Abstract
Background:
Basal cell carcinoma (BCC) can present with indolent or aggressive subtypes. These subtypes usually display vascular features, which are often readily identified using dermatoscopy.
Objective:
Dermatoscopy vascular features of aggressive BCC were compared to superficial, superficial and nodular, and nodular BCC for diagnostic discrimination.
Method:
Dermatoscopy vascular features were recorded live direct from the patient for 1,098 consecutive BCC. Cases with potential confounding influences were excluded. These tumor vascular features included branching (arborizing), serpentine, dot, coil (glomerular), loop (hairpin) and linear vessels. The proportion of pink within the tumor, central versus peripheral tumor vessel distribution and the presence of large vessels within the tumor boundary were also recorded.
Results:
Different subtypes of BCC have distinctive vascular features. Aggressive BCC (n=213) displays a tumor area with no pink (12.2%) or less than half the area pink (27.2%) and absent vessels in the central tumor area (22.1%, CI 17.0%–28.1%, P<0.001) compared to other subtypes. Superficial BCC (n=284) have more than half the tumor area pink (84.9%) and absent large vessels (92.6%), CI 89.0%—95.1%. Nodular BCC (n=230) is characterized by larger vessels (45.7%, CI 39.3%–52.1%, P<0.001) as compared to other subtypes, as well as less dot, coil and loop vessels. Kappa values for all recorded features ranged from 0.48 to 1.0.
Limitations:
Aggressive BCCs within the combined aggressive group were not assessed separately.
Conclusions:
Diagnostic discrimination between different subtypes of BCC is facilitated by vascular feature assessment. Compared to other subtypes, aggressive BCC displays less or no pink and less or absent central tumor vessels.
Keywords: superficial nodular aggressive basal cell carcinoma, dermatoscopy, vascular, vessels
Introduction
Basal cell carcinoma (BCC) is a common encounter in practice, presenting as either a whole single subtype or as various subtype combinations within a single lesion. This study focuses on dermatoscopy vascular features of BCC subtypes with the intention of improving subtype diagnostic discrimination and aggressive subtype identification.
Superficial and nodular BCC subtypes behave with relatively indolent malignant behaviour. More aggressive BCC subtypes include micronodular, infiltrating, morphoeic or sclerosing, and BCC with squamous differentiation—these aggressive subtypes were assessed combined as “aggressive subtype,” in this study. The distribution of BCC subtypes have anatomical site variation, with nodular and morphoeic BCC being more common on the head and neck and superficial BCC more common on the trunk and limbs [1]. Aggressive BCC subtypes have higher rates of recurrence and excision margin involvement [2]. Infiltrative BCC has the highest representation in perineural invasion, one series reported over 30% of cases of perineural invasion were infiltrating BCC [3]. Squamous differentiation indicates a nonexclusive increased risk for the quite rare event of metastasis in BCC [4]. Optimal management of BCC can be facilitated by early recognition and by priority given to appropriate treatment of aggressive BCC subtypes. There are other rare variants of BCC with differentiation representing various cutaneous structures. These rare variants were not covered in this study.
Most early dermatoscopy studies on BCC focused on pigmented features [5,6,7] with the recurrent purpose of diagnostic separation from melanoma. Only a few studies investigate the dermatoscopy vascular features of individual different BCC subtypes. Superficial [8,9,10] and nodular BCC [11,13] dermatoscopy vascular features have been reported, while aggressive BCC subtypes have been relatively neglected. Multiple studies have consistently reported branching vessels as a very characteristic vessel form associated with BCC.
Methods
This project was prospective and data collection was direct from patients, not from digital photographs. Studies on vessels usually record retrospective data from photographs with a fixed focus. This study recorded data direct from patients using “live” variable focus dermatoscopy. Cases came from primary care and referral patients from two centres in Sydney, Australia. During the project time window, all excisions where BCC was either the obvious diagnosis or in the differential diagnosis, were considered for inclusion. After the application of exclusion criteria, the defined vascular features of the remaining cases were recorded direct from each patient with a Heine Delta 20 non-polarized dermatoscope (Heine, Optotechnic GmbH, Herrsching, Germany), just prior to anaesthetic injection, excision and submission for routine histopathological examination. Transparent ultrasound gel was applied between the dermatoscope glass plate and the skin in all cases to enhance resolution by maximizing light transmission and reducing compression on vessels. All excised tissue was submitted for histopathological examination. Histopathology determined non-BCC entities were removed from the data resulting in a final total of 1,098 consecutive BCC cases. Each of these 1,098 BCC cases were allocated into one of the following subtype categories: (1) superficial (no other subtype present), (2) superficial and nodular (no other subtypes present), (3) nodular (only subtype), or (4) aggressive subtypes (collective cases of infiltrating, micronodular, morphoeic or sclerosing BCC and BCC with squamous differentiation).
