Abstract
Aims
Prior reports using pacing manoeuvres, demonstrated an up to 42% prevalence of residual pulmonary vein to left atrium (PV–LA) exit conduction after apparent LA–PV entry block. We aimed to determine in a two-centre study the prevalence of residual PV–LA exit conduction in the presence of unambiguously proven entry block and without pacing manoeuvres.
Methods and results
Of 378 patients, 132 (35%) exhibited spontaneous pulmonary vein (PV) potentials following circumferential PV isolation guided by three-dimensional mapping and a circular mapping catheter. Pulmonary vein automaticity was regarded as unambiguous proof of LA–PV entry block. We determined the prevalence of spontaneous exit conduction of the spontaneous PV potentials toward the LA. Pulmonary vein automaticity was observed in 171 PVs: 61 right superior PV, 33 right inferior PV, 47 left superior PV, and 30 left inferior PV. Cycle length of the PV automaticity was >1000 ms in all cases. Spontaneous PV–LA exit conduction was observed in one of 171 PVs (0.6%). In a subset of 69 PVs, pacing from within the PV invariably confirmed PVLA exit block.
Conclusion
Unidirectional block at the LA–PV junction is unusual (0.6%). This observation is supportive of LA–PV entry block as a sufficient electrophysiological endpoint for PV isolation.
Keywords: Fibrillation, Electrophysiology, Atrium
What's new?
This is the first study in men evaluating bidirectional conduction properties at the LA–PV junction without relying on ambiguous entry or exit pacing manoeuvres.
We showed that, in case of unequivocal entry block, residual PV–LA conduction is an extremely rare condition (‘entry block implies exit block’).
The exceptionally low prevalence of unidirectional block is supportive of LA–PV entry block as a sufficient electrophysiological endpoint for PVI.
Introduction
Catheter-based pulmonary vein (PV) ablation has emerged as an accepted therapy in patients with drug-resistant, symptomatic, paroxysmal, and persistent atrial fibrillation (AF).1,2 Bidirectional PV isolation (PVI), defined as the absence of conduction into the PV from the left atrium (LA–PV entry block) and in the opposite direction (PV–LA exit block), is an established endpoint of PV ablation.2,3
Assessment of LA–PV entry block by complete elimination or dissociation of PV potentials on a circular mapping catheter (CMC) is the primary endpoint of PVI.2 On the other hand, assessment of PV–LA exit block seems imperative given the nature of initiation of AF by ectopy from within the PVs.4 In this respect, Gerstenfeld et al.5 reported that in the presence of apparent LA–PV entry block during sinus rhythm up to 42% of the PVs exhibited residual exit conduction during pacing within the encircled region, a phenomenon referred to as unidirectional LA–PV block. Pacing within the PVs is limited by the ability to capture local venous myocardium following catheter ablation while avoiding capturing neighbouring tissue with high-pacing outputs. Therefore, the existence of true unidirectional LA–PV block remains controversial.
The aim of the present two-centre study was to assess the prevalence of residual PV–LA exit conduction in the presence of LA–PV entry block. For this purpose, PV automaticity after circumferential PVI was studied. We hypothesized that PV automaticity can be regarded as a valid model of unambiguous proof of entry block and a model of randomly applied spontaneous premature stimuli to the PV to determine residual PV–LA exit conduction. This model is completely devoid of pacing manoeuvres.
Methods
Patient population
Data were retrospectively analysed from two centres. At the St Jan Hospital Bruges, 95 of 289 consecutive patients [102 of 1156 (9%) PVs] exhibited PV automaticity on the CMC following circumferential PVI for drug-refractory, symptomatic, and recurrent AF. At the Alfred Hospital, 37 of 89 consecutive patients [69 of 372 (18%) PVs] exhibited dissociated pulmonary vein potentials (PVPs) on the CMC following PVI.6 Any necessary ethics committee approval (St Jan Bruges Ethics Committee and the Alfred Research and Ethics Committee) was secured for the study reported. Informed consent was obtained from all patients.
