Introduction
Nail matrix melanoma, otherwise known as subungual melanoma, is unique in that the actual primary cutaneous melanoma is occult, being covered by the nail plate and the proximal nail fold. Fortunately, the concealed melanoma may produce melanin, which then appears as longitudinal pigmentation in the nail plate. Thus melanin-producing nail matrix melanoma has a distinctive ‘signature’ to the informed observer.
Acral lentiginous melanoma was first described as a subgroup in 1976 by Reed [1]. Prior to that Clark had proposed three histologic sub-types: superficial spreading melanoma, lentigo maligna melanoma and nodular melanoma [2]. About half of all hand and foot melanomas are of the acral lentiginous subtype. A Scottish study restricted to subungual melanoma showed that 45% were acral lentiginous, 27% nodular and 20% superficial spreading in type [3].
We present two consecutive cases of nail matrix melanoma from a general practice. The first patient was referred to the practice and the second patient was recommended to the practice by the first.
Case report 1
A 25-year-old high school science teacher was referred with a lesion on his right thumbnail (Figure 1A), arriving by a circuitous route. One of his teenage students had seen a photograph of a nail melanoma on a patient education poster in the waiting room of a doctor’s surgery office and thought the photo looked like her teacher’s thumb. At first he was somewhat sceptical, but she persisted until he saw his GP, who referred him to a specialized melanoma centre. They, in turn, referred him to the general practice of author CR for a nail matrix biopsy because their preferred plastic surgeon had been temporarily incapacitated by an injury.
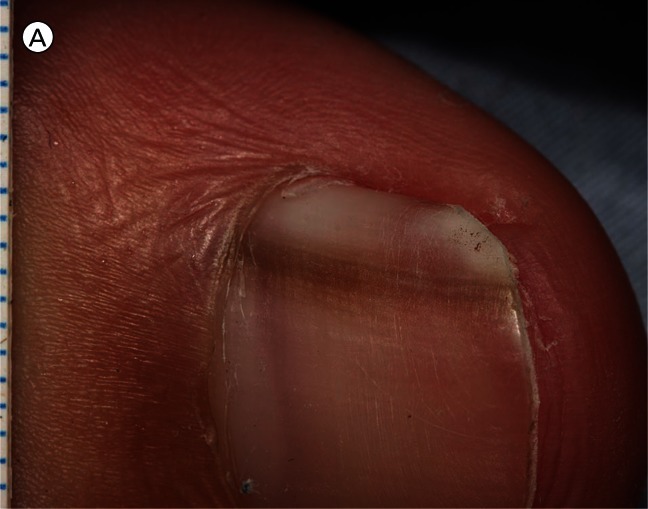
Figure 1.

(A) Close-up image of longitudinal melanonychia on the right thumbnail of a 25-year-old man. This pigment band on the nail plate appeared and widened over the preceding ten months. Pigment is visible through the translucent cuticle and proximal nail fold (false Hutchinson’s sign) but there is no apparent pigmentation of these structures. The pigment band tapers from 3 mm wide proximally to 2 mm distally. (B) Dermatoscopic image of the surface of the nail plate. Lines parallel vary in width, interval and colour. Colours present—black, brown and gray—are the colours of melanin. The overall band of pigment is broader proximally, consistent with origin from a growing lesion in the nail matrix. [Copyright: ©2012 Rosendahl et al.]
The patient had first noticed a fine band of pigment extending the full length of the nail 10 months earlier that had become progressively wider since then.
Dermatoscopy of the surface of the nail plate (Figure 1B) revealed longitudinal melanonychia (also known as melanonychia striata) with lines parallel stretching from the proximal nail fold to the free edge of the nail. The parallel lines varied in width, colour (black, dark brown, light brown and gray) and interval. The pigmented band was wider proximally than distally, consistent with a growing lesion in the nail matrix.
Braun et al [4] have demonstrated how dermatoscopy of the free edge of the nail plate can be used to precisely predict tumour location in the nail matrix. Pigment located deeply in the nail plate (Figure 2) corresponds to a tumour located in the distal nail matrix.
Figure 2.

Dermatoscopy of the free edge of the nail plate shows that melanin has been preferentially incorporated into the deeper portion of the nail plate. This indicates that it is originating from a lesion in the distal portion of the nail matrix.
A provisional diagnosis of nail matrix melanoma was supported by both the history of a new and progressively widening nail plate pigmentation and the dermatoscopy showing lines parallel of varying thickness interval and colour. The required diagnostic procedure of nail matrix biopsy was explained to the patient. He consented, understanding it was likely to result in permanent nail dystrophy.
The procedure was performed under sterile conditions in the operating room of the general practice. Anesthesia was achieved using a digital block with 2% plain xylocaine, and a tourniquet was applied. An incision was made on each side of the proximal nail fold, which was then separated from the nail plate by sweeping the scalpel blade over its surface. The proximal nail fold was retracted with two sutures, exposing that part of the nail plate overlying the nail matrix.