The Ethics Committee of The University of Queensland, Australia, granted formal approval for this project prior to data collection. Data collection ran from July 2008 to December 2010.
Exclusion criteria
Patient informed consent, conforming to the Ethics Committee requirements, was required—only one case was excluded due to an inability to obtain consent. Other exclusion criteria were carefully selected to exclude cases with potential confounding influences. The intention was to only accept BCCs displaying vascular features due to the tumor presence or influence.
The exclusion criteria included the following: collision situations where a BCC was in combination with a non-BCC entity, in either clinical or dermatoscopy examination or in histopathology sections; tumors where exogenous-applied preparations (for example, “make up” or “fake tan”) obscured dermatoscopy detail; residual or recurrent tumors following surgical intervention; tumors that had received previous cryotherapy or other ablative treatments; tumor areas following topical pharmacological treatment, either physician or patient initiated; tumors within fields of previous photodynamic therapy or radiotherapy; and sites juxtaposed to a scar, associated with tattoos or with mucosal involvement.
Vascular features defined
The following defined tumor vascular features and anatomical sites were recorded for each case. Every case was allocated into one of the following categories: (1) head and neck, (2) shoulder, (3) abdomen and back, (4) upper limb, (5) lower limb. The data from all anatomical sites were also combined into one data set per vascular feature.
Pink areas: Pink within the tumor area was categorized into either: (1) no pink present, (2) less than 50% of the tumor pink, or (3) pink present in 50% or more of the tumor area.
Central versus peripheral distribution of vessels: Each tumor dermatoscopy silhouette was divided into a central and peripheral area. A central area occupied the central half of the tumor diameter; the peripheral area occupied the outer half of the tumor diameter, to the tumor edge, as an annular shape, adapted to the tumor shape. Vessel presence was recorded in quadrants for both central and peripheral areas. Nil equals no vessels identified in any quadrant. Vessels present in 1 quadrant were recorded as 1. Vessels present in 2 quadrants were recorded as 2. Vessels present in 3 or in all 4 quadrants were recorded as 3.
Vessel morphology: Branching (arborizing), dot, coil (glomerular) and loop (hairpin) vessel forms are well established and reported in the dermatoscopy literature. Serpentine vessels are defined as having one or more bends, to any degree, not convoluted, not parallel and not compact—no branching permitted. Linear vessels are elongated, any length or diameter, with no curves or branches.
Large vessels: Any vessel inside the tumor boundary with a diameter larger than all visible vessels in the background area surrounding the tumor, out to 10 mm from the boundary, was recorded.
Data validation
To assess inter-observer agreement on the vascular features studied, Kappa values were calculated for all the defined vascular features for 108 consecutive BCC cases examined by JP and DS. These two observers and the statistician (JCW) performing the calculations were all blinded to each other during data collection and results calculations.
Statistical analysis
Inference from proportion
The sample proportion p, of tumors with a certain vascular feature observed in the tumors is calculated by:
This is an estimation to the occurrence rate (denoted as π) of a certain vascular feature being seen in a cutaneous tumor. These vascular features include: linear, branching, serpentine, hairpin/loop, dot, large vessels and the proportions of pink in the tumor. The 95% confidence interval of the occurrence rate π is calculated by the Wilson’s score method without continuity correction
where z.975 ≅1.96 is the 97.5 percentile point of the standard normal distribution [1,2].
Kappa measure of agreement
The Kappa (κ) measure of agreement is used to access the agreement between two clinicians (observers) for the presenting of a certain vascular feature, given the assumption that both clinicians assess n patients independently. If clinicians agree purely by chance, they are not really “agreeing” at all. Only the agreement beyond that expected by chance can be considered as “true” agreement. Kappa is such a measure of “true” agreement. It indicates the proportion of agreement beyond that expected by chance. Its confidence interval takes the form:
In practical situations, the value of lower bounds of confidence interval should usually be higher than 0.4 to be considered as clinically acceptable. All statistical analyses were performed using R software [11].