Electrophysiological study and ablation procedure
The procedure was performed under conscious sedation or general anaesthesia with arterial pressure monitoring. Treatment with anti-arrhythmic drugs except amiodarone was discontinued at least 4–5 half-life periods before the ablation procedure. Following femoral venous access a decapolar catheter was positioned within the coronary sinus (CS) and a quadripolar catheter was positioned at the His bundle. Two separate transseptal punctures were performed using a modified Brockenbrough technique under the guidance of pressure or transoesophageal echocardiography using two long vascular sheaths (SL1, St Jude Medical). After puncture, a continuous infusion of heparin was started to maintain an activated clotting time above 350 s throughout the procedure. A three-dimensional (3D) reconstruction of the LA was made guided by a 3D non-fluoroscopic navigation system (CARTO or NavX) using a 3.5 mm irrigated ablation catheter (Navistar Thermocool, Biosense-Webster or Coolpath, St Jude Medical). A previously performed magnetic resonance imaging or computed tomography scan was integrated into the 3D LA map in all cases (CARTO-Merge or NavX Fusion).
The ablation procedure consisted of encircling ipsilateral PVs as a single unit by a continuous circular lesion set during sinus rhythm. Except for the anterior aspect of the left PVs, lesions were created 10–20 mm outside of the ostia as defined from the 3D map. The continuous lesion set was made by point-by-point radiofrequency (RF) applications (20–35 W, 30–60 s, max 48°C, flow 17–20 mL/min). The PVs were continuously assessed for electrical disconnection using a decapolar CMC (Lasso, 2515 variable catheter, Biosense Webster; Reflexion spiral catheter, St Jude Medical) placed ∼5 mm within the PV ostium. The endpoint for ablation was LA–PV entry block, defined as elimination of all ostial PV potentials during sinus rhythm, pacing at CS and differential pacing at the left atrial appendage and/or posterior wall. If no entry block was obtained after completing the ipsilateral ablation circle, residual conduction gaps at the circumference were identified by the aid of the CMC and targeted by selective RF lesions. Ablation at the intervenous ridge was allowed if entry block could not be achieved by encircling only.7 No isoproterenol was given. All bipolar electrograms were analysed on-line using the EP laboratory system (Bard Electrophysiology, Lowell, MA, USA; EP Med-system, St Jude Medical and Prucka CardioLab, GE Medical) at a sweep speed of 150–200 mm/s and filter settings of 10–250 or 30–500 Hz.
Pulmonary vein automaticity
Following electrical isolation, the CMC was positioned in each PV to look for PV automaticity. Pulmonary vein automaticity was defined as a stable rhythm independent of the sinus rhythm or CS pacing cycle length. Pulmonary vein automaticity was regarded as unambiguous proof of entry block (Figure 1). Throughout a waiting period of at least 30 min following successful PVI, we determined the prevalence of spontaneous exit conduction of the spontaneous PV beats towards the LA. Exit conduction was defined as spontaneous PV beats which advanced local CS activation together with a change in the CS activation pattern.
Figure 1.
Pulmonary vein automaticity (*) as an unambiguous proof of LA–PV entry block (right tracing) in the RSPV. Note the complete elimination of PV potentials compared with the baseline recording (left tracing) and the progressive LA–PV delay before isolation (middle tracing). PV, pulmonary vein; CS, coronary sinus; RSPV, right superior PV; LA, left atrium.
Off-line analysis on the Bard EP system was performed to determine the mean cycle length of underlying sinus rhythm and the mean cycle length of automaticity. For each PV automaticity beat, the preceding LA–PV ‘coupling interval’ was calculated as the interval between local LA activation (recorded on proximal CS for right PVs and distal CS for left PVs) and the earliest PV activation on the CMC (Figure 2, left panel). In a subset of 69 PVs, exit block was verified by pacing (S1-S1:600 ms, from 2 mA up to 20 mA) from the ablation catheter positioned within the PV distal to the ablation line with the circular PV catheter in the same vein to assist in assessing local capture.6 Pacing was performed at several sites within the encircled region until there was proof of capture of the local venous myocardium (i.e. clear PV potentials close to the stimulus artefact) together with absence of capture of far-field regions (surface ECG, CS activation pattern).
Figure 2.
Randomly applied premature PV stimuli. Left panel: for each PV automaticity beat, the preceding LA–PV ‘coupling interval’ was calculated as the interval between local LA activation (recorded on proximal CS for right PVs and distal CS for left PVs) and the earliest PV activation on the CMC. Due to complete dissociation between LA sinus rhythm and PV rhythm, a wide range of LA–PV coupling intervals were present. Right panel: random distribution of all spontaneously applied and wide range of coupling intervals. Out of 1975 analysed PV automaticity beats, 1292 had a preceding coupling interval of ≥300 ms suggesting that these beats fell within the local excitable period of the LA. CS, coronary sinus; L, lasso; LA, left atrium; PV, pulmonary vein; CI, coupling interval; CMC, circular mapping catheter.
