Abstract
Aims
For most elderly pacemaker patients, evaluation of rate-adaptive pacing using treadmill and bicycle tests is impractical and not representative of typical daily activities. This study was designed to compare the performance and physiological response of the closed-loop stimulation (CLS) rate-adaptive sensor to accelerometer (XL) and no rate sensor (DDD) during typical daily activity testing.
Methods and results
Subjects recently implanted with a Cylos pacemaker completed timed activities of daily life testing, which included walking, sweeping, and standing from a seated position. Activity performance and physiological response from each sensor mode was evaluated for subjects requiring ≥80% pacing. Overall, 74 subjects needed ≥80% pacing during at least one test. An increase in the area swept (CLS vs. XL, 1.67 m2 difference, P = 0.009; CLS vs. DDD, 1.59 m2 difference, P = 0.025) and a decrease in the prevalence of orthostatic hypotension (OH) after standing 1 min (CLS vs. XL, odds ratio = 0.16, P = 0.006; CLS vs. DDD, odds ratio = 0.18, P = 0.012) was observed in the CLS mode as compared with XL and DDD. No statistical difference in walk distance was observed between CLS and XL or CLS and DDD.
Conclusion
In acute testing, as compared with XL and DDD, CLS provides a more physiological response during the performance of activities of daily living for subjects with ≥80% pacing. This is clinically reflected in better performance during the sweep test as well as a decrease in the prevalence of OH in our elderly population.
Clinicaltrials.gov identifier: NCT00355797
Keywords: Rate-adaptive pacing, Closed-loop stimulation, Accelerometer, Orthostatic hypotension
What's new.
Novel and less-demanding testing scheme for evaluating rate-adaptive sensors in elderly patients needing pacemaker-mediated chronotropic enhancement.
Benefit provided by closed-loop stimulation rate response was most evident in low-energy activities, as shown by a reduction in orthostatic hypotension upon standing and an increased sweep performance.
Introduction
Symptom-limited exercise tests, including bicycle ergometry and the chronotropic assessment exercise protocol,1 have been commonly used to study pacemaker rate-adaptive sensors in subjects with chronotropic incompetence. While the sensors have been shown to increase heart rate, the increase is not always proportional to exertion and exercise performance is not always improved.2–8 These traditional tests are routinely used in healthy and active individuals, but the typical elderly pacemaker patient may find them difficult or impossible to perform.
As a result, many studies have started using sub-maximal testing protocols as an alternative. Six-minute walk test performance has shown good correlation with bicycle ergometry and more closely reflects daily activity levels.9 Studies have shown that rate-adaptive sensors, including sensors utilizing closed-loop stimulation (CLS), minute ventilation, and accelerometers (XL), can improve the distance walked.10–12
The physical performance of daily activities can also be influenced by physiological parameters, such as blood pressure. Data obtained from the Honolulu Heart Program found that elderly men with orthostatic hypotension (OH) are more likely to exhibit increased frailty, decreased walk performance, and reduced hand grip strength.13 Orthostatic hypotension is relatively common in the elderly, with prevalence ranging from 6.9 to 30%.13–15 For pacemaker subjects with OH, the fall in blood pressure can be moderated with tachypacing or overdrive algorithms.15–18 Rate-adaptive sensors activated by changes in body position or myocardial contractility may provide a similar, and possibly a more refined, benefit.
Closed-loop stimulation is designed to monitor and process intracardiac impedance signals associated with myocardial contraction dynamics on a beat-to-beat basis. As opposed to typical impedance-driven minute ventilation sensors where signal injection and sampling are remote, CLS samples at the site of injection therefore maximizing the myocardial contraction signal. Additional processing filters out any remote signal (e.g. respiration). As contraction dynamics are controlled by the autonomic nervous system, the impedance signals interpreted by CLS are directly linked to other physiological responses, such as heart rate, blood pressure by vasoconstriction and vasodilation, and respiration. The body's natural feedback loop, the parasympathetic and sympathetic nervous systems, allows CLS to increase or decrease the rate response as needed to match cardiac demand. Thus, CLS can provide a physiological rate response during activities where motions are reduced or absent,7 for events causing acute mental stress,19 and during vasovagal syncope.20 In contrast, XL-based sensors respond to upper body motions and adjust the rate response according to the amount of motion detected. As a result, low-motion activities, such as arm-waving and picking up objects, may not produce a physiological increase in the pacing rate.7
The CLEAR (CyLos REsponds with Physiologic RAte Changes DuRing Daily Activities) study was designed to directly compare CLS with XL and no rate sensor (DDD) with regard to physiological response and performance of daily activities. By using standardized and timed tests representing a range of daily activities, rate-adaptive sensor performance during typical daily life can be examined.
