Abstract
Aims
Clinical trials have shown that anticoagulation with vitamin K antagonists (VKAs), e.g. warfarin, decreases the risk of stroke in patients with atrial fibrillation (AF); however, increased bleeding risk is one of the safety concerns. The primary objective was to conduct a systematic review of the published literature, assessing the risk of major bleeding and mortality in patients with AF treated with VKAs.
Methods and results
Online searches of MEDLINE, EMBASE, BIOSIS, and the Cochrane Library were performed to a pre-specified protocol from 1960 to March 2012 for randomized controlled trials (RCTs) and from January 1990 to March 2012 for observational studies. A total of 47 studies (16 RCTs and 31 observational studies) were included. Cumulative follow-up was 61 563 patient-years for RCTs and 484 241 patient-years for observational studies. The overall median incidence of major bleeding was 2.1 per 100 patient-years (range, 0.9–3.4 per 100 patient-years) for RCTs and 2.0 per 100 patient-years (range, 0.2–7.6 per 100 patient-years) for observational studies. With study year as a proxy for changing management patterns, some evidence of bleeding rates and/or their reporting increasing over time was noted. Mortality rates from observational studies were inadequately reported to allow comparison with those from RCT data.
Conclusion
The median rate of major bleeding in observational studies and RCTs is similar. The larger heterogeneity in bleeding rates observed in a real-life setting could reflect a high variability in standard of care of patients on VKAs and/or methodological differences between observational studies and/or variability in data sources.
Keywords: Atrial fibrillation, Bleeding, Mortality, Systematic review, Observational studies, Randomized studies
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia encountered in clinical practice, represents a growing clinical and economic burden.1 Atrial fibrillation is considered an epidemic, affecting 1 to 1.5% of the population in the developed world, and is projected to grow at least three-fold by 2050.2 The prevalence of AF is markedly increasing in our ageing society. The Rotterdam study, a large, European, population-based study, reported an overall prevalence of 5.5%, with age-specific prevalence ranging from 0.7% of people aged 50–59 years to 17.8% of those aged 85 years and older.3 Strongly associated with increased risk of mortality and morbidity due to stroke and other vascular complications, AF is a major public health burden.4
Although many clinical trials have shown that anticoagulation with warfarin and other vitamin K antagonists (VKAs) decreases the risk of ischaemic stroke in patients with AF, concerns remain regarding their long-term safety, mainly increased risk of bleeding, and prescribing practices for VKAs. Warfarin therapy is complicated because of the wide inter-individual variation in response and dose requirements for adequate anticoagulation, narrow therapeutic range [international normalized ratio (INR), 2.0–3.0 for AF], need for chronic anticoagulation monitoring, potentially life-threatening interaction with numerous foods and drugs, and long onset and offset of action. These factors all contribute to the significant underuse of warfarin in patients with AF at risk for stroke despite clear indication for its use.5,6 Vitamin K antagonists are also one of the drug classes creating the highest rates of emergency admissions to the hospital in the elderly.7
A number of published studies have reported risk of bleeding and mortality associated with warfarin and other VKAs; however, no systematic review has been performed to date to collate the available evidence. Thus, this study systematically assembled the best evidence from randomized clinical trials (RCTs) and observational studies conducted in the real-life setting to evaluate the risk of bleeding and mortality in the population with AF treated with warfarin and other VKAs.
Methods
Literature search and data extraction
This review was performed systematically in an unbiased manner by using a pre-specified protocol and an explicit, reproducible plan for literature search and synthesis. Study designs such as RCTs, prospective cohort studies, and retrospective study designs were included in this review. Searches to identify RCTs were of literature published from 1960 to 7 March 2012; searches to identify observational studies were of literature published from January 1990 to 7 March 2012, as observational clinical data published before 1990 are unlikely to be relevant to current treatment practices and resource patterns. No limitations on publication language or geographical perspective were applied. Translation of the articles to English was done as required.
The following electronic databases were searched: MEDLINE, EMBASE, BIOSIS, and the Cochrane Library. In addition, conference abstracts from 2010 to March 2012 from the American Heart Association, International Society on Thrombosis and Haemostasis, and European Society of Cardiology were searched. Search terms included combinations of free text and medical subject headings. Separate sets of terms were used for the health condition of interest (AF), the treatment of interest (VKAs), and the study types of interest (RCTs and observational studies). The specific search strategies for MEDLINE are shown in Appendix 1. Study inclusion was performed at two levels in parallel by two researchers. At level 1, titles and abstracts of all identified articles were screened. Articles were excluded if their abstracts fit any of the pre-specified exclusion criteria: case reports, letters, comments, editorials, and reviews. The full text of all papers determined to be eligible at level 1 was reviewed at level 2 to ensure that they met the inclusion criteria. All disagreements at various stages were resolved by consensus with input from an experienced senior researcher.
For each eligible study that passed both levels of screening, data were extracted by one researcher and verified with the original sources by a second researcher. To focus the review on large, comprehensive studies, only studies that included 300 or more patients receiving VKA treatment were selected. This sample size cut-off point was chosen so that, in the absence of an event, the upper limit of the 95% confidence interval (CI) for the event would be 1%, according to Hanley's rule.8
Data synthesis
Results of this systematic literature search were mainly summarized qualitatively using descriptive statistics. Qualitative synthesis consisted of detailed evidence tables and figures that included information on the study design, population size and characteristics (INR target range, percentage of time in INR range, CHADS2 criteria, and prescribing information), and results (i.e. mortality and bleeding in AF). Some data imputations were necessary to derive number of events, total patient-years, and rates per 100 patient-years. If not directly reported, patient-years for the group were calculated from the sum of the length of follow-up for each participant. When only two of these three estimates (number of events, total patient-years, and rate) were reported, the third was imputed using simple calculations. Univariate summaries, including the median, interquartile range (IQR), and weighted mean (weighted by sample size), were produced and are presented in a box-and-whisker plot.
This review assessed a potentially optimized VKA usage within the last two decades that might have impacted the incidence of bleeding and mortality. To assess this, we used a weighted linear regression model. For RCTs, the publication year was used, while for observational studies the midpoint of the observed period was taken, as these studies included data over a longer time period. Where observational studies did not give the start and end dates of the observation period, we imputed these dates from the year of publication and the average follow-up for patients.