A 6 mm biopsy punch was then used to fenestrate the nail plate overlying the predicted location of the tumour—the distal nail matrix in line with the longitudinal melanonychia (Figure 3). The punch was rotated while applying gentle pressure. The circular incision was probed with the scalpel blade at intervals until it was found to have penetrated at one point. The circular plug of nail was lifted away from the matrix with fine forceps used as an elevator. The incision through the remainder of the nail plate was completed using the scalpel, taking care not to incise the underlying mail matrix. The circular plug of nail plate was lifted out to reveal a densely pigmented lesion in the underlying nail matrix (Figure 3). This was circumscribed with a 4 mm biopsy punch and dissected from underlying periosteum with a scalpel blade. This achieved an excision biopsy of the entire visible pigmented lesion.
Figure 3.
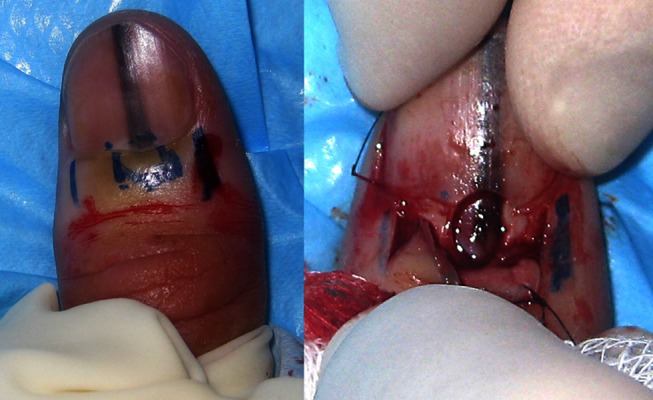
The image on the left shows the proposed incision lines on each side of the proximal nail fold. The marks in the centre are not for incision but indicate the anticipated location of the target lesion in the distal nail matrix. The image on the right shows the proximal nail fold retracted and a densely pigmented lesion in the nail matrix underlying a fenestration in the nail plate. [Copyright: ©2012 Rosendahl et al.]
Both the excised nail matrix and the plug of nail plate were submitted in formalin. The laboratory was contacted to ensure the two different portions received the different processing they required.
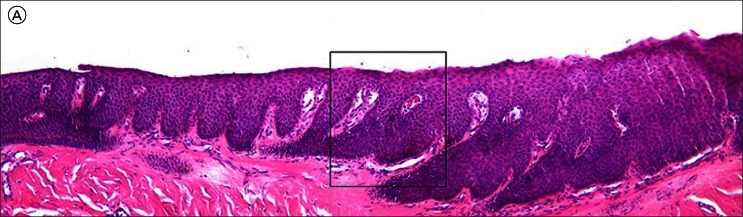
Histopathology (Figures 4 and 5) revealed an increased number of atypical melanocytes in lentiginous array. There were prominent thick dendrites, which were visible on hematoxylin and eosin (H&E) staining because of the amount of melanin they contained. Because it can be difficult to distinguish melanocytes from keratinocytes, immunostaining was employed (Figure 6) with the pan-melanoma antibody cocktail and, quoting from the pathology report, this showed “both basal and suprabasal melanocytes with focal junctional confluence indicative of level 1 (in-situ) subungual melanoma.”
Figure 4.
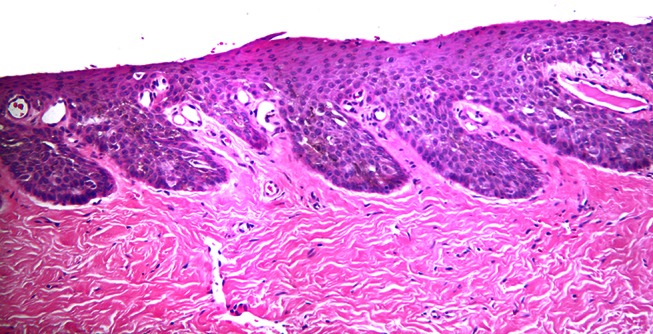
Low power micrograph of an H&E stained specimen of nail matrix from Case 1. It is difficult to distinguish melanocytes from keratinocytes. [Copyright: ©2012 Rosendahl et al.]
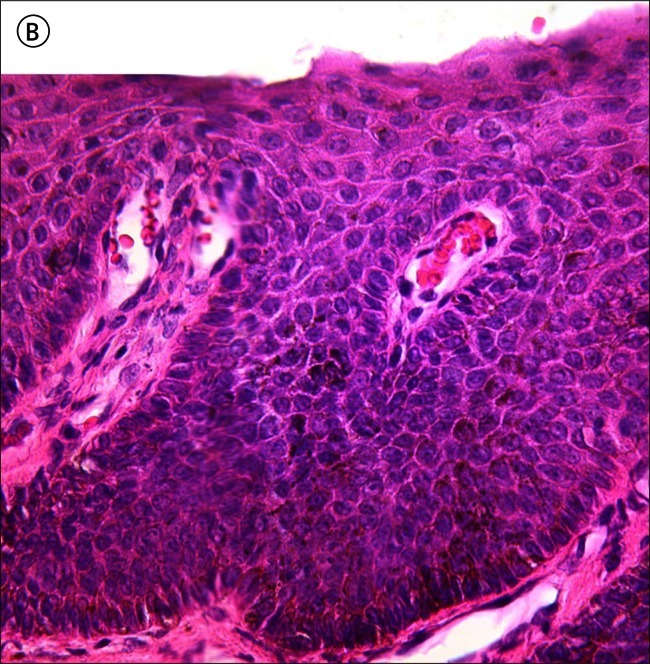
Figure 5.