Methods
Study design
CLEAR was a randomized, prospective, single-blinded, multi-center study designed to enrol a total of 1500 subjects with typical pacing indications. The study consisted of a long-term randomized evaluation of outcome measures, including but not limited to, quality of life, changes in 6 min walk distance, and atrial fibrillation burden. In addition, the first 500 subjects enrolled were required to complete a within-subject comparison designed to evaluate the acute effect of rate-adaptive sensors on activities of daily living. The results of this within subject testing are presented below.
Subject selection and randomization
At each of the US centers involved, the study was approved by the institutional review board. Subjects implanted within 45 days with a pacemaker capable of CLS and XL (Cylos VR, DR, or DR-T, BIOTRONIK, Inc.) were eligible for enrolment. All participants provided written informed consent, were ≥18 years old, and physically able to perform all tests. Subjects performed all three daily activity tests in all three sensor modes. Block randomization was used to determine the sensor order.
Device programming and daily activity testing
Testing occurred within 45 days of enrolment and after an initial CLS calibration period of 7 days. During testing, the default sensor gain setting of 4 was utilized and automatic sensor gain was turned off to ensure the setting was not auto-adjusted. Single-chamber pacing was used for subjects experiencing atrial fibrillation during testing. Though other device programming, including basic/lower rate, was at the discretion of the investigator, site personnel were instructed to keep these settings consistent among all three testing modes.
Subjects were blind to sensor mode. All testing was completed during one or two visits (up to 72 h apart) with all three tasks being completed in the first randomized sensor mode before starting in the second. The three tests, listed in order, were (i) 6 min walk test, (ii) stand and go test, and (iii) sweep test. The 6 min walk test has previously been described;9 however, the sweep test is novel to this study. The stand-and-go test is a modified and timed version of the up-and-go test,21 used to evaluate fall risk in the elderly. A brief description of each test is provided below:
Six-minute walk. Subjects were asked to walk a 15.2 m (50 ft) course at a brisk, but not uncomfortable pace, for 6 min. The total distance walked was recorded.
Stand and go. Blood pressure was recorded once while the subject was seated, once immediately upon standing, and once after standing for 1 min. Thereafter, the subject was instructed to walk 3.0 m (10 ft), return to starting position, sit in a chair, then stand as many times as possible within the remaining 5 min.
Sweep. Subjects were asked to entirely sweep an area of 2.3 m2 (25 ft2), marked by tape, as many times as possible in 5 min. The number of times the area was swept was recorded.
Heart rate and pacing percentage
Pacemaker statistics collected immediately after each test were used to determine the mean heart rate and percentage of pacing. Collection of mean heart rate data has been described previously.19 Pacing percentage was identified directly from the statistics with the atrial pacing percentage recorded for dual-chamber modes and ventricular pacing percentage for single-chamber modes.
For the purposes of this study, we elected to use the pacing percentage as an indication of subjects needing chronotropic enhancement. For each test group, only those subjects with a pacing percentage of ≥80% in both the CLS and XL sensor modes were considered to be in need of pacemaker-mediated chronotropic enhancement. A similar approach, using <10% atrial sensing, has been previously used to identify subjects with moderate to advanced atrial chronotropic disease.11 Age-, gender-, and disease-matched control groups for the walk and sweep tests were selected from subjects with a pacing percentage of ≤20% in CLS and XL. Subjects included in this control group were considered chronotropically competent.
Orthostatic hypotension
Orthostatic hypotension was defined as a decrease of ≥20 mmHg in systolic blood pressure or ≥5 mmHg in diastolic blood pressure after standing. Orthostatic hypotension was evaluated during the stand-and-go test immediately and after 1 min of standing.
Statistical analysis
Continuous data are expressed as mean and standard deviation, while categorical data are expressed as number and percentage. Paired Student's t-test and Fisher's exact tests were used to compare unadjusted test results. Because block randomization of sensor order was determined for the overall population (including subjects subsequently defined as having <80% pacing), sensor order for subjects with ≥80% pacing was disproportionate. Therefore, mixed effect models analysis was performed to provide outcomes adjusted for sensor order effect. Comparison of walk and sweep test baseline demographics used unpaired Student's t-tests or Fisher's exact tests, depending on the type of data analysed. For all analyses, a P value ≤0.05 was used to denote statistical differences.
Results
Participant flow and demographics
Of the 494 subjects consented at the 42 participating centers, 325 completed the within-subject testing. Test results were incomplete or ineligible due to failure to follow instructions in 76 subjects, withdrawal of consent in 53, testing not done in 35, and subject death in 5. If specific tasks were not completed properly, which occurred in 18 walk tests, 29 sweep tests and 23 stand-and-go tests, subjects were not included within the affected test group. A total of 74 subjects needed ≥80% pacing for at least one test, with a majority (43, 58%) of those needing ≥80% pacing for more than one test and over a quarter (20, 27%) needing ≥80% pacing for all three tests (Figure 1). Each test group (walk, sweep, and stand and go) was made up of all subjects that required ≥80% pacing while in CLS and XL during the specific test. Therefore, the walk test group consisted of 57 subjects, while the sweep and stand-and-go test groups included 34 subjects and 46 subjects, respectively. Subjects who needed ≥80% pacing for the sweep test were the most likely to also need ≥80% pacing for another test.
