Abstract
Background:
Deciding whether a skin lesion requires biopsy to exclude skin cancer is often challenging for primary care clinicians in Australia. There are several published algorithms designed to assist with the diagnosis of skin cancer but apart from the clinical ABCD rule, these algorithms only evaluate the dermatoscopic features of a lesion.
Objectives:
The BLINCK algorithm explores the effect of combining clinical history and examination with fundamental dermatoscopic assessment in primary care skin cancer practice.
Patients/Methods:
Clinical and dermatoscopic images of 50 skin lesions were collected and shown to four primary care practitioners. The cases were assessed by each participant and lesions requiring biopsy were determined on separate occasions using the 3-Point Checklist, the Menzies method, clinical assessment alone and the BLINCK algorithm.
Results:
The BLINCK algorithm had the highest sensitivity and found more melanomas than any of the other methods. However, BLINCK required more biopsies than the other methods. When comparing diagnostic accuracy, there was no difference between BLINCK, Menzies method and clinical assessment but all were better than the 3-Point checklist.
Conclusions:
These results suggest that the BLINK algorithm may be a useful skin cancer screening tool for Australian primary care practice.
Keywords: melanoma, skin cancer, diagnostic algorithm, BLINCK, primary care
Introduction
Most GP skin cancer training courses in Australia encourage beginners to use dermatoscopy algorithms when deciding if a lesion requires biopsy to exclude skin cancer. Indeed, the ability to recognise dermatoscopic criteria correctly and apply a dermatoscopic algorithm is often seen as the sine qua non of excellence in primary care skin cancer practice.
The benefits of using a scored “dermatoscopy-only” algorithm are well documented [1,2]. However, this approach does not score often useful clinical information such as history of lesion change, the “ugly duckling” sign [3,4] and even the patient’s own instinct regarding the lesion. The authors feel that a more “holistic” diagnostic approach, where these clinical aspects are also scored, may reduce the chances of the student missing less obvious skin cancers.
Methods
We performed a retrospective analytical trial to see if a new algorithm, incorporating clinical as well as dermatoscopic criteria, was any different to the three methods commonly used in Australia primary care practice to diagnose skin cancer. The new algorithm, BLINCK, was developed and compared to two “dermatoscopy-only” algorithms, (the 3-Point Checklist and the Menzies method), and clinical assessment alone. The BLINCK algorithm was designed as an assessment tool for primary care skin cancer clinicians and does not require the user to be an “expert” in dermatoscopy. In contrast to most existing algorithms, the distinction between melanocytic and non-melanocytic [5] lesions is not necessary and both pigmented and non-pigmented lesions may be assessed.
The acronym, BLINCK, refers to six questions that should be asked when assessing a skin lesion and includes both clinical and dermatoscopic features. B – Benign: Is the lesion immediately recognisable as a common benign tumour, on clinical and dermatoscopic examination, with other similar lesions being present on that part of the body, e.g., typical solar lentigo, seborrheic keratosis, haemangioma or dermatofibroma? If ‘yes’, no further action is required. If ‘no’, then proceed to the following four questions. L – Lonely: Is this lesion, clinically and dermatoscopically, the only one of its type on that region of the body, i.e., an “outlier” or “ugly duckling”? ‘Yes’ scores 1. I – Irregular: For pigmented lesions, is the lesion dermatoscopically irregular, that is, does it have an asymmetrical pigmentation pattern and more than one colour? For non-pigmented lesions, is there an irregular vascular pattern? ‘Yes’ scores 1. N – Nervous: Is the patient nervous or concerned that this particular lesion may be a skin cancer? (This excludes the “generally anxious” patient or patients with hypochondriasis). C – Change: Does the patient, or another observer, feel that the lesion is changing? (Note that only a total score of 1 can be given if either or both of these last two questions are answered ‘yes’.) K – Known clues: Does the lesion definitely have any one of the following dermatoscopic “clues” to malignancy?