Results
Study identification and characteristics
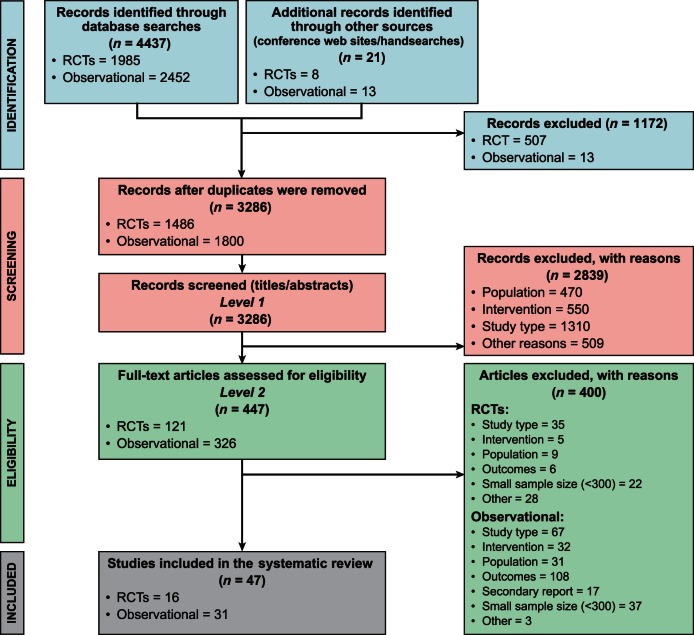
A total of 3286 records were retrieved after the searches were performed, and 47 studies with a sample size of at least 300 patients were included in the review. Of the included studies, 16 were RCTs and 31 were observational studies with either a prospective or retrospective study design. The search results are summarized in Figure 1, PRISMA flowchart. Detailed summaries of the included studies are provided in Appendices 2 and 3.
Figure 1.
Flow chart for study inclusion and exclusion. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCTs, randomized controlled trials.
Randomized controlled trials
All included RCTs were active-controlled trials that were published from 1989 through 2011 and designed to compare newer drugs with VKAs. Their primary objective was to evaluate frequency of fatal and non-fatal stroke (ischaemic or haemorrhagic), intracranial haemorrhage, or other clinically significant arterial embolism in patients who were treated with dose-adjusted VKA. The sample size in the warfarin treatment arm ranged from 319 to 9081 and the cumulative patient-years were 61 563. In all studies, the mean age of patients was greater than 60 years (range, 63–82 years) and most had a majority of male patients (range, 49 to 70%). Study length ranged from 5 months to 3 years. Among patients in the VKA group, the proportion of time in which the intensity of anticoagulation was in the therapeutic range calculated from all INR values during the study ranged from 42 to 86%.9,10 In most RCTs, the target therapeutic INR range of 2.0–3.0 was used, with the exception of four studies. In one study, warfarin dosage was adjusted to achieve an INR range of 1.7–3.0;11 whereas in three other studies, warfarin dosage was adjusted to achieve an INR range of 2–4.5.9,10,12
Observational studies
All 31 observational studies were published during the years 2001 to 2011. Data sources for these studies included registries, claims databases, hospital and anticoagulation clinic electronic records (inpatient and outpatient), and national mortality statistics databases. The year midpoint during which the data were collected for these studies was from 1997 to 2009. Most of the published studies were from data sources in the USA; other countries included the UK, Italy, Sweden, Denmark, Norway, Japan, Hong Kong (China), Australia, and Israel. The sample size in these studies ranged from 328 to 70 057 and the cumulative patient-years was 484 241. The included studies had a mean age range of 64–83 years and a greater proportion of male patients (>50%).
Bleeding
All randomized trials provided a definition for major bleeding, which was consistent across most trials (Table 1). The most frequent definition for major bleeding was bleeding that was fatal or overt bleeding with a drop in haemoglobin level of at least 20 g/L or requiring transfusion of at least 2 units packed blood cells, or haemorrhage into a critical anatomical site (e.g. intracranial, retroperitoneal). The overall median incidence of major bleeding in the included RCTs was 2.1 per 100 patient-years (range, 0.9–3.4 per 100 patient-years; IQR, 1.5–3.1 per 100 patient-years) (Figure 2), and the weighted mean was 2.8 per 100 patient-years.
Table 1.
Major bleeding data—RCTs
| Primary author (trial name) | Publication year | Patients with ≥1 major bleed | Patients (N) | Rate per 100 patient-years | Definition of major bleeding |
|---|---|---|---|---|---|
| Albers (SPORTIF V) 13 | 2005 | 84 | 1962 | 3.1 | Bleeding that was fatal or clinically overt and associated with either transfusion of ≥2 units of blood or a decrease in Hb ≥ 20 g/L, or bleeding that was intracranial, retroperitoneal, spinal, ocular, pericardial, or atraumatic articular (intracranial bleeding excludes intracerebral haemorrhages, which were counted as primary events) |
| Bousser (AMADEUS)14 | 2008 | 29 | 2293 | 1.4 | Bleeding that was fatal, intracranial, or affected another critical anatomical site, or overt bleeding with a drop in Hb ≥20 g/L or requiring transfusion of ≥2 units of erythrocytes |
| Chen (NA)11 | 2009 | 23 | 659 | 1.2 | Cerebral or gastric haemorrhage |
| Connolly (ACTIVE W)15 | 2006 | 93 | 3371 | 2.2 | Any bleeding requiring transfusion of ≥2 units of RBCs or equivalent of whole blood, or which was severe. Severe bleeding associated with any of the following: death, drop in Hb of ≥50 g/L, substantial hypotension with the need for inotropic agents, intraocular bleeding leading to substantial loss of vision, bleeding requiring surgical intervention (other than vascular site repair), symptomatic intracranial haemorrhage, or requirement for a transfusion of ≥4 units of blood |
| Connolly (RE-LY)16 | 2009 | 421 | 6022 | 3.4 | A reduction Hb of ≥20 g/L, transfusion of ≥2 units of blood, or symptomatic bleeding in a critical area or organ. Life-threatening bleeding was a subcategory of major bleeding that consisted of fatal bleeding, symptomatic intracranial bleeding, bleeding with a decrease in the Hb level of at least 50 g/L, or bleeding requiring transfusion of ≥4 units of blood or inotropic agents or necessitating surgery |
| Granger (ARISTOTLE)17 | 2011 | 462 | 9052 | 3.1 | Major bleeding was defined according to the ISTH criteria: clinically overt bleeding accompanied by a decrease in the Hb level of ≥2 g/dL or transfusion of ≥2 units of packed red cells, occurring at a critical site, or resulting in death |