Figure 1.
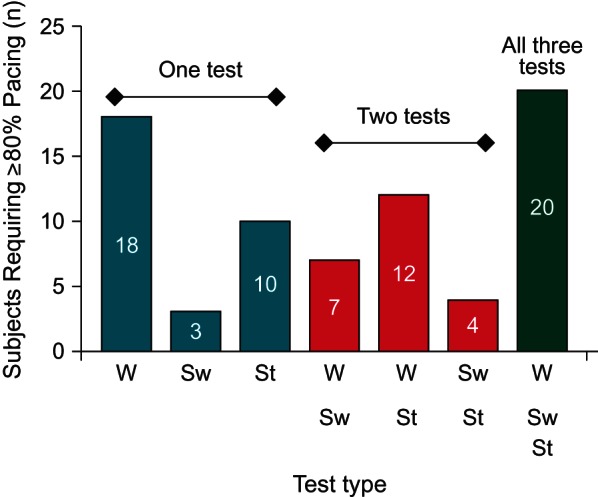
Subjects requiring ≥80% pacing during the walk, sweep, and stand-and-go tests. Subjects may have required ≥80% pacing for one test only, two tests, or all three tests. W denotes the walk test, Sw denotes the sweep test, and St denotes the stand-and-go test.
A summary of baseline characteristics and randomization assignments for the three test groups (walk, sweep, and stand and go) and for the 125 control subjects (who required ≤20% pacing in CLS and XL) are provided in Table 1.
Table 1.
Subject demographics for subjects in various test groups
| ≥80% pacing |
≤20% pacing controls for walk and sweep (n = 125) | |||
|---|---|---|---|---|
| Walk (n = 57) | Sweep (n = 34) | Stand and go (n = 46) | ||
| Age, years | 74.9 ± 12.6 | 79.6 ± 8.9 | 76.6 ± 11.5 | 71.1 ± 13.9 |
| Male, n (%) | 35 (61.4) | 20 (58.8) | 29 (63.0) | 71 (56.8) |
| Dual chamber pacing, n (%) | 47 (82.5) | 28 (82.4) | 37 (80.4) | 117 (93.6) |
| Left ventricular ejection fractiona, % | 58.1 ± 10.4 | 57.3 ± 11.8 | 57.4 ± 12.9 | 60.7 ± 11.4 |
| NYHA, n (%) | ||||
| Class I | 36 (63.2) | 17 (50.0) | 26 (56.5) | 79 (63.2) |
| Class II | 14 (24.6) | 11 (32.4) | 13 (28.3) | 41 (32.8) |
| Class III/IV | 7 (12.3) | 6 (17.6) | 7 (15.2) | 5 (4.0) |
| Reason for implant, n (%) | ||||
| Sinus node dysfunction | 40 (70.2) | 26 (76.5) | 32 (69.6) | 101 (80.8) |
| Heart block | 9 (15.8) | 7 (20.6) | 8 (17.4) | 32 (25.6) |
| Atrial fibrillationb | 9 (15.8) | 3 (8.8) | 10 (21.7) | 12 (9.6) |
| Other | 7 (12.3) | 2 (5.9) | 4 (8.7) | 15 (12.0) |
| Cardiovascular history, n (%) | ||||
| Coronary artery disease | 32 (56.1) | 19 (55.9) | 30 (65.2) | 51 (40.8) |
| Hypertension | 27 (47.4) | 21 (61.8) | 22 (47.8) | 71 (56.8) |
| Congenital heart disease | 10 (17.5) | 6 (17.6) | 7 (15.2) | 18 (14.4) |
| Cardiomyopathy (various) | 7 (12.3) | 3 (8.8) | 5 (10.9) | 6 (4.8) |
| Other | 13 (22.8) | 4 (11.8) | 9 (19.6) | 21 (16.8) |
| None | 6 (10.5) | 2 (5.9) | 4 (8.7) | 19 (15.2) |
| Sensor order, n (%) | ||||
| CLS/XL/DDD | 9 (15.8) | 7 (20.6) | 9 (19.6) | 25 (20.0) |
| CLS/DDD/XL | 7 (12.3) | 2 (5.9) | 7 (15.2) | 21 (16.8) |
| XL/CLS/DDD | 14 (24.6) | 8 (23.5) | 11 (23.9) | 16 (12.8) |
| XL/DDD/CLS | 10 (17.5) | 4 (11.8) | 5 (10.9) | 20 (16.0) |
| DDD/CLS/XL | 8 (14.0) | 5 (14.7) | 4 (8.7) | 21 (16.8) |
| DDD/XL/CLS | 9 (15.8) | 8 (23.5) | 10 (21.7) | 22 (17.6) |
Subjects may have multiple reasons for implant or be included in multiple cardiovascular history groups.