Atypical network—unmistakable variation in thickness of network line
Pseudopods or streaks—segmental
Black dots, globules or clods—irregular and peripheral
Eccentric structureless zone
Blue or grey colour—irregular distribution
Vessels—1. Polymorphous; 2. finely focused and arborizing; 3. glomerular (coiled) shaped
Acral lesions—1. parallel ridge pattern; 2. diffuse irregular brown/black pigmentation
‘Yes’ to any one of these scores 1 (maximum score of 1). A total score of 2 or more out of 4 requires biopsy.
To compare BLINCK with the other diagnostic methods a pilot trial was conducted using images of skin lesions typically seen in Australian primary care skin cancer practice. From June 1 to July 6, 2009, all skin lesions consecutively excised to exclude skin cancer were recorded by an experienced skin cancer doctor, (A.C.), working in a dedicated skin cancer practice in Brisbane, Australia. Clinically obvious basal cell carcinomas which could be easily diagnosed without dermoscopy were not included in the collection set. High quality clinical and dermatoscopic photographs of 50 skin lesions were obtained, (non-polarised dermatoscopic images taken with a non-polarised Dermlite Foto attachment, or Dermlite Fluid dermatoscope, (3Gen, LLC), and Canon D40 digital camera, (Tokyo, Japan). Written patient consent was obtained in every case and any history of lesion change or patient concern was documented, as well as whether the lesion was thought to be an “ugly duckling” by the original examiner. As common lesions such as the dermatofibroma, seborrhoeic keratosis and congenital naevus sometimes pose a diagnostic challenge for inexperienced clinicians, an example of each, seen during the collection period, was included in the set of 50. These three cases were assessed as being obviously benign by A.C. and not biopsied. As well, a flat naevus that was unchanged on sequential digital monitoring was included in the set without biopsy. Histopathological examination of the other 46 lesions revealed 19 to be skin cancers with nine being melanomas (eight in situ and one invasive). Figures 1 and 2 show an example of a melanoma case from the trial.