Statistical analysis
Categorical variables are expressed as frequencies and percentages. In the case of a normal distribution, continuous data are presented as mean value ± standard deviation. Otherwise, median and interquartile ranges are reported.
Results
Patient and pulmonary vein characteristics
Mean age was 58 ± 9 years, with 78% males and 80% paroxysmal AF. Pulmonary vein automaticity was observed in 171 PVs: 61 right superior PV (RSPV), 33 right inferior PV, 47 left superior PV, and 30 left inferior PV (Table 1). Cycle length of PV automaticity was >1000 ms in all cases. At the completion of a 30 min waiting period, PV automaticity only persisted in 33 out of 171 veins (19%). Acute reconnection was seen in 46 out of 171 veins (27%).
Table 1.
Characteristics of the pulmonary vein automaticity observed in 171 pulmonary veins
| N (%) | |
|---|---|
| RSPV, n (%) | 61 (36%) |
| RIPV, n (%) | 33 (19%) |
| LSPV, n (%) | 47 (27%) |
| LIPV, n (%) | 30 (18%) |
| PV–LA exit conduction, n (%) | 1 (0.6%) |
| PV–LA exit block, n (%) | 170 (99.4%) |
Data are presented as number with percentages.
RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; LA, left atrium.
In all patients, previously ineffective anti-arrhythmic drugs were re-started immediately after the ablation and discontinued after 1 month. After at least 1-year follow-up, freedom of AF was observed in 102 out of 128 patients (80%).
Characteristics of the observed pulmonary vein automaticity
The mean cycle length of the spontaneous PVPs was 3770 ± 1995 ms (range: 1032–12 554 ms) with a standard deviation of 182 ± 275 ms. The mean cycle length of PV automaticity was not different between patients without recurrence (3916 ± 2032 ms) and with recurrence (3413 ± 1891 ms, NS). The mean number of analysed PV automaticity beats per vein was 20 ± 15 (median = 16, p25 = 6, p75 = 33). Due to complete dissociation between sinus rhythm and PV rhythm, a wide range of LA–PV coupling intervals were present (Figure 2, left panel). Mean LA–PV coupling interval was 459 ± 299 ms. The distribution of all spontaneously applied coupling intervals is given in Figure 2 (right panel). From the histogram, it is clear that there was an at-random distribution of coupling intervals over a wide range. Out of 1975 analysed PV automaticity beats, 1292 had a preceding coupling interval of ≥300 ms suggesting that these beats fell within the local excitable period of the LA.8 Considering the individual spectrum of LA–PV coupling intervals, the mean number of PV automaticity beats with a coupling interval of ≥300 ms per vein was 11 ± 9 (median = 11, p25 = 3, p75 = 18).
Residual pulmonary vein–left atrium exit conduction after proven left atrium–pulmonary vein entry block
We observed that in 170 out of 171 PVs with automaticity (99.4%), PV beats did not conduct to the LA. A representative example of bidirectional block is given in Figure 3. Absence of PV–LA conduction was confirmed by the demonstration of exit block during pacing from within 69 PVs.
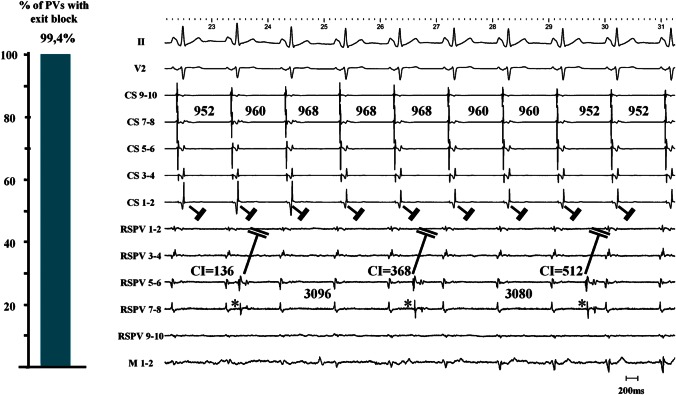
Figure 3.