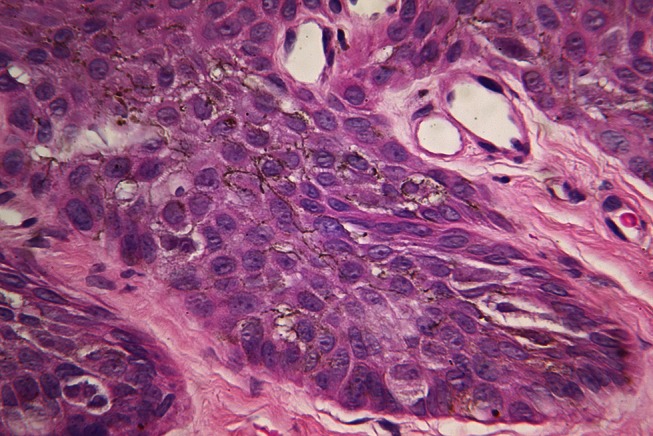
High power micrograph of the H&E stained section shown in Figure 4 shows plump pale melanocytes at the dermo-epidermal junction with prominent heavily pigmented dendrites seen in the centre of the image. [Copyright: ©2012 Rosendahl et al.]
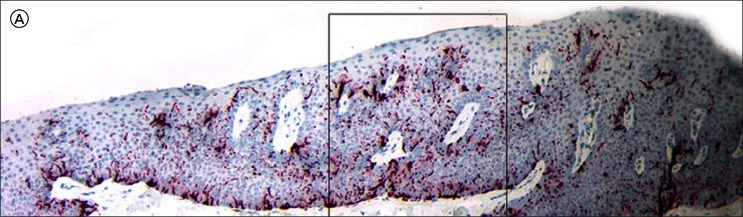
Figure 6.
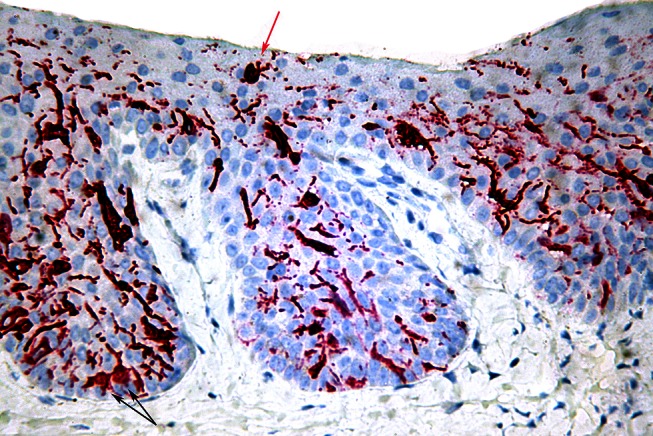
A micrograph of the lesion shown in Figure 3 stained with pan-melanoma immunoperoxidase stain shows prominent dendrites with focally confluent plump junctional melanocytes (black arrows) and a Pagetoid melanocyte (red arrow). [Copyright: ©2012 Rosendahl et al.]
The patient was referred to a plastic surgeon for definitive treatment. After discussion with the patient a decision was made not to amputate the digit, but to perform a conservative excision with 5 mm lateral margins and to excise down to the periosteum in depth. The procedure was performed under sterile conditions in the operating theatre of a day-centre under local anesthetic. The nail plate was removed before the excision was performed, allowing an intact specimen to be submitted in formalin, along with an intact nail plate. The surgical defect was covered with a full-thickness skin graft harvested from the contralateral forearm. The pathologist found no residual tumour in the re-excision specimens.
Case report 2
A 30-year-old female patient presented with a pigmented lesion on the nail of her right hallux (Figure 7A) because of her knowledge of the experience of an acquaintance, the patient presented in Case 1. The lesion had appeared approximately five years earlier but had not concerned her until she learned that the rather similar lesion on her acquaintance’s thumb was an early melanoma. She was not aware of recent change but readily conceded that she had not paid particular attention to it.
Figure 7.
(A) Close up image of longitudinal melanonychia on the right hallux of a 30-year- old woman. This pigment band on the nail plate had been present for approximately five years with no reported recent change. The pigment band tapers only slightly from 2mm wide proximally to a little less than 2 mm distally. (B) Dermatoscopy of the lesion shown in Figure 7A. Lines parallel show only slight variation in width, interval and colour. Colours present—brown and gray—are the colours of melanin. The pigment band is wider proximally than distally. [Copyright: ©2012 Rosendahl et al.]