| Lip (NCT00684307)18 | 2009 | 2 | 318 | 1.4 | Fatal bleeding, clinically overt bleeding causing a fall in Hb level of ≥20 g/L (1.24 mmol/L) or leading to transfusion of ≥2 units of whole blood or red cells, bleeding in areas of special concern or bleeding causing permanent treatment cessation; safety analysis population |
| Mant (BAFTA)19 | 2007 | 25 | 488 | 1.9 | Major haemorrhages were intracranial, haemorrhagic stroke or major extracranial haemorrhage (defined as a fatal haemorrhage or one that resulted in the need for transfusion or surgery), other admissions to hospital for haemorrhage, hospital admission or death as a result of a non-stroke vascular event, and all-cause mortality |
| SPAF Investigators (SPAF II)10 | 1994 | 34 | 555 | 3.1 | Major haemorrhage was assessed by the criteria of Landefeld et al.20 |
| SPAF Investigators (SPAF III)21 | 1996 | 12 | 523 | 2.1 | A bleeding event was called major when it involved the central nervous system; required hospitalization, blood transfusion, and/or surgical intervention; or resulted in permanent functional impairment to any degree. All intracranial haemorrhages were confirmed by neuroimaging. Major haemorrhage was assessed by the criteria of Landefeld et al.20 |
| Morocutti (SIFA)12 | 1997 | 4 | 454 | 0.9 | Non-cerebral and non-fatal bleeding events were classified as major if they were severe, i.e. they made it necessary to hospitalize the patient, administer a blood transfusion, or perform surgery |
| Olsson (SPORTIF III)22 | 2003 | 41 | 1703 | 1.8 | Major bleeding included fatal bleeding; clinically overt bleeding associated with a reduction in Hb ≥ 20 g/L; clinically overt blood loss needing transfusion of ≥2 units of whole blood or erythrocytes; bleeding involving critical anatomical sites (intracranial, intraspinal, intraocular, retroperitoneal, pericardial, or atraumatic intra-articular haemorrhage) |
| Pérez-Gómez (NASPEAF)23 | 2004 | 23 | 479 | 2.0 | Severe bleeding (bleeding was considered severe when requiring hospital admission, blood transfusion, or surgery) |
| Patel (ROCKET-AF)24 | 2011 | 386 | 7125 | 3.4 | Major bleeding was defined by any of the following: a fatal outcome; involvement of a critical site (i.e. intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal bleeding); or clinically overt (≥2 g/dL fall in Hb, or requiring the transfusion of ≥2 units of packed RBCs or whole blood) |
Figure 2.
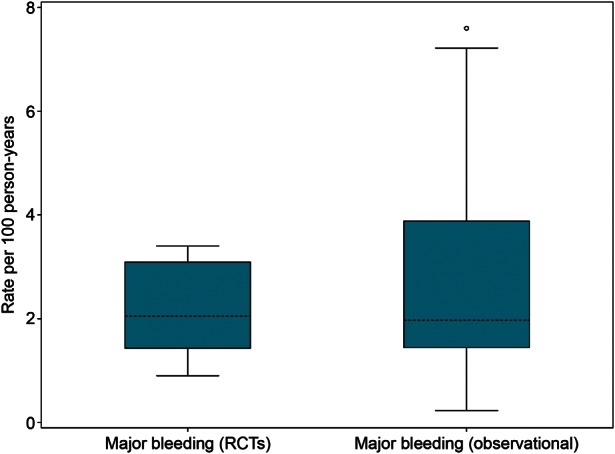
Box-and-whisker plot to summarize major bleeding rates per 100 Patient-years. RCTs, randomized controlled trials. The shaded boxes display the range of the 25th and 75th percentiles (IQR); the dashed line is the median value. The ‘whiskers’ (lines with horizontal caps) indicate the range of values within 1.5 times the IQR outside the IQR, and the circles indicate data points that fall outside the range of the whiskers, i.e. potential outliers. The weighted mean rates per 100 patient-years were 2.8 for major bleeding in RCTs and 4.4 for major bleeding in observational studies.
In observational studies, the definition of major bleeding varied across studies (Table 2). Some studies focused mainly on gastrointestinal bleeding,30,35,46,51,52 whereas other studies included patients that had bleeding from other anatomical sites. The incidence of major bleeding in the observational studies was similar to that in the RCTs—median 2.0 per 100 patient-years (range, 0.2–7.6 per 100 patient-years; IQR, 1.5–3.8 per 100 patient-years); weighted mean, 4.4 per 100 patient-years—but variation was greater in the observational studies (Figure 2).
Table 2.
Major bleeding data—observational studies
| Primary author | Publication year (midpoint of follow-up) | Patients, (N) | Major bleeding events (n) | Total patient-years | Rate per 100 patient-years | Definition of major bleeding |
|---|---|---|---|---|---|---|
| Abdelhafiz26 | 2004 (2001) | 402 | 11 | 634 | 1.74 | Major bleeding complications were defined as bleeding that led to hospital admission, emergency procedure, and/or blood transfusion |
| Blich27 | 2004 (1999) | 506 | 51 | 1228.5 | 4.15 | NR |
| Bosch28 | 2002 (1998) | 1283 | 119 | 4672 | 2.55 | Major haemorrhage in hospital setting |
| Boulanger29 | 2006 (2000) | 2568 | 103 | 3665 | 2.81 | Bleeding events included intracranial or GI haemorrhage and other bleeding episodes (e.g. haemopericardium, haematuria, haemarthrosis, epistaxis and haemoptysis) |
| Cheung30 | 2005 (2001) | 555 | 8 | 893 | 0.90 | GI bleeds |
| Copland31 | 2001 (1999) | 328 | 9 | 458 | 1.97 | Haemorrhages leading to fall in Hb level 2 g/L or transfusion (intracerebral and subdural not included) |
| Currie32 | 2005 (1999) | 1513 | 68 | 8500 | 0.80 | NR |
| Darkow33 | 2005 (2001) | 4895 | NR | NR | 2.68 | A haemorrhagic event was defined as acute inpatient hospitalization with a primary diagnosis of intracranial haemorrhage or other major bleeding |
| Fang34 | 2007 (1997) | 13 559 | 98 | 15 370 | 0.64 | Bleeding was defined as major extracranial haemorrhage, fatal, requiring transfusion of ≥2 units of packed red blood cells, or haemorrhage into a critical anatomical site, such as the retroperitoneum. To restrict analyses to the most serious haemorrhages, events not leading to hospitalization or death were excluded |
| Ghate35 | 2011 (2005) | 37 756 | 531 | 21 423 | 2.48 | Major GI bleeding events were defined as GI bleeding that required hospitalization, identified based on inpatient claims associated with an ICD-9 code for GI bleeding |