NYHA, New York Heart Association.
aLeft ventricular ejection fraction available for 47 (82.5%) subjects in the walk group, 29 (85.3%) in the sweep group, 39 (84.8%) in the stand and go group, and 87 (69.6%) in the ≤20% pacing group.
bWith slow ventricular response, long pauses, or other implant indication.
Six-minute walk and sweep results
As shown in Table 2, the distance walked was identical for XL and DDD modes and slightly further, but not statistically different, for CLS. However, during the sweep test, subjects were able to sweep a statistically greater area while in CLS as compared with XL or DDD.
Table 2.
Average distance walked and area swept
| Average | Unadjusted results |
Adjusted for order effect |
|||
|---|---|---|---|---|---|
| Δ (95% CI) CLS vs. |
P value CLS vs. |
Δ (95% CI) CLS vs. |
P value CLS vs. |
||
| Walk distance, m (n = 57) | |||||
| CLS | 262.6 ± 110.9 | ||||
| XL | 256.4 ± 112.2 | 6.17 (−4.50, 16.83) | 0.252 | 5.19 (−5.67, 16.05) | 0.345 |
| DDD | 256.1 ± 109.0 | 6.51 (−4.50, 17.52) | 0.241 | 6.81 (−4.00, 17.63) | 0.215 |
| Sweep area, m2 (n = 34) | |||||
| CLS | 22.3 ± 15.9 | ||||
| XL | 20.6 ± 15.0 | 1.67 (0.45, 2.89) | 0.009 | 1.64 (0.38, 2.90) | 0.012 |
| DDD | 20.7 ± 15.6 | 1.59 (0.21, 2.97) | 0.025 | 1.51 (0.24, 2.78) | 0.021 |
CLS, closed-loop stimulation; CI, confidence interval.
Mean heart rates obtained during the walk and sweep tests are compared in Figure 2. Subjects included in each control test group required ≤20% pacing in CLS and XL during the respective test and were matched for age, gender, and disease history. In both test groups, the highest mean heart rate was obtained for the matched controls. Heart rates obtained during testing in CLS were the second highest (i.e. most similar to but still lower than the controls) and were statistically higher than the mean heart rates obtained during testing in XL or DDD.
Figure 2.
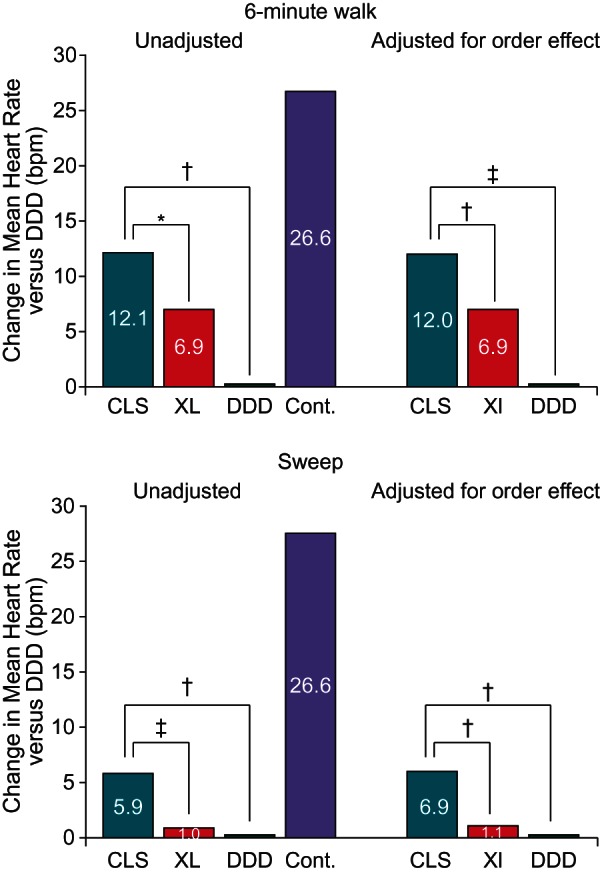
Change in mean heart rate from DDD during walk and sweep tests. Heart rate was lowest with DDD and highest for the control groups. Mean heart rate data could not be digitized for four subjects in the walk group and one subject in the sweep group. *P value = 0.003. †P value < 0.0001. ‡P value = 0.001.