Results
Patient ages ranged from 29 to 98 for the total BCC cases (n=1098), the median age was 64 years, the mean 64.8 years, 67.9% were males. Aggressive BCC (n = 213) had 67.9% male representation (n = 142). Although assessed combined, the aggressive BCC subtypes were represented in decending order of frequency by infiltrating BCC (64.8%, n = 138), micronodular BCC (27%, n = 57), BCC with squamous differentiation (17%, n = 37) and morphoeic BCC (3%, n = 7); some BCC contained more than one aggressive subtype.
Anatomical distribution of basal cell carcinoma subtypes
Aggressive BCC (n = 213) were recorded with the highest incidence on the head and neck 49.3% (n = 105) compared to all other sites. Nodular BCC (n = 230) was also recorded with maximum incidence on the head and neck 48.3% (n = 111). Superficial and nodular BCC (n = 371) peak incidence was on the trunk 28.3% (n = 105) and head and neck 27.5% (n = 102). In contrast, superficial BCC (n=284) were more prominent on the trunk 33% (n = 94) and upper limbs 25% (n = 70).
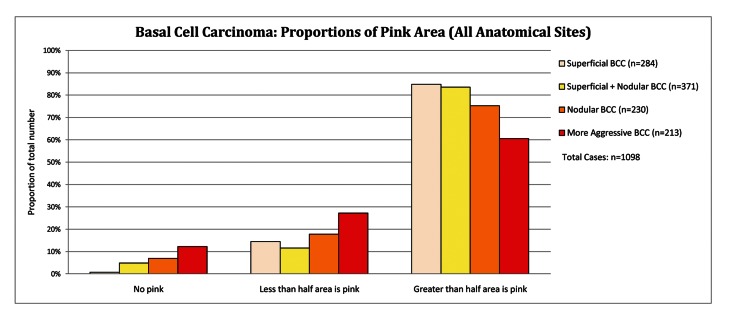
Proportions of pink in basal cell carcinoma subtypes
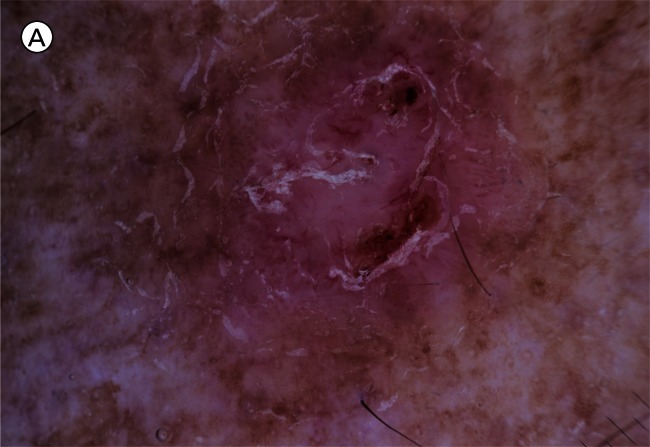
Aggressive BCC had a tumor area with no pink or less than 50% pink in 39.4% cases compared to 18.2% in all other subtypes, P<0.001. Superficial BCC together with superficial and nodular BCC have more than 50% pink in the tumor area in 84.1 % of cases compared to 60.6% of cases with aggressive BCC (P< 0.001), as set out in Figure 1. An example with dermatoscopic-pathologic correlation is given in Figure 2.
Figure 1.
Basal cell carcinoma: proportions of pink areas (all anatomical sites). [Copyright: ©2012 Pyne et al.]