| Hansen36 | 2010 (2002) | 50 919 | 3642 | 93 492 | 3.90 | Bleeding was defined as admission to a Danish hospital, excluding emergency department visits, with a bleeding diagnosis (primary or secondary and classed as airway, intracranial, GI, urinary tract), a non-fatal bleeding episode, or a diagnosis of bleeding as the cause of death reported in the National Causes of Death Register (i.e. a fatal bleeding episode). |
| Ho37 | 2011 (2004) | 476 | 33 | 1 941 | 1.70 | Major bleeding was defined as intracranial bleeding, subarachnoid haemorrhage, subdural haematoma, haemorrhagic transformation of a primary ischaemic stroke (as documented by computed tomography scan, magnetic resonance imaging or autopsy) or any bleeding leading to transfusion of ≥2 units of whole blood or erythrocytes or bleeding requiring surgical or angiographic intervention, or bleeding resulting in permanent disability or involving a critical anatomical site |
| Hylek38 | 2007 (2002) | 472 | 26 | 360 | 7.22 | Major haemorrhage was defined as bleeding that was fatal, required hospitalization with transfusion of >2 units of packed RBCs, or involved a critical site (i.e. intracranial, retroperitoneal, intraspinal, intraocular, pericardial, or atraumatic intra-articular haemorrhage) |
| Jackson39 | 2001 (1998) | 505 | 9 | 267.7 | 3.40 | Bleeding complications were considered major if these involved intracranial or intracerebral haemorrhage, were life-threatening, or required blood transfusion |
| Mercaldi40 | 2011 (2005) | 70 057 | 12 039 | 158 408 | 7.60 | Major bleeding events included extracranial haemorrhages resulting in hospitalization or an emergency room visit |
| Naganuma41 | 2012 (2004) | 845 | 28 | 1900 | 1.47 | Major bleeding events were defined as intraocular haemorrhages leading to a substantial loss of vision, GI haemorrhage or other severe haemorrhage that was fatal or required endoscopic haemostasis, surgical intervention, hospital admission, or blood transfusion |
| Nichol42 | 2008 (2003) | 1107 | 84 | 2083 | 4.00 | The first diagnosis for a bleed resulting in hospitalization occurring 30 or more days after the index date was classified as an event (ICD-9 codes) |
| Njaastad43 | 2006 (1999) | 421 | 4 | 475.2 | 0.84 | Bleeding was defined as major if it was associated with at least one of the following: death; intracranial, retroperitoneal, intraocular, or intra-articular bleeding; a decrease in haemoglobin level ≥20 g/L; need for transfusion of ≥2 units of blood; or need for surgical or medical intervention |
| Olesen44 | 2011 (2003) | 37 425 | 5183 | 133 614 | 3.88 | Bleeding included GI bleeding, intracranial bleeding, bleeding from the urinary tract, and airway bleeding |
| Pengo45 | 2001 (1999) | 433 | 11 | 615 | 1.79 | The following were considered to be major bleeding events: fatal (death due to haemorrhage); intracranial (documented by CAT and/or NMR); ocular (with blindness); articular; retroperitoneal; bleeding requiring surgery or angiographic intervention to stop bleeding; bleeding leading to haemoglobin reduction of ≥2 g/dL and/or need for transfusion of ≥2 blood units |
| Poli46 | 2005 (2002) | 364 | 2 | 859 | 0.23 | GI bleeds |
| Poli47 | 2009 (2003) | 783 | 37 | 2567 | 1.44 | Bleeding was classified as major when fatal, intracranial (documented by imaging), ocular causing blindness, articular, or retroperitoneal; when surgery or transfusion of >2 blood units were required or when haemoglobin was reduced by ≥2 g/dL |
| Poli48 | 2011 (2009) | 3015 | 90 | 7630 | 1.18 | Major endpoints of the study were first major bleeding, defined fatal, ocular causing blindness, articular, or retroperitoneal bleeding; when surgery or an invasive manoeuvre was necessary to stop bleeding; when transfusion of >2 units of blood was required; or when Hb was reduced by >2 g/dL. |
| Poli49 | 2011 (2008) | 3302 | 97 | 10 019 | 0.97 | Bleeding was classified as ‘major’ when it was fatal, intracranial (documented by imaging), ocular causing blindness, articular, or retroperitoneal; when surgery or transfusion of >2 blood units was required; or when Hb was reduced by >2 g/dL. |
| Rose50 | 2008 (2001) | 3396 | 55 | 2892.1 | 1.90 | Major haemorrhage was defined according to the ISTH definition: a fatal event, an event requiring hospitalization with transfusion of at least 2 units of packed red blood cells, or bleeding involving a critical anatomical site such as the cranium or the retroperitoneum |
| Rosenman51 | 2009 (2003) | 1485 | 127 | 3364 | 3.80 | GI bleeds |
| Shireman52 | 2004 (1999) | 8131 | 98 | 2004 | 4.89 | Major bleeds included GI haemorrhages that resulted in an inpatient admission. Only the first episode of a major bleed per cohort member during the study period was included. Number of major bleeds and patient-years were imputed from the N and %, which in turn enabled rate per 100 patient-years to be imputed |
| Suzuki53 | 2007 (2005) | 667 | 9 | 503 | 1.79 | Major bleeding was defined as bleeding that required emergent hospitalization and included extracranial haemorrhages (GI haemorrhages, haematuria, haemoptysis) |
| Wess54 | 2008 (2000) | 501 | 52 | 876 | 5.94 | All GI bleeds and intracranial haemorrhages based on ICD-9-CM codes recorded on inpatient hospitalization claims |
| Wieloch55 | 2011 (2008) | 2491 | 53 | 2043 | 2.59 | ISTH guidelines include central nervous system, GI, and other bleeds |
| Yousef56 | 2004 (1999) | 739 | 28 | 1484 | 1.89 | Any bleeding event leading to hospitalization |
AF, atrial fibrillation; CAT, computed axial tomography; GI, gastrointestinal; Hb, haemoglobin; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ISTH, International Society on Thrombosis and Haemostasis; NMR, nuclear magnetic resonance (imaging); NR, not reported; RBC, red blood cells.