Left panel: in 170 out of 171 PVs we observed no residual PV–LA exit conduction after proven LA–PV entry block. Right panel: after encircling the right-sided PVs, a stable, regular, and slow PV rhythm (*) appeared in the RSPV (2651 ± 68 ms) during underlying sinus rhythm (976 ± 24 ms). Together with the elimination of PV potentials (only far-field potentials resided on the CMC with timing consistent with far-field RA activation), this unambiguously proved LA–PV entry block. Three PV automaticity beats with a coupling interval of 136, 368, and 512 ms are shown. None of these PV beats advanced LA activation or altered the CS activation pattern. CS, coronary sinus; RSPV, right superior pulmonary vein; M, mapping catheter; LA, left atrium; PV, pulmonary vein; CMC, circular mapping catheter.
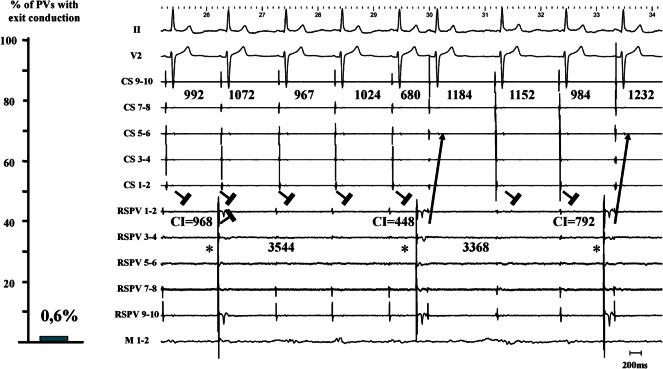
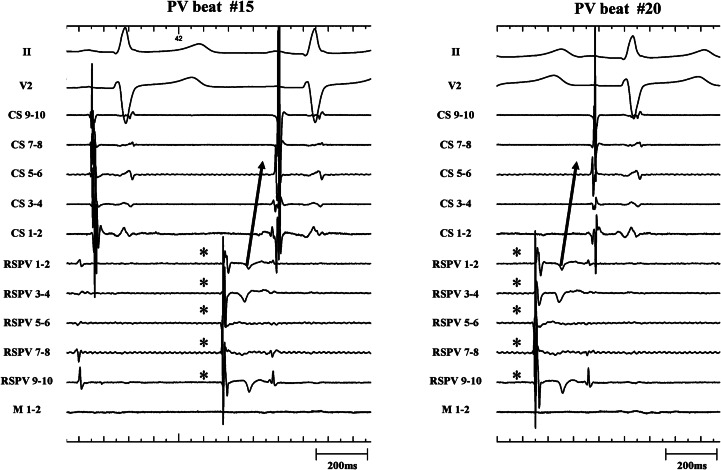
Only 1 PV out of 171 PVs revealed residual PV–LA exit conduction (0.6%) after proven LA–PV entry block (Figure 4, left panel). The corresponding electrogram tracing is shown in Figure 4 (right panel). A stable, regular, and slow PV rhythm (3486 ± 200 ms) was observed in the RSPV. Together with elimination of PV potentials (only far-field potentials with a biphasic electrogram morphology and a timing consistent with far-field RA activation), this unambiguously proved LA–PV entry block. The last two PV beats advanced LA activation with a consistent change in CS activation pattern. This proved residual PV–LA conduction in the presence of unidirectional block. In this patient, out of 33 analysed PV automaticity beats with an LA–PV coupling interval ranging from 56 to 1088 ms, 15 beats did capture the LA with the shortest coupling interval being 320 ms. Capture of the LA by PV beats was characterized by a consistent change in CS activation pattern and P-wave (Figure 5, high sweep speed image). Finally, in this patient, exit pacing from within the encircled region (10 mA, 2 ms pulse width pacing from the ablation catheter) paralleled the observation of unidirectional block at the LA–PV junction (Figure 6).
Figure 4.
Left panel: only 1 single PV out of 171 PVs revealed residual PVLA exit conduction (0.6%) after proven LA–PV entry block. Right panel: the corresponding electrogram tracing is shown. PV automaticity beats (*) with a coupling interval of 968, 448, and 792 ms are shown. The two last beats advanced LA activation with a clear and consistent change in CS activation pattern. This proved residual PV–LA conduction and the presence of unidirectional block. CS, coronary sinus; RSPV, right superior pulmonary vein; M, mapping catheter; LA, left atrium; PV, pulmonary vein.