Figure 1.
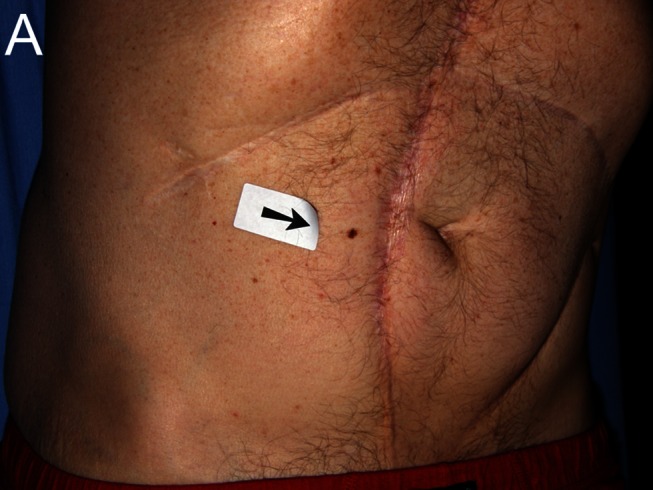
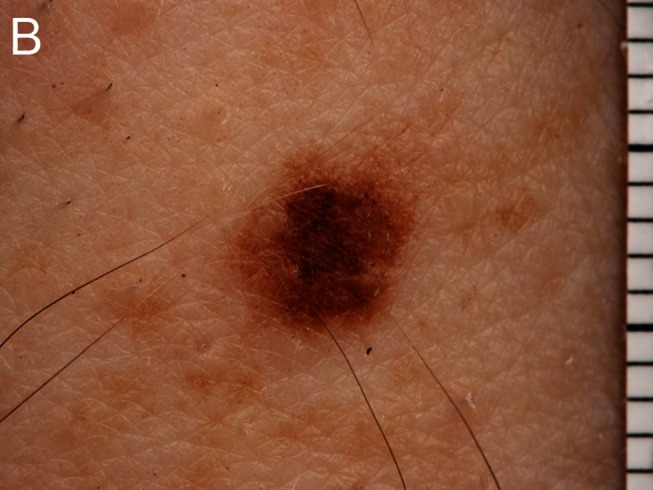
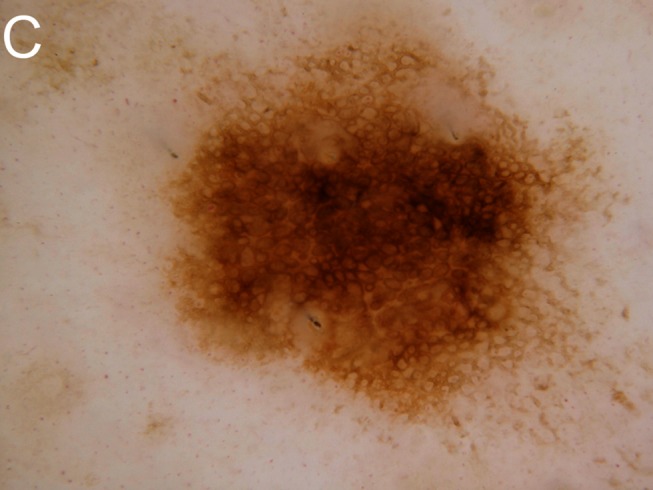
Despite being dermatoscopically bland, this lesion had changed and was “lonely,” scoring 2 in the BLINCK method and mandating biopsy. Histopathology is shown demonstrating melanoma in situ. A: Clinical view. B: Macro view. C: Dermatoscopic view. [Copyright: ©2012 Bourne et al.]
Figure 2.


Dermatoscopic view. A: Histology slide 1. B: Histology slide 2. C: Histology slide 3. [Copyright: ©2012 Bourne et al.]
Four primary care clinicians, (three GPs and a clinical nurse), with varying levels of dermatoscopic experience, were asked to review the photographs and select which lesions were suspicious for malignancy, hence requiring biopsy. This assessment was done on four occasions, each time using a different diagnostic approach. The following methods were used in this order.
3-Point Checklist—Only dermatoscopic images were shown.
Menzies method—Only dermatoscopic images were shown.
Clinical assessment alone—Only clinical images were shown.
BLINCK—Dermatoscopic and clinical images were supplied as well as information regarding reported lesion change, “ugly duckling” sign or patient concern as recorded by original examiner.
The clinicians received prior instruction on the use of the three algorithms, and Excel answer sheets for each method listed the various criteria used in that algorithm. The clinicians were asked to decide if these criteria were present or not and the spreadsheet was used to calculate the results. The clinician’s response for each case was compared to the correct diagnosis and graded as true positive, false negative, false positive or true negative. The number of cancers and melanomas correctly detected and the number of biopsies indicated for each clinician and each method were also recorded (Table 1).
TABLE 1.
True and false positives and negatives for the four methods by the four clinicians with sensitivity, specificity, melanomas found and biopsies indicated.