Stand and go test results
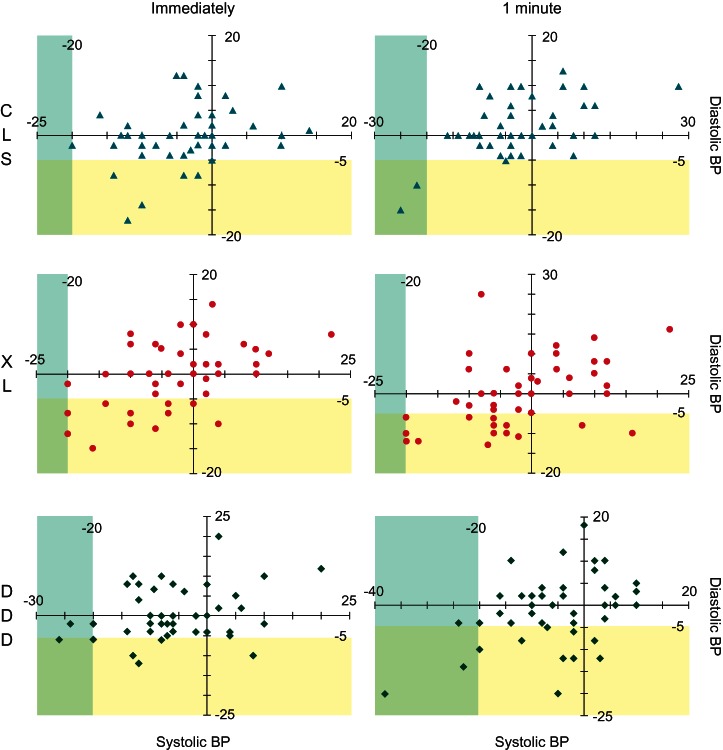
Fewer individuals met the definition of OH upon standing when in CLS than when programmed to either XL or DDD; though, a statistical difference was observed only after subjects had been standing for 1 min (Table 3). Changes in blood pressure for each subject in the stand-and-go test group are displayed in Figure 3. Out of the 46 subjects included in the stand-and-go test group, a total of 30 subjects (65%) met the definition of OH in at least one mode and one time point. About half of these subjects (14/30, 47%) were at a single mode and single time point. Almost all subjects meeting the definition of OH (29/30, 97%) displayed a decrease of ≥5 mmHg in diastolic blood pressure, either alone (20/30, 67%) or in conjunction with a decrease of ≥20 mmHg in systolic blood pressure (9/30, 30%).
Table 3.
Prevalence of OH during stand-and-go test
| Unadjusted results |
Adjusted for order effect |
||||
|---|---|---|---|---|---|
| OH, n (%) | OR (95% CI) CLS vs. |
P value CLS vs. |
OR (95% CI) CLS vs. |
P value CLS vs. |
|
| Immediately after standing (n = 46) | |||||
| CLS | 6 (13.0) | ||||
| XL | 12 (26.1) | 0.43 (0.14, 1.25) | 0.188 | 0.40 (0.12, 1.31) | 0.126 |
| DDD | 10 (21.7) | 0.54 (0.18, 1.63) | 0.410 | 0.48 (0.14, 1.62) | 0.228 |
| After standing 1 min (n = 46) | |||||
| CLS | 3 (6.5) | ||||
| XL | 14 (30.4) | 0.16 (0.04, 0.60) | 0.006 | 0.10 (0.02, 0.53) | 0.008 |
| DDD | 13 (28.3) | 0.18 (0.05, 0.67) | 0.012 | 0.10 (0.02, 0.53) | 0.008 |
OH, orthostatic hypotension; OR, odds ratio; CI, confidence interval; CLS, closed-loop stimulation.
Figure 3.
Change in blood pressure from baseline for each subject included in the stand-and-go test. Systolic blood pressure is displayed on the x-axis, while diastolic blood pressure is displayed on the y-axis. The blue shading denotes the systolic blood pressure OH criteria of a decrease of ≥20 mmHg, while the yellow shading denotes the diastolic blood pressure OH criteria of a decrease of ≥5 mmHg.
Discussion
In the CLEAR study, three tests, designed to simulate typical activities for our elderly study population, were used to determine whether CLSbenefits subjects requiring ≥80% pacing. Within-subject evaluations were performed for walking, sweeping, and standing. During the walk, CLS provided a small but non-statistical improvement in the distance walked. These results differ from previous studies in which rate-adapting sensors, including CLS and XL, were able to significantly increase walk distance as compared with DDD.10–12 The discrepant results may be due to differences in test design and subject population. For CLEAR, all walk group subjects, except for one, performed the three walk tests on the same day and within 90 days of implant. In contrast, subjects enrolled in two of the studies completed the DDD walk at 1 month after implant, and the sensor mode walk at month 4 or at months 4 and 7.11,12 The latter design allowed for a period of cardiovascular training related to enhanced chronotropism, thus stacking the deck in favour of the subsequently tested sensor mode(s). Subjects in the CLEAR walk group were also slightly older (2.5–2.9 years on average) and walked less distance in comparison (68–138 m less on average in any mode). This suggests that the CLEAR subjects were generally more frail and less conditioned than subjects evaluated in previous studies and that chronotropic incompetence is only one of many competing physical conditions limiting subject mobility.