Figure 5.
Representative electrograms at higher sweep speed illustrating the single case of unidirectional LA–PV block (same patient as Figure 4). Two PV automaticity beats (*) are shown. Clearly there is consistency in CS activation pattern and P-wave during capture. The CS activation pattern is different from the activation during sinus rhythm (left panel). CS, coronary sinus; LA, left atrium; PV, pulmonary vein.
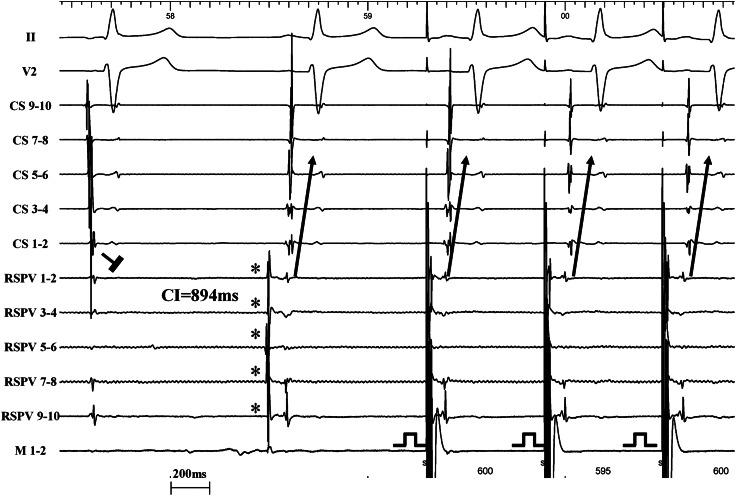
Figure 6.
Representative electrograms illustrating the single case of unidirectional LA–PV block (same patient as Figure 4). The first beat shows entry block. The second beat reveals a PV automaticity beat (*) with PV–LA exit conduction after a LA–PV coupling interval of 894 ms. The third beat shows exit pacing from the mapping catheter within the encircled region which results in clear capture of the PV myocardial sleeves and 1 : 1 exit conduction towards the LA with the same activation pattern on the CS as the one observed during exit conduction from the PV automaticity. CS, coronary sinus; RSPV, right superior pulmonary vein; M, mapping catheter; LA, left atrium; PV, pulmonary vein.
Discussion
In a large multicentre series of patients undergoing PV ablation for drug-resistant AF we observed that unidirectional block at the LA–PV junction is unusual (0.6%). Pulmonary vein to LA conduction was assessed in a pacing-free model of both unambiguous proof of entry block and randomly applied spontaneous premature stimuli to the PV.
Catheter ablation, bi- and unidirectional block
Catheter ablation aims to create a continuous barrier prohibiting transmission of electrical conduction with the electrophysiological endpoint of bidirectional block as the hallmark of completion of the lesion set. Unidirectional conduction block following ablation may be determined by inherent anatomic differences in fibre orientation and tissue characteristics favouring impulse transmission in a particular direction.9 The above principles are applicable to linear or circumferential ablation with the classic example being cavotricuspid isthmus (CTI) ablation for typical atrial flutter. Unidirectional block in the setting of CTI ablation is unusual. Although several groups reported residual latero-medial conduction after obtaining medio-lateral CTI block (apparent unidirectional block),10,11 other groups reported complete absence of unidirectional block when assessed with multipolar catheters meticulously positioned in relation to the ablation line.12,13
In the setting of PV ablation, bidirectional block at the LA–PV junction is defined as the absence of conduction from the LA to PV (entry block) and in the opposite direction from PV to LA (exit block).2,3 Entry block during ablation can be recognized by the complete elimination of the PVP on the CMC or the appearance of dissociated PVPs. This is commonly used as the simple and primary endpoint in AF ablation. However, Gerstenfeld et al.5 reported residual PV–LA exit conduction (assessed by exit pacing from the ablation catheter or CMC within the circle at 10 mA amplitude and 2 ms pulse width) after LA–PV entry block in 42% of the PVs. In theory, unidirectional block at the LA–PV may be explained by tissue characteristics favouring impulse transmission in a particular direction.9 Using a basket catheter, Kumagai et al.14 observed that sites of entry breakthrough may differ from exit breakthrough sites. Takahashi et al.15 reported residual PV–LA conduction via residual intervenous conduction in 14% of patients after apparent entry block at the superior PV ostium. Therefore, segmental ablation of entry fibres might be associated with residual exit conduction. In circumferential PV ablation, unidirectional conduction block is expected to be less common due to the underlying source–sink mismatch imposed by abrupt tissue expansion from the myocardial PV sleeves towards the LA.13 It would be unlikely that after ablation-induced block of preferential conduction from the LA towards PV, the PVs would still be capable of exciting the large LA. On the other hand, macroscopic anatomy might not necessarily reflect local source-sink mismatches and on a microscopic scale a well-connected group of cells within the PV may connect to a small exit pathway to the LA. Finally, ablation itself may transiently or permanently alter anatomy and excitability favouring impulse transmission in a particular direction.