Dermatoscopy of the surface of the nail plate (Figure 7B) revealed longitudinal melanonychia with lines parallel stretching from the proximal nail fold to the free edge of the nail. There was some variation in the width and interval of the lines but it was not marked. Colors included brown and a suggestion of gray. The pigment was visible through the translucent nail cuticle (pseudo-Hutchinson sign), although that structure did not appear to be pigmented.
Dermatoscopy of the free edge of the nail plate (Figure 8) revealed the presence of pigment preferentially in the superficial layers, suggesting that the tumour would be located in the proximal nail matrix.
Figure 8.

Dermatoscopy of the free edge of the nail plate shows that melanin has been preferentially incorporated into the superficial portion of the nail plate. This indicates that it is originating from a lesion in the proximal portion of the nail matrix. [Copyright: ©2012 Rosendahl et al.]
On the basis of longitudinal melanonychia present for approximately five years with no reported recent change, combined with dermatoscopic findings of only slight variability of line width, interval and colour, a provisional diagnosis was made of nail matrix nevus. However, nail matrix melanoma could not be excluded with any degree of certainty so a nail matrix biopsy was proposed to the patient, including an explanation that it would cause a degree of permanent nail dystrophy.
The patient agreed and the procedure was performed two weeks later under sterile conditions in the operating room of the general practice using a similar method to the one described in the Case 1. Author AC attended and photo-documented the procedure, which is presented in Figures 9 and 10.
Figure 9.
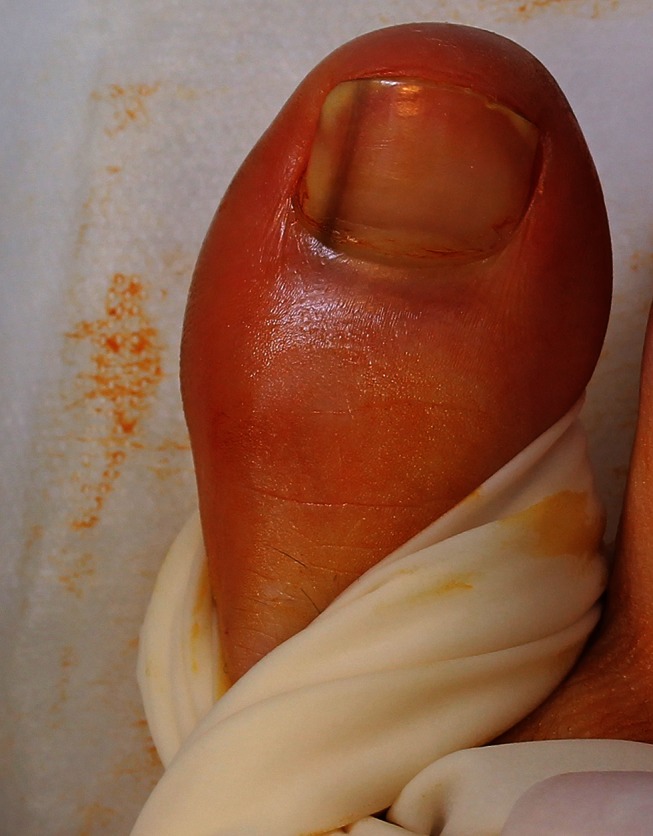
Following skin preparation with betadine and a local anesthetic digital block with 2% plain xylocaine a tourniquet (sterile glove) is applied and secured with artery forceps. [Copyright: ©2012 Rosendahl et al.]
Figure 10.