Regression models (weighted) were used to examine the relationship between potentially optimized VKA usage over time and major bleeding, and results showed that bleeding rates or bleeding reporting tended to increase over the last decade in both RCTs and observational studies; the increase was statistically significant in observational studies (Figure 3). The regression model estimates the increase in major bleeding rate over a period of 10 years to be 3.84 per 100 patient-years (95% CI, 0.68 to 7.00, P = 0.019), for observational studies and 1.00 per 100 patient-years (95% CI, −0.05 to 2.05, P = 0.061) for RCTs. Although some observations on the scatter plots lie outside the CIs, these may have minimal impact on the fitted regression if the sample sizes are relatively small, as these are weighted regressions.
Figure 3.
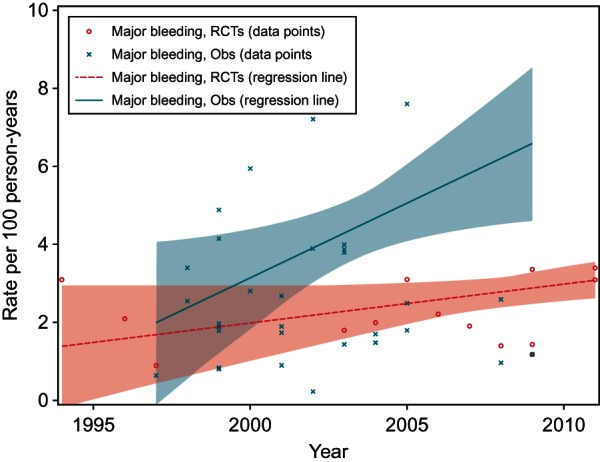
Weighted regression of major bleeding rates in RCTs and observational studies. Obs, observational studies; RCTs, randomized controlled trials. This figure presents the rates of major bleeding observed by year of study. The shaded areas indicate 95% CIs of the fitted regression line.
Mortality
In most clinical studies, mortality was evaluated as a secondary endpoint and was commonly defined as death due to vascular diseases or all-cause mortality. Of the 16 RCTs, 15 reported all-cause mortality and 11 reported vascular mortality, of which 10 reported both all-cause and vascular mortality; data are presented in Table 3. To assess and compare all-cause and vascular mortality, summary data are presented for the 10 trials that report both. Median all-cause mortality in patients treated with VKAs was 4.5 per 100 patient-years (range, 2.9–8.0 per 100 patient-years; IQR, 3.8–5.8 per 100 patient-years) (Figure 4) and the weighted mean was 4.3 per 100 patient-years. Incidence of vascular mortality was 2.6 per 100 patient-years (range, 1.5–6.7 per 100 patient-years; IQR, 2.1 to 3.2 per 100 patient-years) (Figure 4) and the weighted mean was 2.3 per 100 patient-years. As a proportion of all-cause mortality, 52% (weighted mean) of deaths were classified as vascular (minimum, 31%; median, 62%; and maximum, 91%; Table 3). Although not investigated statistically, it is possible that the proportion of all deaths classified as vascular has reduced over time (Figure 5). In observational studies, all-cause mortality was reported in only 11 of 31 studies. Owing to heterogeneity in the methodology and the large differences in the reliability and validity of death reporting in the data sources (and hence in the reported mortality rates) and the relatively low proportion of included observational studies reporting mortality, we could not further summarize these data in a meaningful way.
Table 3.
Mortality data—randomized controlled trials
| All-cause mortality |
Vascular mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| Primary author (trial name) | Publication year | Deaths (n) | Patients (n) | Rate per 100 patient-years | Deaths (n) | Patients, (n) | Rate per 100 patient-years | Percentage of all-cause deaths that were vascular |
| Albers13 | 2005 | 123 | 1962 | 3.8 | NR | NR | NR | NR |
| Bousser14 | 2008 | 61 | 2293 | 2.9 | 33 | 2293 | 1.5 | 54.1 |
| Chen11 | 2009 | 10 | 659 | 0.5 | NR | NR | NR | NR |
| Connolly15 | 2006 | 158 | 3371 | 3.8 | 106 | 3371 | 2.5 | 67.1 |
| Connolly16 | 2009 | 487 | 6022 | 4.1 | 317 | 6022 | 2.7 | 65.1 |
| Granger17 | 2011 | 669 | 9081 | 3.9 | 343 | 9081 | 2.0 | 51.3 |
| Hu25 | 2006 | 4 | 335 | 0.8 | NR | NR | NR | NR |
| Lip18 | 2009 | 2 | 318 | 1.4 | NR | NR | NR | NR |
| Mant19 | 2007 | 107 | 488 | 8.0 | 41 | 488 | 3.1 | 38.3 |
| Morocutti12 | 1997 | 32 | 454 | 7.4 | 29 | 454 | 6.7 | 90.6 |
| Patel24 | 2011 | 632 | 7090 | 4.9 | 193 | 7082 | 1.7 | 30.6 |
| Pérez-Gómez23 | 2004 | 43 | 479 | 3.7 | 28 | 479 | 2.4 | 65.1 |
| Petersen9 | 1989 | NR | NR | NR | 3 | 335 | 0.5 | NR |
| SPAF Investigators10 | 1994 | 62 | 555 | 5.6 | 36 | 555 | 3.3 | 58.1 |
| SPAF Investigators21 | 1996 | 35 | 523 | 5.9 | 27 | 523 | 4.6 | 77.1 |
| Olsson22 | 2003 | 79 | 1703 | 3.2 | NR | NR | NR | NR |
NR, not reported.
Figure 4.
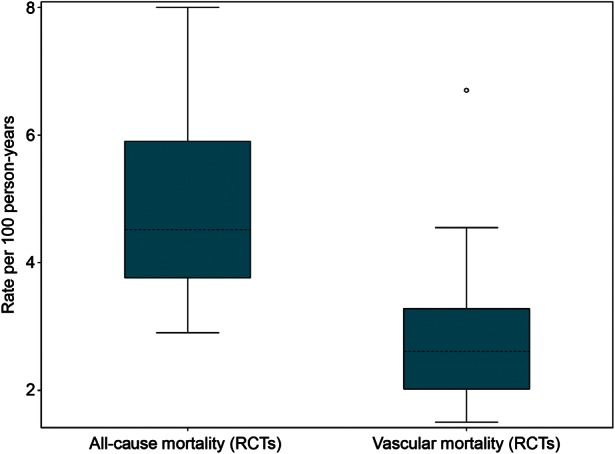
Box-and-whisker plot to summarize all-cause and vascular mortality rates per 100 patient-years. RCTs, randomized controlled trials. The shaded boxes display the range of the 25th and 75th percentiles (IQR); the dashed line is the median value. The ‘whiskers’ (lines with horizontal caps) indicate the range of values within 1.5 times the IQR outside the IQR, and the circles indicate data points that fall outside the range of the whiskers, i.e. potential outliers. The weighted mean rates per 100 patient-years were 4.3 for all-cause mortality and 2.3 for vascular mortality.