Statistically more area (average of 1.6 m2 or 17.2 ft2) was swept while subjects were in CLS as compared with XL and DDD. The percentage increase of 7% is comparable with significant increases seen in other performance-based tests.10–12 Six-minute walk test results presented by Abraham et al.22 for the MIRACLE Study found CRT group patients increased the distance walked by 29 m (∼10% improvement) at 6 months. Walk distance improvements were even smaller, ∼4 and 7%, at 1 and 3 months, respectively. While the percent change was low, the results were used to show that CRT devices provide significant clinical improvement in the patient group studied.
In contract to the walk group, the sweep group subjects were, on average, 4.7 years older, more likely to be New York Heart Association Class II, III, or IV, had a higher prevalence of hypertension, and a lower prevalence of atrial fibrillation. However, analysis of subject demographics revealed no statistical differences between the walk and sweep groups (age, P = 0.055; all other demographics, P > 0.2). In addition, 27/34 sweep group subjects were also included in the walk group. Other than subject selection, the motions and physical exertion required to perform each activity are quite different and may well have contributed to the differences in walk vs. sweep performance. As opposed to walking, sweeping requires motions that are less routine in daily living which leads to sub-optimal conditioning of activity-specific skeletal muscles. In addition, the overall metabolic demands required with sweeping are less due to the minimal use of the bulky lower body muscles. The ability to increase heart rate relatively during the less routine and less energy consuming activities (such as sweeping) may provide a greater benefit, especially in the elderly.
Results from the stand-and-go test reveal that CLS may benefit pacemaker subjects with OH. Use of CLS resulted in over a 75% reduction in the prevalence of OH after standing 1 min as compared with XL and DDD. Upon standing, intrathoracic and intracardiac volumes decrease, which should increase intracardiac impedance and consequently provide an increase in the CLS driven heart rate. An early increase in heart rate may slightly decrease stroke volume early after standing, thus accelerating recruitment of carotid baroreceptor adrenergic reflexes. These early adjustments are clinically very relevant as most symptoms occur early after standing, as opposed to a few minutes later when autonomic accommodation has finally occurred.
The remarkable decrease in OH when using CLS is consistent with the premise that activities requiring a lower energy expenditure (standing < sweeping < walking) are more dramatically affected by improvements in the chronotropic cardiac response. This may significantly benefit the quality of life for the elderly and frail (like those in the CLEAR study group) as they tend to spend most of their day participating in low-energy activities.
Head-up tilt test studies have shown that CLS is able to increase the atrial paced rate immediately upon tilting.23–25 Cron et al.24 reported that tilting with an active CLS sensor resulted in a heart rate increase of >20 b.p.m. within 1 min and a return to baseline within 2–3 min. A slightly greater increase (>25 b.p.m.) was observed in another study.25 The increased heart rate with CLS seen during these studies is similar to successful overdrive pacing algorithms used for patients with OH whereby the heart rate is increased by 35 b.p.m. for 2 min before slowly decaying back to the base rate.15 For our present study, the device statistics were able to detect a maximum average increase of 12.3 b.p.m. after standing 1 min with CLS, with six subjects experiencing a maximum increase of >20 b.p.m. after standing 1 min. For the other pacing modes, the increases were smaller (9.1 b.p.m. increase for XL and 7.3 b.p.m. increase for DDD). The smaller increase in heart rate with CLS seen in this study is probably due to the use of device statistics, which provides incremental averages instead of a continuous heart rate, combined with the fact that standing, a dynamic activity, requires muscle contractions and activates numerous neurohumoral responses not initiated during passive head-up tilt tests.
For all tests, the disproportion in sensor order (as seen in Table 1) did not have much influence on the outcomes. Results adjusted for sensor order were similar to unadjusted results, suggesting that changes in performance are correlated with the sensor mode and not with the sensor order, training effects, or subjects becoming tired during later tests.
The results of this study emphasize the importance test selection plays in identifying pacemaker patients who may benefit from rate adaptation. Not all of our test group subjects required ≥80% pacing during all three tests, suggesting that pacing requirements are not the same for different levels and types of activity. This is consistent with clinical observations of patients labelled as chronotropically incompetent by standard clinical Holter or stress testing criteria. In addition, results for CLS indicate that non-traditional tests, including low energy consuming activities, may be required to effectively evaluate possible benefits provided by rate-adaptive sensors.