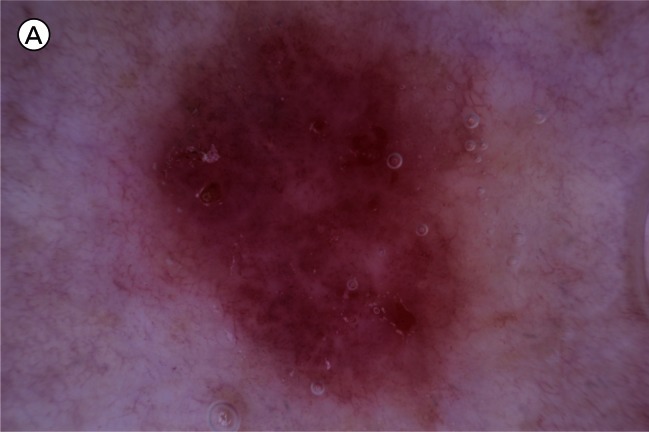
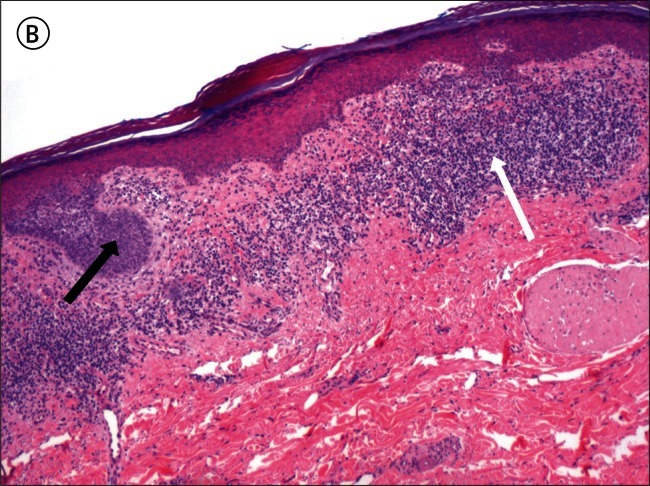
Figure 2.
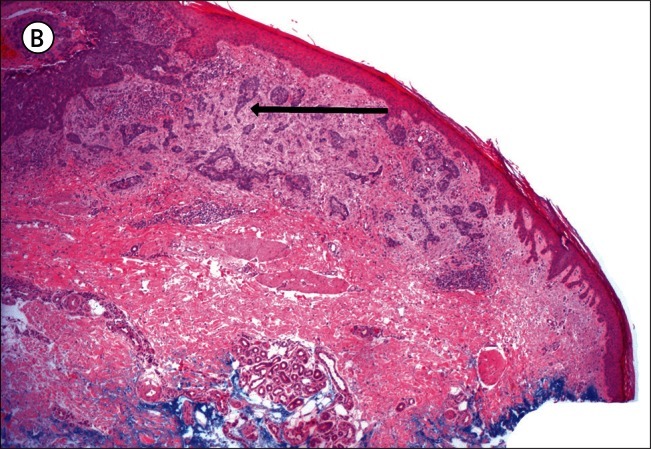
A) Superficial basal cell carcinoma: dermatoscopy. This example demonstrates pink occupying considerably greater than 50% of the dermatoscopy identified tumor area. [Copyright: ©2012 Pyne et al.] B) Superficial basal cell carcinoma: histopathology (same lesion as Figure 2A). Hematoxylin and eosin stain. Black arrow to basaloid tumor cells, white arrow to the lichenoid response. [Copyright: ©2012 Pyne et al.]
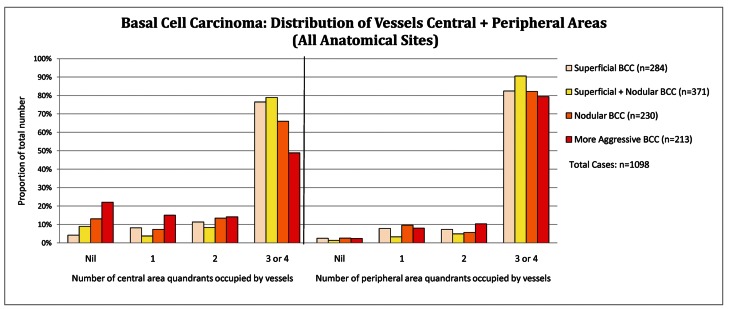
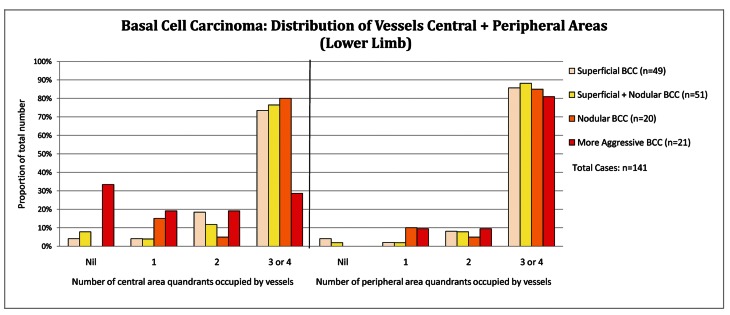
Distribution of tumor vessels: central versus peripheral location
In the central tumor areas, aggressive BCC (n = 213) had no vessels in 22.1%, CI 17.0%–28.1% of cases compared to 8.5%, CI 6.8%–10.5% in other subtypes (P<0.001) as displayed below in Figure 3A (all anatomical sites data combined). On the lower limb, 33% (7 out of 21) of aggressive BCCs display no vessels in the central tumor area (Figure 3B). The Infiltrating BCC in Figure 4A (dermatoscopy image) and Figure 4B (histopathology of the same lesion) illustrate the tendency for aggressive subtypes of BCC to display reduced pink and reduced vessels in the central area of the tumor.