Figure 5.
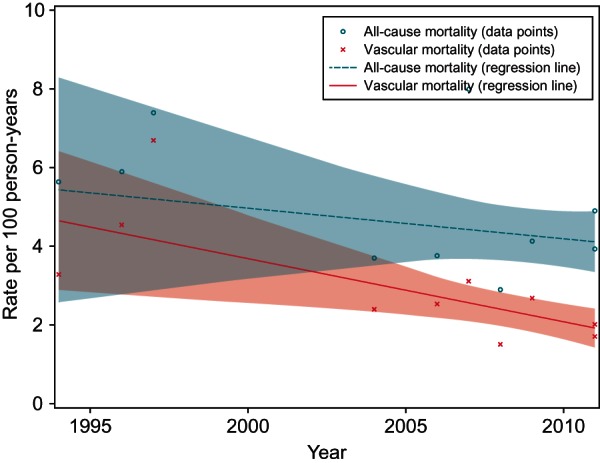
Weighted regression of mortality rates in RCTs by year. RCTs, randomized controlled trials. This figure presents the rates of mortality observed by year of reporting. The shaded areas indicate 95% CIs of the fitted regression line.
Within the RCT data, by using the publication year as a proxy to changing VKA management patterns over time, we found no clear evidence of a relationship with all-cause mortality, but some evidence of decreasing vascular mortality over time (Figure 5). Regression models (weighted) were used, and the models estimated a non-significant decrease in all-cause mortality rate over a period of 10 years to be −0.78 (95% CI, −2.66 to 1.09, P = 0.362) and a significant decrease in the vascular mortality rate over a period of 10 years to be −1.60 (95% CI, −2.77 to −0.44, P = 0.013).
Discussion
This systematic review of patients with AF confirms the assertion that there is a risk of major bleeding when treated with VKAs; this was confirmed by the overall incidence rates reported in RCTs and in observational studies conducted in the real-life clinical setting. The overall median rate of major bleeding was similar in the RCTs and the observational studies, but there was greater variation in the results reported in the observational studies. A sensitivity analysis performed in RCTs also including studies with smaller sample sizes (<300) gave very similar results. The IQRs of major bleeding rates were similar in RCTs (1.5–3.1) and observational studies (1.5–3.8), suggesting that the observed increased variability in observational studies are in the extremes. The largest observed major bleeding rate in observational studies occurred in the largest study.40 Including this study from the US-Medicare claims database considerably increased the weighted mean bleeding rate from 3.1 to 4.4.
We critically examined some of the potential reasons for heterogeneity in the bleeding and mortality rates observed in the publications using the study year as a proxy to changing management patterns in clinical practice. Over the years, there has been greater awareness of the warfarin benefit-to-risk ratio, and there are efforts to stay within a narrow therapeutic range (INR, 2.0–3.0 for AF) by stringently monitoring anticoagulation parameters, and scrutinizing administration of co-medications and dietary products. Regression models (weighted) examined this relationship and results showed that bleeding rates tended to increase over time in both RCTs and observational studies; the increase was statistically significant in observational studies. A number of factors could all potentially contribute to this increase over time: changing definition of major bleeding over time; heightened awareness of major bleeding and therefore increased reporting; and changes in patterns of prescribing VKAs due to greater awareness of the positive benefit-to-risk ratio, leading to the more likely treatment of vulnerable patients that are at high risk of stroke, but also at risk of bleeding.
Other potential factors may include concurrent use of drugs that interact with VKAs, thereby increasing the risk of serious bleeding, which is a widespread problem in clinical practice. Many drugs interact with the metabolism of VKAs, and the number increases as new drugs enter the market. These drugs can lead to over-anticoagulation, under-anticoagulation, or increased bleeding risk by pathways independent of INR, such as altered platelet function. Enhanced sensitivity to oral anticoagulation with VKAs, resulting in a higher risk for bleeding that varies across patient populations, may be attributed to co-morbid conditions such as chronic renal failure,57 hepatic dysfunction, and old age. Not all studies have shown consistent results, making it difficult to get a clear view of the association of VKA use in real-life clinical settings with major bleeding and mortality.
The evidence gathered in this review suggests that there is a paucity of high-quality and consistently reported data for major bleeding. One of the limitations of this review is heterogeneity across the included observational studies in terms of patient population, study objectives, year of publication (which, for example, influences how VKA treatment is monitored), definitions of outcomes, and length of follow-up. In addition, quality of design and execution of observational studies can differ dramatically. These sources of heterogeneity are evident in the differences in the minimum and maximum major bleeding rates observed in the RCTs and observational studies. There was also lack of mortality data in observational studies. For studies that used claims database records, there is uncertainty about the accuracy and completeness of medical record documentation. Also, information of variables known to be associated with the risk of bleeding (such as specific co-morbidities58 or concomitant use of antiplatelets) was not reported sufficiently to incorporate them into our analyses.
Published after the cut-off date for the searches conducted for this study, a large (over 48 000 patients receiving VKAs only), well-conducted, high-quality observational study reported a major bleeding rate of 1.9 per 100 patient-years in patients treated with VKAs only over an observation period of 2005–2008.59 This finding is consistent with the median major bleeding rate observed across our included studies (2.0 per 100 patient-years). Another large observational study, published after the cut-off date, was in an AF cohort of 125 195 Canadian patients starting warfarin therapy.60 In this study the bleeding rate per 100 patient-years was 3.8, with major bleeding defined as a visit to an emergency department or an admission to hospital for haemorrhage during warfarin therapy. Bleeding rates were highest in the first 30 days of warfarin therapy (11.8 per 100 patient-years) compared with the remainder of the 5-year follow-up period (3.4 per 100 patient-years).
It could have been hypothesized that the bleeding rates in observational studies would be higher than those in RCTs, given that patients in RCTs are typically a more selective group than those receiving anticoagulation in routine care. In addition, it is widely known that VKA control in RCTs is systematically better than in routine care, probably relating to study investigators being more skilful in the handling of warfarin.61 That this did not lead to markedly different bleeding rates may be attributed to the fact that adverse event reporting in RCTs is typically more systematic and complete than in observational studies. Also, bleeding events can be prospectively defined and recorded in RCTs (often even with adjudication by an independent review board). Such consistent criteria are more difficult to apply in retrospective database studies.