Study strengths and limitations
The testing scheme used in this study defines a unique approach that we feel provides an empiric and reproducible patient group benefitting from rate-adaptive pacing. Our test groups (≥80% pacing) and the control group (≤20% pacing) were retrospectively identified and were not expected to identify all subjects that meet the traditional treadmill-based definition of chronotropic incompetence or chronotropic competence. This may have resulted in a larger group of individuals than would have been prospectively identified by traditional protocols. On the other hand, the use of traditional protocols may not have identified subjects benefitting during the sweep or orthostatic tests. In addition, these traditional protocols are not representative of daily activities for most elderly patients.
It is unclear if the results from our elderly population can be generalized to a younger pacemaker population where the current standard techniques for identifying chronotropic incompetence are generally viable options. As with many clinic-based studies, it is not known if the improved performance demonstrated with CLS during this testing will translate to improved performance of activities or quality of daily living at home.
A major strength of the CLEAR study design is the use of within subject analysis (each subject completed each test in all three sensor modes). This paired data design requires significantly fewer subjects to obtain statistically valid results. Besides adding statistical power to the study, the within-subject test design also minimizes variations in results obtained across the subject population. In CLEAR, the same subject performed the same tests in each mode on the same day with the same person administering the test and collecting data. An unpaired (between-subject) design would have required more than 15× the number of subjects (>510 subjects) to obtain P < 0.05 with the same sweep test results (mean and standard deviation). Additional power was provided by randomizing the sensor order during testing. However, CLEAR was not intended to be prospectively powered to obtain statistically significant results for the ≥80% pacing test groups, which may account for a lack of significance (statistical and/or clinical) in some data subsets.
Conclusions
The results of the CLEAR study reveal that CLS provides benefits over XL and DDD in patients requiring ≥80% pacing. Specifically, subjects were able to sweep more area, had a reduced prevalence of OH, and exhibited a brisker chronotropic response, as indicated by a closer to ‘normal’ heart rate, during both the walk and sweep activities. Overall, in this elderly deconditioned population, the beneficial effects of an enhanced chronotropic response were more evident in lower energy activities (CLS effect was most prominent in the stand and go test, least with the walk test).
Finally, this study also raises a legitimate question regarding the need of a more ‘tuned’ definition of chronotropic incompetence.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: F.M.A.-S. has received research support from St Jude Medical, Sorin Group, Medtronic, and BIOTRONIK. He is also a member of the speaker's bureau for Sorin Group and St Jude Medical. N.S. has received research support from St Jude Medical, Medtronic, and Sorin Group. C.D.M. is an employee of BIOTRONIK, Inc. In addition to the individual disclosures, the institutions of F.M.A.-S., N.S., B.L.R., and J.V.D. have received research support from BIOTRONIK for the conduct of the CLEAR study.
Funding
This study was supported by BIOTRONIK, Inc., Lake Oswego, OR, USA.
Supplementary Material
References
- 1.Wilkoff BL, Corey J, Blackburn G. A mathematical model of the cardiac chronotropic response to exercise. J Electophysiol. 1989;3:176–80. [Google Scholar]
- 2.Jutzy RV, Florio J, Isaeff DM, Marsa RJ, Bensal RC, Jutzy K, et al. Comparative evaluation of rate modulated dual chamber and VVIR pacing. Pacing Clin Electrophysiol. 1990;13:1838–46. doi: 10.1111/j.1540-8159.1990.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 3.Sulke N, Chambers J, Dritsas A, Sowton E. A randomized double-blind crossover comparison of four rate-responsive pacing modes. J Am Coll Cardiol. 1991;17:696–706. doi: 10.1016/s0735-1097(10)80186-x. [DOI] [PubMed] [Google Scholar]
- 4.Capucci A, Boriani G, Specchia S, Marinelli M, Santarelli A, Magnani B. Evaluation by cardiopulmonary exercise test of DDDR versus DDD pacing. Pacing Clin Electrophysiol. 1992;15:1908–13. doi: 10.1111/j.1540-8159.1992.tb02992.x. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus A, Michell K for the Dromos DR Investigators Group. A prospective multicenter study demonstrating clinical benefit with a new accelerometer-based DDDR pacemaker. Pacing Clin Electrophysiol. 1996;19:1694–7. doi: 10.1111/j.1540-8159.1996.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 6.Candinas R, Jakob M, Buckingham T, Mattmann H, Amann FW. Vibration, acceleration, gravitation, and movement: activity controlled rate adaptive pacing during treadmill exercise testing and daily life activities. Pacing Clin Electrophysiol. 1997;20:1777–86. doi: 10.1111/j.1540-8159.1997.tb03566.x. [DOI] [PubMed] [Google Scholar]