True prevalence of unidirectional block at the left atrium–pulmonary vein junction?
In the present study, we used PV automaticity as a model of unambiguous proof of entry block and a model of randomly applied spontaneous premature stimuli to the PV to determine unidirectional block at the LA–PV junction. Although it is hard to prove that automaticity is completely uninfluenced by excitatory current flowing from the rest of the atrium—because older studies have described ‘triggered automaticity’16,17—pacemaker activity is expected to arise only after creating complete electrical silence downstream.18 As such, PV automaticity together with elimination of all PV potentials on the CMC seems a definite proof of LA–PV entry block. Using this model of PV automaticity, we observed a 0.6% prevalence of residual PV–LA conduction after unambiguously proven LA–PV entry block. These findings strongly suggest that true unidirectional block is an exceptional phenomenon at the LA–PV junction.
The discrepancy between the low prevalence of unidirectional block in the present study of <1% compared with 42% in an earlier study5 may be explained by: (i) differences in technique with segmental ostial ablation using an 8 mm ablation catheter compared with continuous linear encirclement using an irrigated 4 mm ablation catheter in the present study; (ii) underestimate of unidirectional block in the present study: although a wide range of coupling intervals to the LA were documented due to the random nature of spontaneous PVPs, with the majority ≥300 ms and likely to conduct to the atrium in the presence of incomplete conduction block,8 confirmation by PV pacing was not performed in all patients; (iii) overestimate of unidirectional block in the study by Gerstenfeld et al.5 Without careful annotation of baseline electrograms, dedicated differential pacing and verification of the correct position of the CMC, assessment of entry block is subjective and remaining PV potentials may not be recognized. Moreover exit pacing manoeuvres may have led to an erroneous diagnosis of residual PV–LA conduction due to capture of far-field structures neighbouring the PV ostium.
Finally, the low prevalence of unidirectional block in the present study is consistent with more recent observations based upon meticulous pacing manoeuvres in small patient groups. Shah et al.3 performed careful exit pacing in 142 PVs and observed only 3% of residual PV–LA conduction after entry block (unreported data). Similarly, Pratola et al.19 observed that exit block was invariably present in all of the veins where no PV potentials and complete voltage abatement around the PVs were present.
Clinical implications
Creation of permanent exit block at the PV–LA junction remains the ultimate goal in preventing PV foci from initiating AF.4,20,21 Prior reports suggested that demonstration of entry block into the vein is not a sufficient condition to conclude exit block implying that evaluation of exit conduction by pacing manoeuvres is a critical step after obtaining entry block.5 The present study suggests that entry block (i.e. complete elimination of PVP obtained on the CMC) is a sufficient endpoint for PVI. Pulmonary vein pacing maintains an important place in the assessment of PVI in equivocal cases with apparent far-field potentials.
In the present study a range of measures were routinely adopted to achieve a high degree of accuracy in determining LA–PV entry block including: annotation of baseline CMC recordings, a correct and stable position of the CMC left in position via a second transseptal puncture during encirclement, differential pacing manoeuvres at various sites in the LA and observance of PVPs. If these combined criteria are consistent with LA–PV entry block, then evaluation of exit conduction adds little. In many AF ablation procedures however—and especially when using ‘single-shot devices’ (cryoballoon, PVAC)—the CMC (if any) is not at the LA–PV junction during ablation, there is no second LA catheter for differential pacing, or there is no electro-anatomical mapping to tag areas of interest. This may lead to ambiguity in determining LA–PV entry block.22,23 In those cases of uncertainty or at the time of repeat procedures, exit pacing is a suitable alternative most likely to identify persisting bidirectional connection rather than true unidirectional PV–LA block. One must be mindful of the pitfalls of PV pacing such as: far-field capture and the identification of local PV potentials in the presence of stimulus artefact.