The limitations of oral anticoagulation by VKAs, particularly safety concerns, have prompted the development and availability of new oral anticoagulants, such as the oral direct thrombin inhibitors (e.g. dabigatran etexilate) and oral factor Xa inhibitors (e.g. rivaroxaban and apixaban). These new drugs may cause less bleeding and do not require stringent monitoring like VKAs, and therefore may be useful alternatives to VKAs.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: RTI Health Solutions were paid consultants to Boehringer Ingelheim GmbH for this project. B.M. and H.N. are paid employees of BI. N.R. and M.S. are paid employees of RTI Health Solutions.
Funding
This work was supported by Boehringer Ingelheim GmbH, Germany.
Supplementary Material
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, and are fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development.
References
- 1.Iqbal MB, Taneja AK, Lip GY, Flather M. Recent developments in atrial fibrillation. BMJ. 2005;330:238–43. doi: 10.1136/bmj.330.7485.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savelieva I, Camm J. Update on atrial fibrillation: part I. Clin Cardiol. 2008;31:55–62. doi: 10.1002/clc.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeringa J, Conway DS, van der Kuip DA, Hofman A, Breteler MM, Lip GY, et al. A longitudinal population-based study of prothrombotic factors in elderly subjects with atrial fibrillation: the Rotterdam Study 1990–1999. J Thromb Haemost. 2006;4:1944–9. doi: 10.1111/j.1538-7836.2006.02115.x. [DOI] [PubMed] [Google Scholar]
- 4.Khaykin Y, Shamiss Y. Cost considerations in the management of atrial fibrillation—impact of dronedarone. Clinicoecon Outcomes Res. 2012;4:67–78. doi: 10.2147/CEOR.S16675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirolkar SC, Fiuzat M, Becker RC. Dronedarone and vitamin K antagonists: a review of drug-drug interactions. Am Heart J. 2010;160:577–82. doi: 10.1016/j.ahj.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Waldo AL. New possibilities in anticoagulant management of atrial fibrillation. Rev Cardiovasc Med. 2004;5(Suppl. 5):S30–8. [PubMed] [Google Scholar]
- 7.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–12. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 8.Eypasch E, Leferinga R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311:619. doi: 10.1136/bmj.311.7005.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK Study. Lancet. 1989;1:175–9. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 10.Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II study. Lancet. 1994;343:687–91. [PubMed] [Google Scholar]
- 11.Chen X-J, Zhang H, Zhen R-L, Li W-Z, Qian H-D, Lei H-D, et al. The prognostic effect of different warfarin anticoagulation on patients with paroxysmal non-valvular atrial fibrillation. Chin J Evid Based Med. 2009;5:517–21. [Google Scholar]
- 12.Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, et al. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke. 1997;28:1015–21. doi: 10.1161/01.str.28.5.1015. [DOI] [PubMed] [Google Scholar]
- 13.Albers GW, Diener HC, Frison L, Grind M, Nevinson M, Partridge S, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690–8. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- 14.Bousser MG, Bouthier J, Buller HR, Cohen AT, Crijns H, Davidson BL, et al. Comparison of idraparinux with vitamin k antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet. 2008;371:315–21. doi: 10.1016/S0140-6736(08)60168-3. [DOI] [PubMed] [Google Scholar]
- 15.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 17.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 18.Lip GY, Rasmussen LH, Olsson SB, Jensen EC, Persson AL, Eriksson U, et al. Oral direct thrombin inhibitor AZD0837 for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: a randomized dose-guiding, safety, and tolerability study of four doses of AZD0837 vs. vitamin K antagonists. Eur Heart J. 2009;30:2897–907. doi: 10.1093/eurheartj/ehp318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 20.Landefeld CS, Anderson PA, Goodnough LT, Moir TW, Hom DL, Rosenblatt MW, et al. The bleeding severity index: validation and comparison to other methods for classifying bleeding complications of medical therapy. J Clin Epidemiol. 1989;42:711–8. doi: 10.1016/0895-4356(89)90066-8. [DOI] [PubMed] [Google Scholar]
- 21.Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: stroke Prevention In Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–8. [PubMed] [Google Scholar]
- 22.Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–8. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Gómez F, Alegría E, Berjón J, Iriarte JA, Zumalde J, Salvador A, et al. Comparative effects of antiplatelet, anticoagulant, or combined therapy in patients with valvular and nonvalvular atrial fibrillation: a randomized multicenter study. J Am Coll Cardiol. 2004;44:1557–66. doi: 10.1016/j.jacc.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 25.Hu DY, Zhang HP, Sun YH, Jiang LQ. The randomized study of efficiency and safety of antithrombotic therapy in nonvalvular atrial fibrillation: warfarin compared with aspirin [in Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:295–8. [PubMed] [Google Scholar]
- 26.Abdelhafiz AH, Wheeldon NM. Results of an open-label, prospective study of anticoagulant therapy for atrial fibrillation in an outpatient anticoagulation clinic. Clin Ther. 2004;26:1470–8. doi: 10.1016/j.clinthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Blich M, Gross B. Thromboembolic prophylaxis in nonrheumatic atrial fibrillation: utilization patterns, efficacy, and complications in a long-term follow-up of community patients. Int J Cardiol. 2004;96:89–95. doi: 10.1016/j.ijcard.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Bosch M, Hernandez J, Serra-Prat M. Effectiveness and safety of oral anticoagulant therapy in patients with non-rheumatic atrial fibrillation (abstract) Blood. 2002;100:11. [Google Scholar]
- 29.Boulanger L, Hauch O, Friedman M, Foster T, Dixon D, Wygant G, et al. Warfarin exposure and the risk of thromboembolic and major bleeding events among Medicaid patients with atrial fibrillation. Ann Pharmacother. 2006;40:1024–9. doi: 10.1345/aph.1G408. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CM, Tsoi TH, Huang CY. The lowest effective intensity of prophylactic anticoagulation for patients with atrial fibrillation. Cerebrovasc Dis. 2005;20:114–9. doi: 10.1159/000086801. [DOI] [PubMed] [Google Scholar]