Figure 3A.
Basal cell carcinoma: distribution of vessels central and peripheral (all anatomical sites). [Copyright: ©2012 Pyne et al.]
Figure 3B.
Basal cell carcinoma: distribution of vessels central and peripheral (lower limb). [Copyright: ©2012 Pyne et al.]
Figure 4.
A) Infiltrating basal cell carcinoma: dermatoscopy. [Copyright: ©2012 Pyne et al.] B) Infiltrating basal cell carcinoma: histopathology (same lesion as Figure 4A). Hematoxylin and eosin stain. Black arrow to the collagen rich tumor stroma. [Copyright: ©2012 Pyne et al.]
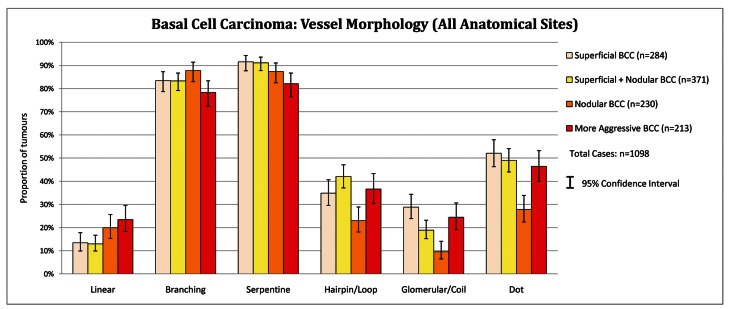
Vessel morphology in basal cell carcinoma subtypes
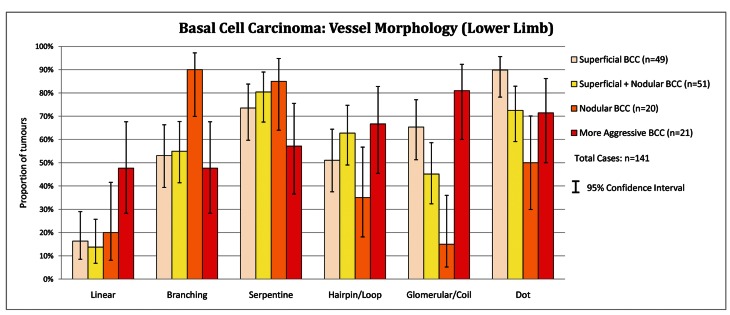
Examination of the vessel morphology data in Figure 5 below reveals the strong dominating representation of branching and serpentine vessels over all other vessel morphologies in all BCC subtypes, for all Anatomical Sites combined. All BCC subtypes listed tended to have the different vessel morphologies present with similar incidences. Aggressive BCC on the lower limb is the exception to this trend. On the lower limb, aggressive BCC (n=21) displays loop (66.7%), coil (81.0%) and dot vessels (71.4%) more frequently than branching (47.6%) and serpentine vessels (57.1%): these vessel morphologies are displayed on Figure 6.
Figure 5.
Basal cell carcinoma: vessel morphology (all anatomical sites). [Copyright: ©2012 Pyne et al.]
Figure 6.
Basal cell carcinoma: vessel morphology (lower limb). [Copyright: ©2012 Pyne et al.]