| Clinician | Method | True Pos | False Neg | False Pos | True Neg | Sens. | Spec. | Number melanomas found | Number biopsies indicated |
|---|---|---|---|---|---|---|---|---|---|
| P.B. | 3point | 11 | 23 | 5 | 6 | 68.8 | 20.7 | 6 | 34 |
| Menzies | 6 | 8 | 10 | 21 | 37.5 | 72.4 | 2 | 14 | |
| Clinical | 9 | 5 | 10 | 26 | 47.3 | 83.9 | 2 | 14 | |
| BLINCK | 19 | 13 | 0 | 18 | 100 | 58.1 | 9 | 32 | |
| C.R. | 3point | 11 | 18 | 5 | 11 | 68.7 | 37.9 | 6 | 29 |
| Menzies | 10 | 6 | 6 | 23 | 62.5 | 79.3 | 5 | 16 | |
| Clinical | 13 | 14 | 6 | 17 | 68.4 | 54.8 | 3 | 27 | |
| BLINCK | 19 | 23 | 0 | 8 | 100 | 25.8 | 9 | 42 | |
| D.B. | 3point | 11 | 18 | 5 | 11 | 68.8 | 37.9 | 5 | 29 |
| Menzies | 11 | 14 | 5 | 15 | 68.8 | 51.7 | 6 | 25 | |
| Clinical | 9 | 6 | 10 | 25 | 47.4 | 80.6 | 2 | 15 | |
| BLINCK | 16 | 15 | 3 | 16 | 84.2 | 51.6 | 8 | 31 | |
| H.C. | 3point | 5 | 8 | 11 | 21 | 31.3 | 72.4 | 2 | 13 |
| Menzies | 8 | 8 | 8 | 21 | 50 | 72.4 | 3 | 16 | |
| Clinical | 9 | 9 | 10 | 22 | 47.4 | 80 | 2 | 18 | |
| BLINCK | 15 | 11 | 4 | 20 | 78.9 | 64.5 | 7 | 26 |
Four clinicians using four methods resulted in 16 contingency tables for sensitivity and specificity. As two of the methods related only to pigmented lesions, (3-Point and Menzies), the five non-pigmented specimens in the set of 50 were excluded from the contingency tables for these methods. Specificity, sensitivity and diagnostic accuracy were calculated according to standard formula. Analysis of variance (ANOVA) was used with the LSD test, (least significant difference test), to detect differences between clinicians and methods. A P-value of <0.05 indicated statistical significance. The means for specificity, sensitivity and diagnostic accuracy are shown with their 95% Confidence Interval, (95% CI). We used the Statistica software package for statistical analysis.
Results
There were no differences between the clinicians regarding sensitivity, specificity, diagnostic accuracy, number of cancers detected, number of melanomas found or biopsies indicated, however, there were significant differences between the four methods (Table 2). BLINCK had higher sensitivity and found significantly more melanomas than the other three methods. However, the Menzies method and clinical only approach had higher specificity and resulted in fewer biopsies than BLINCK. Diagnostic accuracy was the same for BLINCK, Menzies and clinical only, and all were better than the 3-Point checklist. BLINCK had higher sensitivity, diagnostic accuracy, number of cancers found and number of melanomas found than the 3-Point checklist, but had similar specificity and number of biopsies required.
TABLE 2.
Mean values of sensitivity, specificity and diagnostic accuracy with 95% Confidence Intervals (CI) are shown, as well as numbers of melanomas and cancers found and biopsies required.
| Sensitivity (95% CI) | Specificity (95% CI) | Diagnostic Accuracy (95% CI) | Melanomas found (9 total) | Total cancers found (19 total) | Number biopsies (50 total) | |
|---|---|---|---|---|---|---|
| 3-point | 59.4a (52.2–66.5) | 42.2a (35.0–49.4) | 48.3a (44.7–52.0) | 5b | 9a | 26ab |
| Menzies | 54.7a (47.4–62.0) | 69ab (62.2–75.7) | 63.9b (60.4–67.3) | 4ab | 9a | 18a |
| Clinical | 52.6a (40.1–54.7) | 74.8b (74.0–85.7) | 65.0b (61.5–68.5) | 2a | 10a | 18a |
| BLINCK | 90.8b (86.5–95.0) | 50ab (42.7–57.3) | 65.5b (62.1–69.8) | 8c | 17b | 33b |
The 50 lesions used in the trial were sourced from 46 patients, 22 male and 24 female, with ages varying between 30 and 60 years (average 58 years). Anatomical sites of lesions are shown in Table 3.
TABLE 3.
Anatomical location of lesions
| Location | Number |
|---|---|
| face | 8 |
| neck | 1 |
| chest | 3 |
| back | 21 |
| shoulder | 2 |
| arm | 3 |
| thigh | 4 |
| leg | 7 |
| foot plantar | 1 |
Four clinically benign lesions were included in the set without a histological diagnosis, (dermatofibroma, seborrhoeic keratosis, congenital naevus and monitored flat naevus), and the remainder were subjected to histological examination (Table 4).