Finally, it is important to state that the observation of PV automaticity as such (i.e. bidirectional block) does not necessarily obviate the need for testing with adenosine.24 Cheung et al.24 showed that administration of adenosine-induced transient reconduction from the LA to the PV in 9 out of 42 veins with automaticity.
Study limitations
This was a retrospective study. Although pacing manoeuvres were deliberately avoided in the present study, PV pacing in all veins could have added additional information. Furthermore, we did not quantify the different responses to PV pacing (actual capture vs. loss of capture vs. far-field capture). Although the observation of PV automaticity with no left atrial coupling is commonly observed and felt to be consistent with exit block, this question has not been formally studied. Adenosine was not given in the present study. Although adenosine is known to suppress PV automaticity in most veins,24,25 it would have been interesting to study whether in those veins with persisting automaticity transient exit conduction (i.e. transient unidirectional block) could be observed.
Conclusion
Unidirectional block at the LA–PV junction is unusual (0.6%). This observation is supportive of LA–PV entry block as a sufficient electrophysiological endpoint for PVI.
Acknowledgements
Assoc Professor Kistler's contribution is supported in part by the Victorian Government's Operational Infrastructure Support Program. Associate Professor Kistler is supported by a practitioner fellowship from the Australian National Health and Medical Research Council (NHMRC).
Conflict of interest: none declared.
References
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. doi:10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. doi:10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Shah D. Electrophysiological evaluation of pulmonary vein isolation. Europace. 2009;11:1423–33. doi: 10.1093/europace/eup289. doi:10.1093/europace/eup289. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary viens. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. doi:10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenfeld EP, Dixit S, Callans D, Rho R, Rajawat Y, Zado E, et al. Utility of exit block for identifying electrical isolation of the pulmonary veins. J Cardiovasc Electrophysiol. 2002;13:971–9. doi: 10.1046/j.1540-8167.2002.00971.x. doi:10.1046/j.1540-8167.2002.00971.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee G, Kalman JM, Vohra JK, Teh A, Medi C, Ling LH, et al. Dissociated pulmonary vein potentials following antral pulmonary vein isolation for atrial fibrillation: impact on long-term outcome. Heart. 2011;97:579–84. doi: 10.1136/hrt.2010.211367. doi:10.1136/hrt.2010.211367. [DOI] [PubMed] [Google Scholar]
- 7.Marchlinski FE. Atrial fibrillation catheter ablation: learning by burning continues. J Am Coll Cardiol. 2008;51:1011–3. doi: 10.1016/j.jacc.2007.11.042. doi:10.1016/j.jacc.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Jaïs P, Hocini M, Macle L, Choi KJ, Deisenhofer I, Weerasooriya R, et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation. 2002;106:2479–85. doi: 10.1161/01.cir.0000036744.39782.9f. doi:10.1161/01.CIR.0000036744.39782.9F. [DOI] [PubMed] [Google Scholar]
- 9.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. doi:10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cauchemez B, Haissaguerre M, Fischer B, Thomas O, Clementy J, Coumel P. Electrophysiological effects of catheter ablation of inferior vena cava-tricuspid annulus isthmus in common atrial flutter. Circulation. 1996;93:284–94. doi: 10.1161/01.cir.93.2.284. doi:10.1161/01.CIR.93.2.284. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher B, Pfeiffer D, Tebbenjohanns J, Lewalter T, Jung W, Lüderitz B. Acute and long-term effects of consecutive radiofrequency applications on conduction properties of the subeustachian isthmus in type I atrial flutter. J Cardiovasc Electrophysiol. 1998;9:152–63. doi: 10.1111/j.1540-8167.1998.tb00896.x. doi:10.1111/j.1540-8167.1998.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 12.Poty H, Saoudi N, Abdel Aziz A, Nair M, Letac B. Radiofrequency catheter ablation of type 1 atrial flutter. Prediction of late success by electrophysiological criteria. Circulation. 