- 7.Malinowski K. Interindividual comparison of different sensor principles for rate adaptive pacing. Pacing Clin Electrophysiol. 1998;21:2209–13. doi: 10.1111/j.1540-8159.1998.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamas GA, Knight JD, Sweeney MO. Impact of rate-modulated pacing on quality of life and exercise capacity—evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT) Heart Rhythm. 2007;4:1125–32. doi: 10.1016/j.hrthm.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Langenfeld H, Schneider B, Grimm W, Beer M, Knoche M, Riegger G, et al. The six-minute walk—an adequate exercise test for pacemaker patients? Pacing Clin Electrophysiol. 1990;13:1761–5. doi: 10.1111/j.1540-8159.1990.tb06886.x. [DOI] [PubMed] [Google Scholar]
- 10.Provenier F, Jordaens L. Evaluation of six minute walking test in patients with single chamber rate responsive pacemakers. Br Heart J. 1994;72:192–6. doi: 10.1136/hrt.72.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padeletti L, Pieragnoli P, Biase L, Colella A, Landolina M, Moro E, et al. Is a dual-sensor pacemaker appropriate in patients with sino-atrial disease? Results from the DUSISLOG study. Pacing Clin Electrophysiol. 2006;29:34–40. doi: 10.1111/j.1540-8159.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 12.Coenen M, Malinowski K, Spitzer W, Schuchert A, Schmitz D, Anelli-Monti M, et al. Closed loop stimulation and accelerometer-based rate adaptation: results of the PROVIDE study. Europace. 2008;10:327–33. doi: 10.1093/europace/eun024. [DOI] [PubMed] [Google Scholar]
- 13.Maskai KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–5. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 14.Luukinen H, Koski K, Laippala P, Kivela SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–80. doi: 10.1001/archinte.159.3.273. [DOI] [PubMed] [Google Scholar]
- 15.Tse HF, Lau CP, Park E, Bornzin G, Yu C, Benser M, et al. Transient overdrive pacing upon standing prevents orthostatic hypotension in elderly pacemaker patients with chronotropic incompetence. Pacing Clin Electrophysiol. 2007;30:188–92. doi: 10.1111/j.1540-8159.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Abe H, Numata T, Hanada H, Kohshi K, Nakashima Y. Successful treatment of severe orthostatic hypotension with cardiac tachypacing in dual chamber pacemakers. Pacing Clin Electrophysiol. 2000;23:137–9. doi: 10.1111/j.1540-8159.2000.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 17.Tse HF, Siu CW, Tsang V, Yu C, Park E, Bornzin G, et al. Blood pressure response to transition from supine to standing posture using an orthostatic response algorithm. Pacing Clin Electrophysiol. 2005;28(Suppl. 1):S242–5. doi: 10.1111/j.1540-8159.2005.00054.x. [DOI] [PubMed] [Google Scholar]
- 18.Kohno R, Abe H, Oginosawa Y, Nagatomo T, Otsuji Y. Effects of atrial tachypacing on symptoms and blood pressure in severe orthostatic hypotension. Pacing Clin Electrophysiol. 2007;30(1):S203–6. doi: 10.1111/j.1540-8159.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 19.Chandiramani S, Cohorn LC, Chandiramani S. Heart rate changes during acute mental stress with closed loop stimulation: report on two single-blinded, pacemaker studies. Pacing Clin Electrophysiol. 2007;30:976–84. doi: 10.1111/j.1540-8159.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 20.Occhetta E, Bortnik M, Audoglio R, Vassanelli C for the INVASY Study Investigators. Closed loop stimulation in prevention of vasovagal syncope. Inotropy Controlled Pacing in Vasovagal Syncope (INVASY): a multicentre randomized, single blind, controlled study. Europace. 2004;6:538–47. doi: 10.1016/j.eupc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 23.Filho MM, Nishioka S, Lopes H, Oliveira J, Pedros A, Siqueira S, et al. Neurohumoral behavior in recipients of cardiac pacemakers controlled by a closed-loop autonomic nervous system-driven sensor. Pacing Clin Electrophysiol. 2000;23:1778–82. doi: 10.1111/j.1540-8159.2000.tb07017.x. [DOI] [PubMed] [Google Scholar]
- 24.Cron TA, Hilti P, Schachinger H, Pouskoulas CD, Keller DI, Zaugg CE, et al. Rate response of a closed-loop stimulation pacing system to changing preload and afterload conditions. Pacing Clin Electrophysiol. 2003;26:1504–10. doi: 10.1046/j.1460-9592.2003.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- 25.Fortrat JO, Lemarie C, Bellard E, Victor J. Do we need a reflex tachycardia to stand up? Pacing Clin Electrophysiol. 2005;28:962–7. doi: 10.1111/j.1540-8159.2005.00216.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.