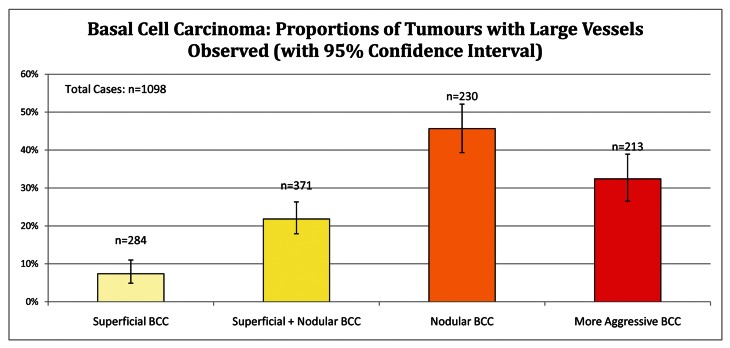
Large vessels in BCC subtypes
Superficial BCCs (n=284) together with superficial and nodular BCCs (n=371) display a relative absence of large vessels compared to nodular together with aggressive BCC subtypes, P< 0.001. Superficial BCC had absent large vessels in 92.6% of cases (CI 89.0%–95.1%). Compared to all other subtypes of BCC, nodular BCC (n= 230) have large vessels present 45.7%, CI 39.3%–52.1%, P < 0.001, as shown in Figure 7.
Figure 7.
Basal cell carcinoma: proportions of tumors with large vessels. [Copyright: ©2012 Pyne et al.]
Vascular feature validation data
Table 1 below sets out the Kappa values for two observers (JP and DS) for BCC (n=108), with 95% confidence intervals. Kappa values from 0.41 to 0.60 indicate moderate agreement between observers, 0.61 to 0.80 are regarded as substantial agreement and from 0.81 to 1.00 almost perfect.
TABLE 1.
Vascular features - Kappa values
| Vascular Feature | Cohen’s Kappa value | Confidence Interval Lower Bound | Confidence Interval Upper Bound |
|---|---|---|---|
| Branching vessels | 0.63 | 0.38 | 0.88 |
| Serpentine vessels | 0.48 | 0.30 | 0.67 |
| Hairpin vessels | 0.70 | 0.56 | 0.83 |
| Glomerular vessels | 0.71 | 0.52 | 0.89 |
| Dot vessels | 0.53 | 0.23 | 0.82 |
| Linear vessels | 1.00 | 1.00 | 1.00 |
| Large vessels | 0.96 | 0.89 | 1.00 |
| Pink areas | 0.66 | 0.19 | 1.00 |
| Central vessels | 1.00 | 1.00 | 1.00 |
| Peripheral vessels | 1.00 | 1.00 | 1.00 |
Discussion
Diagnosing BCC utilizing dermatoscopy is facilitated by the recognition of multiple distinctive features, including pigmented structures, shiny white lines (chrysalis[13] in metaphoric terminology), ulceration and vascular features. Discussion on the practical use of the study of vascular features follows with the intention of assisting in the correct identification of aggressive subtype BCC.
Pink areas
In practice, pink in the tumor area of BCC commonly attracts the clinician’s attention during clinical examination. Aggressive subtypes display absent or less tumor area pink than other subtypes (Figure 1), this may render aggressive subtypes less obvious on clinical examination. Prominent collagen in the tumor stroma of infiltrating (Figure 4B) and morphoeic BCC may correlate with reduced pink areas identified during dermatoscopy (Figure 4A). Although not formally recorded in this study, the non-pink tumor areas where observed to be dominated by white structureless areas. White structureless areas have been reported to be seen more frequently in BCC using contact dermatoscopy compared to polarized non-contact dermatoscopy [14]. Pink represents increased localized vascular perfusion in BCC and is more conspicuous in polarized non-contact dermatoscopy [14].
The finding of 84.9% of superficial BCCs having greater than half the tumor area pink is a useful clue to identifying this tumor (Figure 2A). It is unknown to what extent these pink areas correlate with the commonly associated lichenoid response in the papillary dermis, as shown in Figure 2B, or to more specific tumor factors.
Vessel distribution in central and peripheral tumor areas
Reduced or absent vessels in the central tumor area are also more frequent in aggressive subtypes, particularly the lower limb, where 1 in 3 aggressive BCCs displayed no central vessels (Figure 3B).