TABLE 4.
Breakdown of correct lesion diagnosis of the 50 cases.
| Correct diagnosis | Number |
|---|---|
| Banal naevus | 10 |
| Blue naevus | 1 |
| Naevus and seborrhoeic keratosis/solar lentigo collision | 3 |
| Seborrhoeic keratosis | 5 |
| Solar lentigo | 4 |
| Lichen planus-like keratosis (LPLK) | 4 |
| Dermatofibroma | 1 |
| Psoriasis | 1 |
| Solar keratosis | 2 |
| Intraepidermal carcinoma | 3 |
| Regressed keratoacanthoma | 1 |
| Basal cell carcinoma | 6 |
| Lentigo maligna | 1 |
| Melanoma in situ | 7 |
| Melanoma- invasive - (0.52 mm Breslow) | 1 |
Discussion
Australia has the highest rate of melanoma in the world [6]. It is the third most common cancer in Australia in both men and in women [7]. Approximately two out of every three Australians will be diagnosed with skin cancer before the age of 70 [8] and roughly a million GP visits are made annually for skin cancer. This makes skin cancer the most expensive of all cancers for the Australian health system [9, 10]. More skin cancers are diagnosed and treated in Australia by primary care doctors than by medical specialists [11]. These generalists require a simple yet accurate screening tool that will allow the detection of melanoma and other skin cancers at an early stage when complete cure is possible. Currently, most introductory skin cancer courses in Australia endorse dermatoscopic evaluation of suspicious skin lesions as the preferred screening method, commonly using the 3-Point checklist or the Menzies method [12,13].
Other clinical features that may assist with the diagnosis of skin cancer have been previously studied. The “ugly duckling” sign has been shown to be of possible use in melanoma screening [4]. Importantly, the ability to assess whether a lesion is “different” from surrounding lesions does not appear to require advanced training. Hence, it would seem sensible that primary care clinicians seek out “ugly duckling” lesions when performing a skin examination, (“Lonely” in the BLINCK algorithm). Lesion change is also known to be associated with malignancy, particularly in patients over 50 years of age [14], and consideration of this clinical feature would also seem prudent. A disproportionate amount of concern for a lesion by the patient, (“Nervous”), may have significance for two reasons. Firstly, patients may be uncertain if their lesion has changed or perhaps they may simply fail to volunteer the history of change, bleeding, itch or soreness. They suspect that it is a cancer but assume the doctor is able to make the diagnosis by mere inspection without needing any clinical history. However, this history may be the only clue to malignancy in dermatoscopically bland lesions, and a false negative diagnosis may be made using dermatoscopic assessment alone. Secondly, dismissing a lesion about which the patient is quite concerned may have medico-legal consequences should it prove later to be malignant.
In this small trial, the BLINCK algorithm, which scored these extra clinical features along with basic dermatoscopic assessment, found more skin cancers and melanomas than the commonly endorsed methods in Australia. However, more excisions were required to achieve this result. This raises the question as to what is the best measure of “accuracy” in melanoma diagnosis. Argenziano has suggested that NNE, (number of melanocytic lesions needed to be excised in order to find one melanoma), may be useful for measuring accuracy in melanoma detection and compared the NNE in specialised and non-specialised clinical settings [15]. In his study the NNE reduced over time from 12.8 to 6.8 with specialised clinics but remained unchanged at 29.4 in non-specialised centres. This would seem to suggest that a level around 6.8 may be an appropriate goal for skin cancer clinicians. In our study, the overall NNE for melanoma by all clinicians using the BLINCK algorithm was 6, with the 3-Point checklist 11, the Menzies method 13 and clinical assessment only 22, suggesting that BLINCK may have value as a skin cancer screening tool.