1995;92:1389–92. doi: 10.1161/01.cir.92.6.1389. doi:10.1161/01.CIR.92.6.1389. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, de Chillou C, Basiouny T, Sadoul N, Filho JD, Magnin-Poull I, et al. Cavotricuspid isthmus mapping to assess bidirectional block during common atrial flutter radiofrequency ablation. Circulation. 1999;100:2507–13. doi: 10.1161/01.cir.100.25.2507. doi:10.1161/01.CIR.100.25.2507. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai K, Noguchi H, Ogawa M, Nakashima H, Zhang B, Miura S, et al. New approach to pulmonary vein isolation for atrial fibrillation using a multielectrode basket catheter. Circ J. 2006;70:88–93. doi: 10.1253/circj.70.88. doi:10.1253/circj.70.88. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi A, Iesaka Y, Takahashi Y, Takahashi R, Kobayashi K, Takagi K, et al. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002;105:2998–3003. doi: 10.1161/01.cir.0000019585.91146.ab. doi:10.1161/01.CIR.0000019585.91146.AB. [DOI] [PubMed] [Google Scholar]
- 16.Janse MJ, van Capelle FJ. Electrotonic interactions across an inexcitable region as a cause of ectopic activity in acute regional myocardial ischemia. A study in intact porcine and canine hearts and computer models. Circ Res. 1982;50:527–37. doi: 10.1161/01.res.50.4.527. doi:10.1161/01.RES.50.4.527. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof CJ, Allessie MA. Sinus node automaticity during atrial fibrillation in isolated rabbit hearts. Circulation. 1992;86:263–71. doi: 10.1161/01.cir.86.1.263. doi:10.1161/01.CIR.86.1.263. [DOI] [PubMed] [Google Scholar]
- 18.Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Mechanisms underlying sustained firing from pulmonary veins: evidence from pacing maneuvers and pharmacological manipulation. Pacing Clin Electrophysiol. 2004;27:1120–9. doi: 10.1111/j.1540-8159.2004.00594.x. doi:10.1111/j.1540-8159.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 19.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. doi:10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang F, Antz M, Ernst S, Hachiya H, Deger FT, Schaumann A, et al. Recovered pulmonary vein conduction as a dominant for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from the double Lasso technique. Circulation. 2005;111:136–44. doi: 10.1161/01.CIR.0000151289.73085.36. doi:10.1161/01.CIR.0000151310.00337.FA. [DOI] [PubMed] [Google Scholar]
- 21.De Greef Y, Tavernier R, Vandekerckhove Y, Duytschaever M. Triggering pulmonary veins: a paradoxical predictor for atrial fibrillation recurrence after PV isolation. J Cardiovasc Electrophysiol. 2010;21:381–8. doi: 10.1111/j.1540-8167.2009.01646.x. doi:10.1111/j.1540-8167.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 22.Duytschaever M, Anne W, Papiashvili G, Vandekerckhove Y, Tavernier R. Mapping and isolation of the pulmonary veins using the PVAC catheter. Pacing Clin Electrophysiol. 2010;33:168–78. doi: 10.1111/j.1540-8159.2009.02609.x. doi:10.1111/j.1540-8159.2009.02609.x. [DOI] [PubMed] [Google Scholar]
- 23.Chierchia GB, Namdar M, Sarkozy A, Sorgente A, de Asmundis C, Casado-Arroyo R, et al. Verification of pulmonary vein isolation during single transseptal cryoballoon ablation: a comparison between the classical circular mapping catheter and the inner lumen mapping catheter. Europace. 2012 doi: 10.1093/europace/eus189. (EPUB ahead of print: 6 July 2012). [DOI] [PubMed] [Google Scholar]
- 24.Cheung JW, Ip JE, Chung JH, Markowitz SM, Liu CF, Thomas G, et al. Differential effects of adenosine on pulmonary vein ectopy after pulmonary vein isolation: implications for arrhythmogenesis. Circ Arrhythm Electrophysiol. 2012;5:659–66. doi: 10.1161/CIRCEP.112.971945. doi:10.1161/CIRCEP.112.971945. [DOI] [PubMed] [Google Scholar]
- 25.Marrouche N, Wazni OM, Martin DO, Rossillo A, Saliba W, Erciyes D, et al. Response to pharmacological challenge of dissociated pulmonary vein rhythm. J Cardiovasc Electrophysiol. 2005;16:122–6. doi: 10.1046/j.1540-8167.2005.40333.x. doi:10.1046/j.1540-8167.2005.40333.x. [DOI] [PubMed] [Google Scholar]