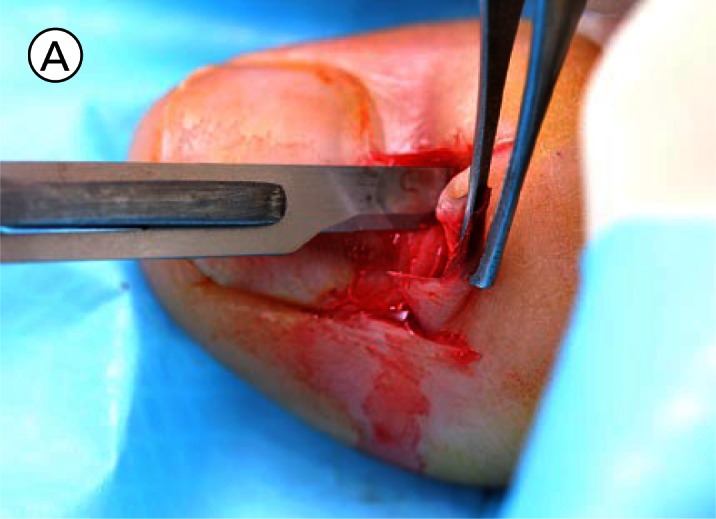
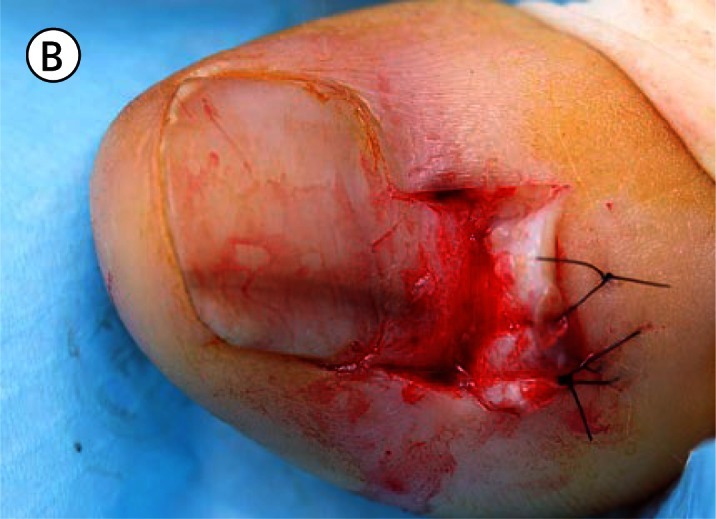
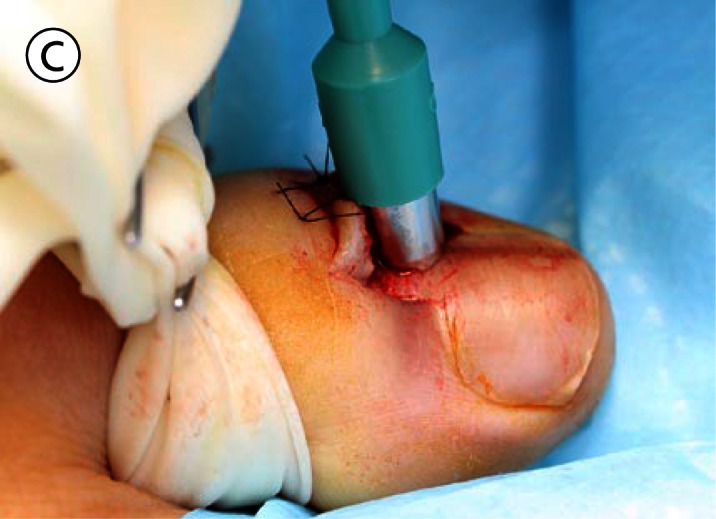
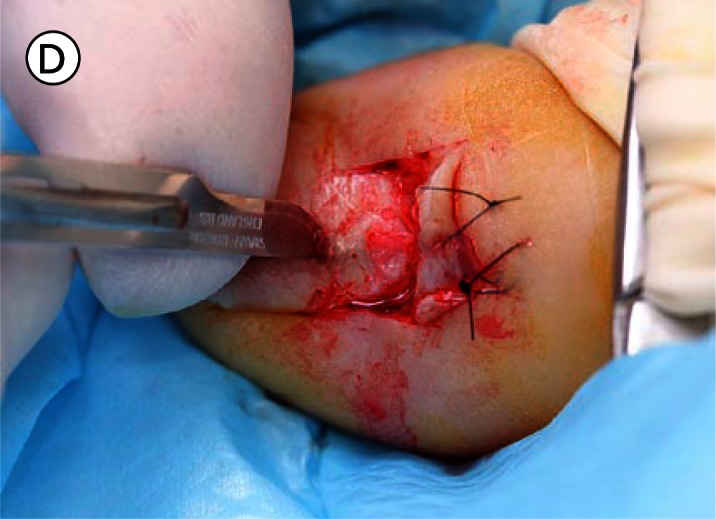

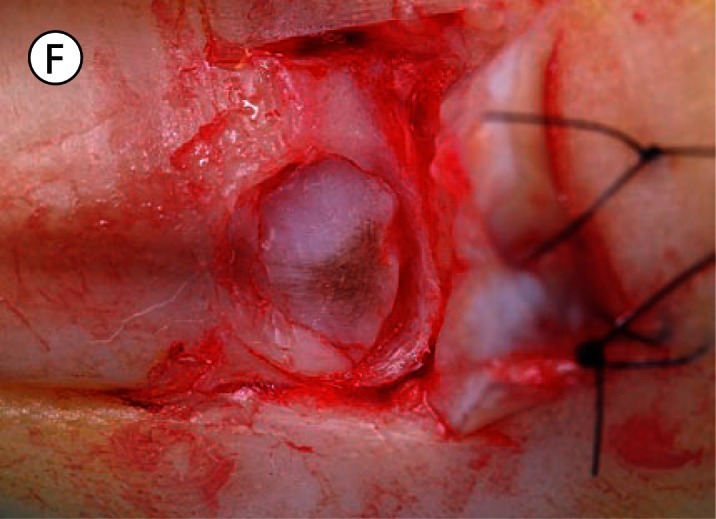

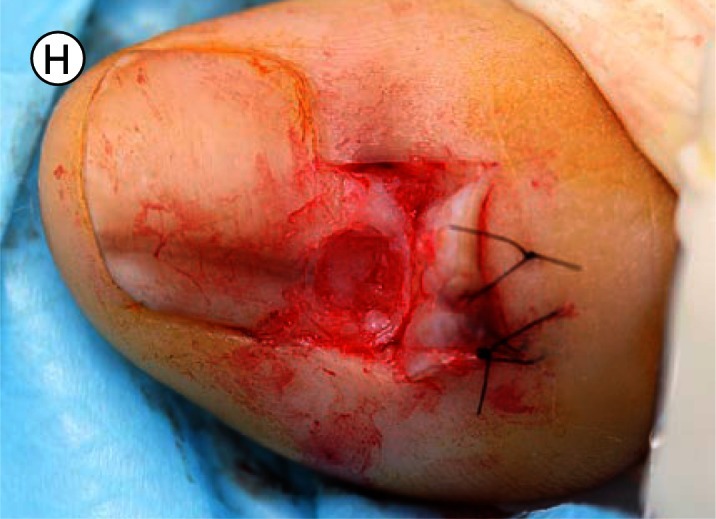

(A) The proximal nail fold is incised on each side and separated from the nail plate with a scalpel. (B) The mobilised proximal nail fold is retracted with two sutures. (C) A 6mm biopsy punch is rotated with gentle pressure applied in the first step in nail plate fenestration. (D) The fenestration incision is tested with a scalpel blade until full thickness penetration is confirmed at this point. (E) Forceps are used as an elevator to lift the fenestrated nail plate away from the underlying nail matrix while fenestration is completed with a scalpel. The cutting edge of the scalpel blade is directed outwards away from the nail matrix. (F) After removal of the nail plate plug a pigmented lesion is clearly seen on the nail matrix. Lines parallel extend distally from the central lesion which appears to have a structure of reticular lines although this is a macroscopic, not a dermatoscopic image. The posterior margin has already been (inadvertently) incised with the biopsy punch and appears just clear of the pigmented lesion. (G) The nail matrix containing the pigmented lesion is removed as an excision biopsy. The only portion to have a visible positive margin is where the parallel lines of pigment extend to the distal margin where they are continuous with lines in the developing nail plate, which in turn appear to be continuous with the lines on the exposed nail plate. The specimen is gripped at one edge, as remote as possible from the pigmented lesion, to avoid crush artefact of the tumour. (H) The excision biopsy has been dissected at the level of the periosteum. Careful inspection of the base reveals no evidence of residual melanin pigment. (I) The proximal nail fold is laid back over the defect and secured with two 5-0 nylon sutures on each side. The tourniquet