Vessel morphology
The presence or absence of the selected specific vessel morphologies alone were ineffective in subtype discrimination between the BCC subtypes assessed in the anatomical sites selected (Figure 5). Linear vessels are more frequent in aggressive subtypes, yet the low incidence (23.5%, in all anatomical sites combined) and lack of statistical significance (overlapping confidence intervals) limits practical use.
Dot, loop and glomerular vessels are usually associated with keratinocytic malignancy [14]. In aggressive BCC on the lower limb, dot (71.4%), hairpin (66.7%) and glomerular (81.0%) vessels were all more frequent than branching (47.6%) and serpentine (57.1%) vessels (Figure 6). In all subtypes of BCC, at all other sites, branching and serpentine vessels predominate over dot, hairpin and glomerular vessels. Thus on the lower limb there is a shift in the aggressive BCC recorded vessel morphology profile towards squamous cell carcinoma.
Large diameter vessels
Large diameter vessels were the hallmark of nodular BCC in this study. This is not surprising given the usual larger volume of tumor per unit area observed during dermatoscopy and in histopathology sections. Microvascular studies [16,17] and reflectance confocal microscopy [18,19] have established that vessels in BCC are located outside the basaloid tumor cell aggregations. Thus, it is not surprising that nodular BCC with a large thicker tumor mass is perfused from large diameter vessels located outside and adjacent to the tumor.
Superficial BCC typically occupies a relatively thin volume in the superficial papillary dermis, thus the finding of large tumor vessels being usually absent from this subtype is also not surprising.
Aggressive BCC is commonly found in combination with nodular BCC. The record of large diameter vessels being associated with aggressive BCC may be confounded by the additional presence of nodular BCC in the same tumor mass.
Defining aggressive BCC subtypes by histopathological appearance does not imply these specific BCCs will always follow a more aggressive course.
Study limitations
All dermatoscopy performed was non-polarized. Ulceration is a common dermatoscopy feature of BCC. Assessment of ulceration details by BCC subtype was not a formal part of this study.
Although potentially confounding previous interventions were to be excluded, some cases of tumors with confounding intervention may have “slipped through” the application of exclusion criteria due to the frequent reliance on history provided by the patient.
No data stratification based on tumor diameter or depth were structured into the data records. Routine sectioning during histopathology preparation may have resulted in presence of some additional subtypes being missed due to sampling error. There is an increased risk of additional subtype diagnostic omission if the mass of this missed tissue is small relative to the total tumor volume.
Aggressive subtypes of BCC were combined together rather than segregated—these subtypes included infiltrating, micronodular, morphoeic or sclerosing BCC and BCC with squamous cell differentiation. Future work may clarify differences in the dermatoscopy appearance between these different aggressive subtypes. Pigmented features in BCC were not formally assessed in this study. Spatial distribution of different vessel morphologies within the tumor mass of BCC and more specific detail on the geometry of vessels (for example, angles between branching vessels) are other possible areas for future investigation.
Conclusion
Aggressive BCC tends to have no or less pink within the tumor area and absent or few vessels in the central tumor area compared to other BCC subtypes. Superficial BCC typically has the dermatoscopy vascular features of increased pink and relative absence of large diameter vessels. Nodular BCC commonly displays large diameter vessels and may be combined with aggressive subtype BCC.
How dermatoscopy features in aggressive BCC correlates with subtype histopathology and thus assists in individual aggressive subtype diagnostic discrimination is worthy of further future investigation.