- 31.Copland M, Walker ID, Tait RC. Oral anticoagulation and hemorrhagic complications in an elderly population with atrial fibrillation. Arch Intern Med. 2001;161:2125–8. doi: 10.1001/archinte.161.17.2125. [DOI] [PubMed] [Google Scholar]
- 32.Currie CJ, McEwan P, Emmas C, Morgan CL, Peters JR. Anticoagulation in patients with non-valvular atrial fibrillation: an evaluation of stability and early factors that predict longer-term stability on warfarin in a large UK population. Curr Med Res Opin. 2005;21:1905–13. doi: 10.1185/030079905X75050. [DOI] [PubMed] [Google Scholar]
- 33.Darkow T, Vanderplas AM, Lew KH, Kim J, Hauch O. Treatment patterns and real-world effectiveness of warfarin in nonvalvular atrial fibrillation within a managed care system. Curr Med Res Opin. 2005;21:1583–94. doi: 10.1185/030079905X61956. [DOI] [PubMed] [Google Scholar]
- 34.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–5. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghate SR, Biskupiak JE, Ye X, Hagan M, Kwong WJ, Fox ES, et al. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann Pharmacother. 2011;45:701–12. doi: 10.1345/aph.1P593. [DOI] [PubMed] [Google Scholar]
- 36.Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 37.Ho LY, Siu CW, Yue WS, Lau CP, Lip GY, et al. Safety and efficacy of oral anticoagulation therapy in Chinese patients with concomitant atrial fibrillation and hypertension. J Hum Hypertens. 2011;25:304–10. doi: 10.1038/jhh.2010.57. [DOI] [PubMed] [Google Scholar]
- 38.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 39.Jackson SL, Peterson GM, Vial JH, Daud R, Ang SY. Outcomes in the management of atrial fibrillation: clinical trial results can apply in practice. Intern Med J. 2001;31:329–36. doi: 10.1046/j.1445-5994.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 40.Mercaldi CJ, Ciarametaro M, Hahn B, Chalissery G, Reynolds MW, Sander SD, et al. Cost efficiency of anticoagulation with warfarin to prevent stroke in Medicare beneficiaries with nonvalvular atrial fibrillation. Stroke. 2011;42:112–8. doi: 10.1161/STROKEAHA.110.592907. [DOI] [PubMed] [Google Scholar]
- 41.Naganuma M, Shiga T, Sato K, Murasaki K, Hashiguchi M, Mochizuki M, et al. Clinical outcome in Japanese elderly patients with non-valvular atrial fibrillation taking warfarin: a single-center observational study. Thromb Res. 2012;130:21–6. doi: 10.1016/j.thromres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Nichol MB, Knight TK, Dow T, Wygant G, Borok G, Hauch O, et al. Quality of anticoagulation monitoring in nonvalvular atrial fibrillation patients: comparison of anticoagulation clinic versus usual care. Ann Pharmacother. 2008;42:62–70. doi: 10.1345/aph.1K157. [DOI] [PubMed] [Google Scholar]
- 43.Njaastad AM, Abildgaard U, Lassen JF. Gains and losses of warfarin therapy as performed in an anticoagulation clinic. J Intern Med. 2006;259:296–304. doi: 10.1111/j.1365-2796.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 44.Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739–49. doi: 10.1160/TH11-05-0364. [DOI] [PubMed] [Google Scholar]
- 45.Pengo V, Legnani C, Noventa F, Palareti G. Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding: a multicenter inception cohort study. Thromb Haemost. 2001;85:418–22. [PubMed] [Google Scholar]
- 46.Poli D, Antonucci E, Cecchi E, Marcucci R, Liotta AA, Cellai AP, et al. Culprit factors for the failure of well-conducted warfarin therapy to prevent ischemic events in patients with atrial fibrillation: the role of homocysteine. Stroke. 2005;36:2159–63. doi: 10.1161/01.STR.0000183620.06179.7b. [DOI] [PubMed] [Google Scholar]
- 47.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Bleeding risk during oral anticoagulation in atrial fibrillation patients older than 80 years. J Am Coll Cardiol. 2009;54:999–1002. doi: 10.1016/j.jacc.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 48.Poli D, Antonucci E, Testa S, Tosetto A, Ageno W, Palareti G. Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation. 2011;124:824–9. doi: 10.1161/CIRCULATIONAHA.110.007864. [DOI] [PubMed] [Google Scholar]
- 49.Poli D, Testa S, Antonucci E, Grifoni E, Paoletti O, Lip GY. Bleeding and stroke risk in a real-world prospective primary prevention cohort of patients with atrial fibrillation. Chest. 2011;140:918–24. doi: 10.1378/chest.10-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose AJ, Ozonoff A, Henault LE, Hylek EM. Warfarin for atrial fibrillation in community-based practise. J Thromb Haemost. 2008;6:1647–54. doi: 10.1111/j.1538-7836.2008.03075.x. [DOI] [PubMed] [Google Scholar]
- 51.Rosenman M, Simon T, Teal E, McGuire P, Jackson J, Tierney W. Atrial fibrillation and warfarin: time in therapeutic range-an electronic medical record system study of real-world practice (abstract) Circulation. 2009;120(18 Suppl. 2):S521. [Google Scholar]
- 52.Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35:2362–7. doi: 10.1161/01.STR.0000141933.75462.c2. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Yamashita T, Kato T, Fujino T, Sagara K, Sawada H, et al. Incidence of major bleeding complication of warfarin therapy in Japanese patients with atrial fibrillation. Circ J. 2007;71:761–5. doi: 10.1253/circj.71.761. [DOI] [PubMed] [Google Scholar]
- 54.Wess ML, Schauer DP, Johnston JA, Moomaw CJ, Brewer DE, Cook EF, et al. Application of a decision support tool for anticoagulation in patients with non-valvular atrial fibrillation. J Gen Intern Med. 2008;23:411–7. doi: 10.1007/s11606-007-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wieloch M, Själander A, Frykman V, Rosenqvist M, Eriksson N, Svensson PJ. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J. 2011;32:2282–9. doi: 10.1093/eurheartj/ehr134. [DOI] [PubMed] [Google Scholar]
- 56.Yousef ZR, Tandy SC, Tudor V, Jishi F, Trent RJ, Watson DK, et al. Warfarin for non-rheumatic atrial fibrillation: five year experience in a district general hospital. Heart. 2004;90:1259–62. doi: 10.1136/hrt.2003.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olesen JB, Kamper AL, Køber L, Lindhardsen J, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–35. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 58.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 59.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 60.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2012 doi: 10.1503/cmaj.121218. doi:10.1503/cmaj.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control. a systematic review and metaregression. Chest. 2006;129:1155–66. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.