is released with consequent brisk bleeding and dressing is delayed until hemostasis has been achieved with the assistance of direct pressure and the passage of time. With the dressing, care is taken not to apply any constriction and the skin of the distal end of the toe is left uncovered. The patient is instructed about checking this for colour and capillary return in the event of undue pain or discomfort. [Copyright: ©2012 Rosendahl et al.]
Histopathology (Figure 11) revealed (quoting from the pathology report) “a mild increase in basal melanocytes.” The pathologist went on to state, “As usual in matrix preparations it is almost impossible to detect any atypia . . . I am performing a battery of four melanocytic markers in an attempt to clarify the pathology.”
Figure 11.
(A) Low power micrograph of the nail matrix specimen from Case 2. (B) High power micrograph of the boxed area in A. Prominent heavily pigmented dendrites raise suspicion of a malignant neoplastic process but a diagnosis of nail matrix melanoma is not rendered on this H&E stained tissue. [Copyright: ©2012 Rosendahl et al.]
Immunostaining was performed (Figure 12) and the follow-up report stated, “Three of the immunoperoxidase stains (S100, panmelanoma and HMB45) show an increase in melanocytes with Pagetoid spread and some plump cells. These changes are those of an evolving level 1 (in-situ) acral melanoma. The Melan A stain shows less Pagetoid spread but still some plump melanocytes. . . . There is no regression, ulceration, significant lymphocytic infiltrate or dermal invasion.”
Figure 12.
(A) A Low power micrograph of nail matrix from Case 2 stained with pan melanoma stain. (B) High power micrograph of the boxed area in A with pan-melanoma stain. Plump atypical melanocytes are seen in lentiginous array (black arrows), in some parts confluent. Pagetoid spread of melanocytes is also seen (red arrows). [Copyright: ©2012 Rosendahl et al.]
The patient was referred to a plastic surgeon for definitive treatment of a level 1 nail matrix melanoma.
