fig. 5).
Fig. 5.
CXR some days after transcatheter embolization of cranial AV malformation showing decreased cardiac size
Discussion
Cerebral AV malformation enlarging the vein of Galen is rare. This defect is the result of an AV malformation or fistula that increases flow through the vein of Galen and deep venous system, causing aneurysmal dilatation. Newborn infants with this disorder often develop symptoms of congestive heart failure and cyanosis. Infants with severe congestive heart failure caused by an intracranial AV malformation are critically ill, and prompt diagnosis is essential. Two-dimensional ultrasonography of the heart and brain provides a rapid, efficient method for detection of intracranial AV malformation. Doppler examination of descending aorta shows evidence of retrograde diastolic flow[6].
The common causes of reversal of flow in the aortic arch are lesions in which a communication exists between the ascending aorta, the proximal aortic arch or its branches, and a lower pressure chamber or channel (such as pulmonary vessels, ventricles, or systemic veins), that results in “stealing” of blood from the distal aortic arch and descending aorta (example: aorticopulmonary window, severe aortic valve regurgitation, systemic arteriovenous (A-V) malformation of the head and neck vessels, coronary artery fistula, and systemic to pulmonary fistula).
The second category comprises lesions with severe obstruction of the left ventricular outflow, such as critical aortic stenosis or hypoplastic left heart syndrome. Because of a severe reduction in the forward flow across the aortic valve, the blood flow to the aortic arch and its branches is supplied in a retrograde manner via the patent ductus arteriosus (PDA) in systole and sometimes in diastole[7]. Severe heart failure associated with cranial AV malformation appears to be more severe than heart failure associated with intracardiac anomalies that place a volume load on the right ventricle. It has been suggested that this is because the volume load is obligatory and continues despite increase in pulmonary artery resistance[8].
After a careful physical examination including cranial auscultation we performed echocardiogram in patients who demonstrated no congenital heart disease as a reason for reverse flow in transverse aortic arch, such as severe aortic insufficiency, aorticopulmonary window and coronary artery fistula. Enlargement of right cardiac chambers and superior vena cava as well as dilated and tortuous ascending aorta and brachiocephalic vessels along with retrograde diastolic flow in descending aorta and right to left shunting via PFO and ductus arteriosus all were indirect evidence for presence of cerebral AV malformation which led to performing cranial ultrasonography and CT scan for detecting more precise anatomy and direct visualization of the lesion. Also in both cases pediatricians forgot to perform an important part of physical examination in children which is the cranial auscultation.
Conclusion
Cranial AV malformation causes volume overloading and cyanosis due to persistent fetal circulation and most of these infants are at first considered to have congenital heart disease. Careful echocardiography, by demonstrating normal intracardiac anatomy via two-dimentional mode and stealing of blood in cranial region by pulsed and color Doppler modes helped us to reach the correct diagnosis promptly.
It should be emphasized that cranial auscultation in children is an important part of physical examination which is ignored many times.
References
- 1.Grifka RG, Preminger TJ. Vascular anomalies. In: Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents Including the Fetus and Young Adult. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 715–28. [Google Scholar]
- 2.Arteriovenous Malformation - My Child Has -Children's Hospital Boston. Available at: http://www.childrenshospital.org/az/Site593/mainpageS593PO.html. Access date: Jan 23, 2010.
- 3.Pellegrino PA, Milanesi O, Saia OS, Carollo C. Congestive heart failure secondary to cerebral arterio-venous fistula. Child's Nervous System. 1987;3(3):141–4. doi: 10.1007/BF00717889. [DOI] [PubMed] [Google Scholar]
- 4.Tessler FN, Dion J, Vinuela F, et al. Cranial arteriovenous malformation in neonates: color doppler imaging with angiographic correlation. Am J Roentgenol. 1989;153(5):1027–30. doi: 10.2214/ajr.153.5.1027. [DOI] [PubMed] [Google Scholar]
- 5.Lehman JS, Chynn KY, Hagstrom JWC, Steinberg I. Heart failure in infancy due to arteriovenous malformation of the vein of Galen: report of a case. Am J Roentgenol. 1966;98(3):653–9. doi: 10.2214/ajr.98.3.653. [DOI] [PubMed] [Google Scholar]
- 6.Snider AR, Serwer GA, Ritter SB. Echocardiography in Pediatric Heart Disease. 2nd ed. Mosby; 1997. Abnormal vascular connections and structures; pp. 452–496. [Google Scholar]
- 7.Khongphatthanayothin A, Lane J, Wong PC, Acherman RJ. Severe pulmonary hypertension as a cause of reverse aortic arch flow in the neonate: report of two cases. J Am Soc Echocardiogr. 1996;9(6):915–7. doi: 10.1016/s0894-7317(96)90492-7. [DOI] [PubMed] [Google Scholar]
- 8.Frawley GP, Dargaville PA, Mitchell PJ, et al. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch Dis Child Fetal Neonatal. 2002;87(2):144–9. doi: 10.1136/fn.87.2.F144. [DOI] [PMC free article] [PubMed] [Google Scholar]