As this trial was limited by the small number of skin cancers and melanomas, and by the fact that it was a virtual study without direct assessment of patients’ lesions, it is difficult to draw definite conclusions regarding the benefits of combining clinical with dermatoscopic features in one diagnostic algorithm. Indeed, it could be said that clinicians using dermatoscopic-only algorithms implicitly incorporate relevant clinical aspects of the case in their decision making process. However, knowing how much weight to give these clinical aspects is often difficult for novices. Being forced to score the clinical with the dermatoscopic findings may help develop a more structured and holistic approach when deciding if a lesion requires biopsy.
Footnotes
Competing interests: All authors declare no conflict of interest with regards the preparation or writing of this paper. They have not accepted from a sponsor, pharmaceutical company or other organization any funds for research, consultancy fees, fellowship/research/education grants, nor hold any stock or shares in any entity that may gain financial benefit or detriment as a result of the deliberations set out in or the conclusions of the study.
Funding: None.
References
- 1.Zalaudek I, Argenziano G, Soyer HP, et al. Three-point checklist of dermoscopy: an open internet study. Br J Dermatol. 2006;154(3):431–7. doi: 10.1111/j.1365-2133.2005.06983.x. [DOI] [PubMed] [Google Scholar]
- 2.Menzies SW, Ingvar C, Crotty KA, McCarthy WH. Frequency and morphologic characteristics of invasive melanomas lacking specific surface microscopic features. Arch Dermatol. 1996;132(10):1178–82. [PubMed] [Google Scholar]
- 3.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134(1):103–4. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 4.Scope A, Dusza SW, Halpern AC, et al. The “Ugly Duckling” Sign: Agreement Between Observers. Arch Dermatol. 2008;144(1):58–64. doi: 10.1001/archdermatol.2007.15. [DOI] [PubMed] [Google Scholar]
- 5.Kittler H. Why the first step should be abandoned! Arch Dermatol. 2010;146(10):1182–3. doi: 10.1001/archdermatol.2010.271. [DOI] [PubMed] [Google Scholar]
- 6.Australian Institute of Health and Welfare and Australasian Association of Cancer Registries . Cancer in Australia 2001. AIHW; Canberra: 2004. AIHW cat. no. CAN 23. [Google Scholar]
- 7.Australian Institute of Health and Welfare (AIHW) and Australasian Association of Cancer Registries (AACR) Cancer in Australia: An Overview. AIHW; Canberra: 2008. Cancer series No 46. [Google Scholar]
- 8.Staples M, Elwwod M. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust. 2006;184(1):6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 9.Australian Institute of Health and Welfare (AIHW) Non-melanoma Skin Cancer: General Practice Consultations, Hospitalisation and Mortality. AIHW; Canberra: 2008. Cancer Series no. 43 Catalogue no. 49. [Google Scholar]
- 10.Australian Institute of Health and Welfare (AIHW) Health System Expenditures on Cancer and Other Neoplasms in Australia, 2000–01. AIHW; Canberra: 2005. Health and Welfare Expenditure Series Number 22. [Google Scholar]
- 11.Askew DA, Wilkinson D. Skin cancer surgery in Australia 2001–2005:the changing role of the general practitioner. Med J Aust. 2007;187(4):210–4. doi: 10.5694/j.1326-5377.2007.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 12.SCCANZ Certificate in Skin Cancer Medicine. SCCANZ web site, http://www.sccanz.com.au. Accessed October 30, 2011.
- 13.UQ Certificate in Primary Care Skin Cancer Medicine. Skin Cancer Courses web site; http://www.skincancercourses.com.au/skc/index.htm. Accessed October 30, 2011.
- 14.Banky P, Kelly JW, English DR, et al. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141(8):998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 15.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: A 10- year survey. J Am Acad Dermatol. 2011. [published online ahead of print 2011 Oct 6] http://dx.doi.org/10.1016/j.jaad.2011.07.019. [DOI] [PubMed]