Discussion
Durbec et al performed a comprehensive literature review of the epidemiology of hand and foot melanoma (HFM) [5]. They conclude that HFM is rare in all populations, with estimates of incidence of HFM ranging from 0.04 to 0.25 per 100,000 per year. They found one study, which specifically addressed the incidence of (invasive) subungual melanoma, with a finding of 0.1 per 100,000 per year (1 in a million per year) [6]. For HFM in general, ultraviolet radiation appears to have, at most, a very limited causative role, with previous trauma and nevi on the soles or toes being the most consistent risk factors across the few relevant studies. In one study nail trauma was recalled by 50% of patients with subungual melanoma [7]. Subungual melanomas were evenly distributed between hand and foot, but hallux and thumbnails were disproportionately affected compared to other digits and there was equal incidence of nail subungual melanoma across all races.
HFM appears to have a worse prognosis than cutaneous melanoma in general. This is thought to be mainly due to delay in diagnosis, with possible contributions from more aggressive tumour behaviour at acral sites or of the acral lentiginous melanoma subtype. While Breslow thickness and tumour ulceration remain the main prognostic factors, Durbec et al found them less reliable for HFM than melanoma in general. No recent population-based studies of subungual melanoma mortality were discovered, but in a cancer registry-based English study on cases from 1984–93, the five-year survival for invasive subungual melanoma was only 51% [6].
Although nail apparatus melanoma is not common, the cardinal clinical sign of longitudinal melanonychia is a distinctive one. Furthermore, the dermatoscopic feature of longitudinal pigmented lines running the full length of the nail plate and varying in width, interval and colour has been shown to assist in differentiating early nail matrix melanoma from other causes of nail pigmentation [8]. In Case 1 these features were clearly present, but in Case 2 they were equivocal. Another described clue to subungual melanoma is onset of longitudinal melanonychia during adulthood [8], and taking this into account, along with equivocal dermatoscopic features, a nail matrix biopsy was appropriate in Case 2.
A method known as the ABCDEF rule has been proposed to assist with early diagnosis of subungual melanoma [9]. In this method the letters have the following relevance: A) Age—peak incidence fifth to seventh decades with Africans, Asians and native Americans (dark skinned races) accounting for up to one third of all cases; B) Black or brown with band width of 3mm or more; C) Change in the nail or lack of improved change with treatment; D) Digit most commonly involved (finger or thumb); E) Extension of pigment onto the proximal or lateral nail-fold (Hutchinson’s sign); and F) Family or personal history of dysplastic nevus or melanoma. We suggest that adherence to this system, apart from the problem of its complexity, may actually delay early diagnosis. Both cases reported by us were in patients with Fitzpatrick type 2 skin and they were aged 25 and 30 respectively. Neither manifested Hutchinson’s sign or had a personal or family history of melanoma. Furthermore, we believe that the routine use of dermatoscopy can simplify the clinical diagnostic process and we propose one clue which should lead to suspicion of nail matrix melanoma: Dermatoscopy of the nail plate revealing the presence of lines parallel, chaotic (chaos being defined as asymmetry of structure or colour). Therefore, lines parallel, varying in width, interval and colour, stretching the full length of a nail should lead to consideration of appropriate biopsy. Longitudinal nail pigmentation not fulfilling these criteria; and with a history of worrying features, such as recent onset or change, could be further assessed by sequential digital photographic monitoring with any increase in bandwidth or band chaos leading to an appropriate biopsy. Of course it should be remembered that nail matrix melanoma can present as an amelanotic tumour, and therefore any unexplained dystrophy of a single nail should lead to consideration of malignancy.
An inadequate biopsy can impair the pathologist’s ability to provide an accurate diagnosis [7] and for this reason the quality of biopsy material is important. Nail plate tissue is routinely placed in a softening agent. If an inexperienced laboratory assistant placed nail matrix in softener, even a carefully collected specimen could be compromised.
As with other melanomas, there is overlap between normal findings and criteria for a diagnosis of melanoma. Both architectural criteria (including with nail matrix melanomas, confluent junctional proliferation of melanocytes and a degree of partial or full-thickness Pagetoid spread) as well as cytological features (including in nail matrix melanomas, plump atypical melanocytes and dendrites packed with melanin), are needed for the diagnosis of melanoma to be made. Furthermore, the differentiation between melanocytes and keratinocytes can be difficult with H&E stained specimens. In both of the cases reported here immunoperoxidase stains were performed to facilitate a confident diagnosis.
Braun et al [7] describe a full range of biopsy techniques suitable for longitudinal melanonychia of varying sizes and locations. The biopsy technique used for these two cases is a modification of the punch method, with the fenestrated portion of nail plate being submitted for histology rather than being placed back into the defect as a “biological dressing.” In both these cases healing proceeded rapidly without replacing the nail plug.
While invasive nail matrix melanomas are inevitably treated by partial or complete amputation of the affected digit according to tumour thickness, in-situ nail matrix melanomas can be treated by conservative excision of the entire nail apparatus (nail plate, bed and matrix) with graft reconstruction [7,10]. As even partial loss of thumb or big toe causes significant disability, diagnosis at the in-situ stage has led to a better functional outcome, as well as a better prognosis for these two patients.