Acknowledgments
The authors are very grateful for the advice and guidance on various technical aspects of this study provided by Prof. H. Peter Soyer from The University of Queensland, Brisbane, Australia.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Betti R, Radaelli G, Bombonato C, Crosti C, Cerri A, Menni S. Anatomic location of basal cell carcinomas may favour certain histologic subtypes. J Cutan Med Surg. 2010;14(6):298–302. doi: 10.2310/7750.2010.09081. [DOI] [PubMed] [Google Scholar]
- 2.Brown CI, Perry AE. Incidence of perineural invasion in histologically aggressive types of basal cell carcinoma. Am J Dermatopath. 2000;22(2):123–5. doi: 10.1097/00000372-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 3.De’ Ambrosis K, De’ Ambrosis B. Nonmelanoma Skin cancer with perineural invasion: report of outcomes in a case series. Dermatol Surg. 2010;36(1):133–8. doi: 10.1111/j.1524-4725.2009.01367.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J Am Acad Dermatol. 2009;60(1):137–43. doi: 10.1016/j.jaad.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136(8):1012–6. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 6.Peris K, Altobelli E, Ferrari A, Farqnoli MC, Piccolo D, Chimenti S. Interobserver agreement on dermoscopic features of pigmented basal cell carcinoma. Dermatol Surg. 2002;28:643–645. doi: 10.1046/j.1524-4725.2002.01302.x. [DOI] [PubMed] [Google Scholar]
- 7.Demirtasoglu M, Ilknur T, Lebe B, Kusku E, Akarsu S, Ozkan S. Evaluation of dermoscopic and histopathologic features and their correlations in pigmented basal cell carcinomas. J Eur Acad Dermatol Venereol. 2006;20(8):916–20. doi: 10.1111/j.1468-3083.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 8.Scalvenzi M, Lembo S, Francia MG, Balato A. Dermoscopic patterns of superficial basal cell carcinoma. Int J Dermatol. 2008;47(10):1015–8. doi: 10.1111/j.1365-4632.2008.03731.x. [DOI] [PubMed] [Google Scholar]
- 9.Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol Surg. 2005;31:1710–3. doi: 10.2310/6350.2005.31314. [DOI] [PubMed] [Google Scholar]
- 10.Pan Y, Chamberlain AJ, Bailey M, Chong AH, Haskett M, Kelly JW. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque—features distinguishing superficial basal cell carcinoma, intra epidermal carcinoma and psoriasis. J Am Acad Dermatol. 2008;59(2):268–74. doi: 10.1016/j.jaad.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 11.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2011. R: A language and environment for statistical computing. URL http://www.R-project.org/. [Google Scholar]
- 12.Micantonio T, Gulia A, Altobelli E, Di Cesare A, Fidanza R, Riitano A, Fargnoli MC, Peris K. Vascular patterns in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2011;25(3):358–61. doi: 10.1111/j.1468-3083.2010.03734.x. [DOI] [PubMed] [Google Scholar]
- 13.Leibman TN, Rabinovitz HS, Balagula Y, Jaimes-Lopez N, Marghoob AA. White shiny structures in melanoma and BCC. Arch Dermatol. 2012;148(1):146. doi: 10.1001/archdermatol.2011.618. [DOI] [PubMed] [Google Scholar]
- 14.Liebman TN, Jaimes-Lopez N, Balagula Y, et al. Dermoscopic features of basal cell carcinomas: differences in appearance under non-polarized and polarized light. Dermatol Surg. 2012;38(3):392–99. doi: 10.1111/j.1524-4725.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 15.Zalaudek I, Giacomel J, Schmid K, et al. Dermatoscopy of actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J Am Acad Dermatol. 2012;66(4):589–97. doi: 10.1016/j.jaad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Grunt TW, Lametschwandtner A, Staindl O. The vascular pat-tern of basal cell tumors: light microscopy and scanning electron microscopic study on vascular corrosion casts. Microvas Res. 1985;29(3):371–86. doi: 10.1016/0026-2862(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 17.Chin CW, Foss AJ, Stevens A, Lowe J. Differences in the vascular patterns of basal and squamous cell skin carcinomas explain their differences in clinical behaviour. J Pathol. 2003;(3):308–13. doi: 10.1002/path.1363. [DOI] [PubMed] [Google Scholar]
- 18.Agero ALC, Cuevas J, Jaen P, Marghoob AA, Gill M, Gonzalez S. Basal cell carcinoma. In: Gonzalez S, Gill M, Halpern A, editors. Reflectance Confocal Microscopy of Cutaneous Tumors. Informa; 2008. pp. 60–75. [Google Scholar]
- 19.Ahlgrimm-Siess V, Cao T, Oliviero M, Hofmann-Wellenhof R, Rabinovitz HS, Scope A. The vasculature of Nonmelanocytic skin tumors in reflectance confocal microscopy: vascular features of basal cell carcinoma. Arch Dermatol. 2010;146(3):353–4. doi: 10.1001/archdermatol.2009.380. [DOI] [PubMed] [Google Scholar]