Conclusion
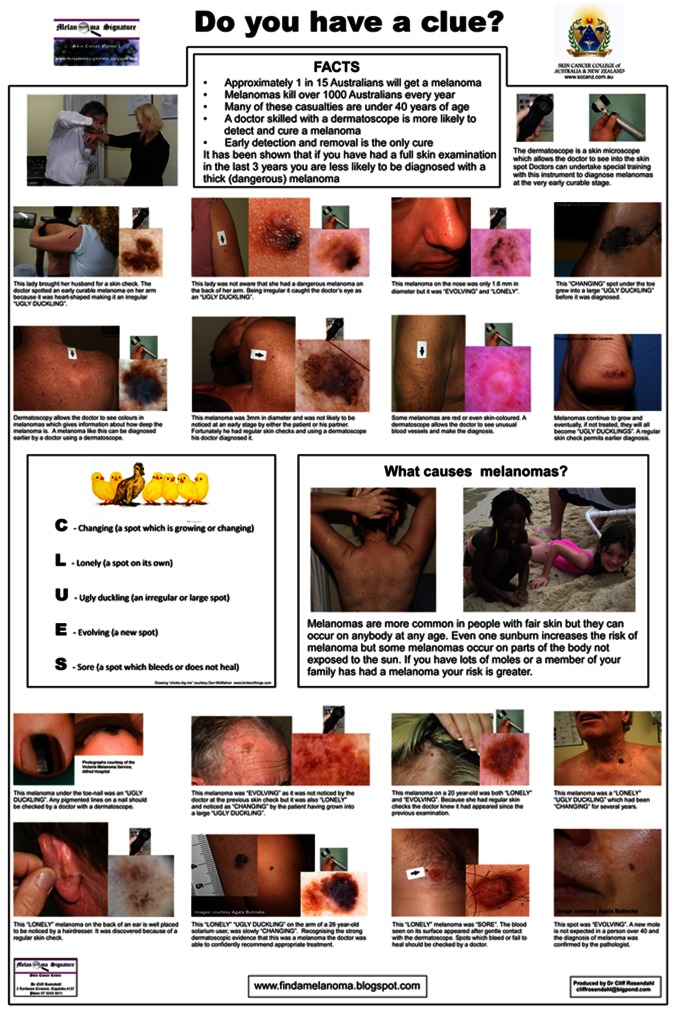
An uncommon, occult form of melanoma was diagnosed at an early stage in two young adults because an informed teenager recognised its signature. Her discovery also made this presentation possible. A simple image-based patient education initiative (Figure 13) paid a huge dividend.
Figure 13.
The patient education poster, which prompted a teenager to suggest that her teacher consult his general practitioner about a discoloured thumbnail. [Copyright: ©2012 Rosendahl et al.]
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Reed RJ. Acral lentiginous melanoma. In: Hartman W, Kay S, Reed RJ, editors. New Concepts in Surgical Pathology of the Skin. New York: John Wiley & Sons, Inc.; 1976. pp. 89–90. [Google Scholar]
- 2.Clark WH, Jr, From L, Bernardino EA, Mihm MC., Jr The histogenesis and biologic behaviour of primary malignant melanomas of the skin. Cancer Res. 1969;29(23):705–27. [PubMed] [Google Scholar]
- 3.Blessing K, Kernohan NM, Park KG. Subungual malignant melanoma: clinicopathological features of 100 cases. Histopathology. 1991;19(5):425–9. doi: 10.1111/j.1365-2559.1991.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 4.Braun RP, Baran R, Saurat JH, Thomas L. Surgical Pearl: Der-moscopy of the free edge of the nail to determine the level of nail plate pigmentation and the location of its probable origin in the proximal or distal nail matrix. J Am Acad Dermatol. 2006;55(3):512–3. doi: 10.1016/j.jaad.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Durbec F, Martin L, Derancourt C, Grange F. Melanoma of the hand and foot Epidemiologic, prognostic and genetic features: a systematic review. Br J Dermatol. In press. Accepted for publication 2-12-2011. [DOI] [PubMed]
- 6.Banfield CC, Redburn JC, Dawber RP. The incidence and prognosis of nail apparatus melanoma. A retrospective study of 105 patients in four English regions. Br J Dermatol. 1998;139(2):276–9. doi: 10.1046/j.1365-2133.1998.02365.x. [DOI] [PubMed] [Google Scholar]
- 7.Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56(5):835–47. doi: 10.1016/j.jaad.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Thomas L, Dalle S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol Ther. 2007;20(1):3–10. doi: 10.1111/j.1529-8019.2007.00106.x. [DOI] [PubMed] [Google Scholar]
- 9.Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000;42(2 Pt 1):269–74. doi: 10.1016/S0190-9622(00)90137-3. [DOI] [PubMed] [Google Scholar]
- 10.Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. Br J Dermatol. 2011;165(4):852–8. doi: 10.1111/j.1365-2133.2011.10477.x. [DOI] [PubMed] [Google Scholar]