Abstract
Homogenates of oat (Avena sativa cv. Goodfield) roots contained at least five membrane-associated adenosine triphosphatase (ATPase) activities. The membrane-bound ATPases were separated on sucrose gradients and distinguished by membrane density, pH optima, sensitivity to monovalent salts, and substrate specificity.
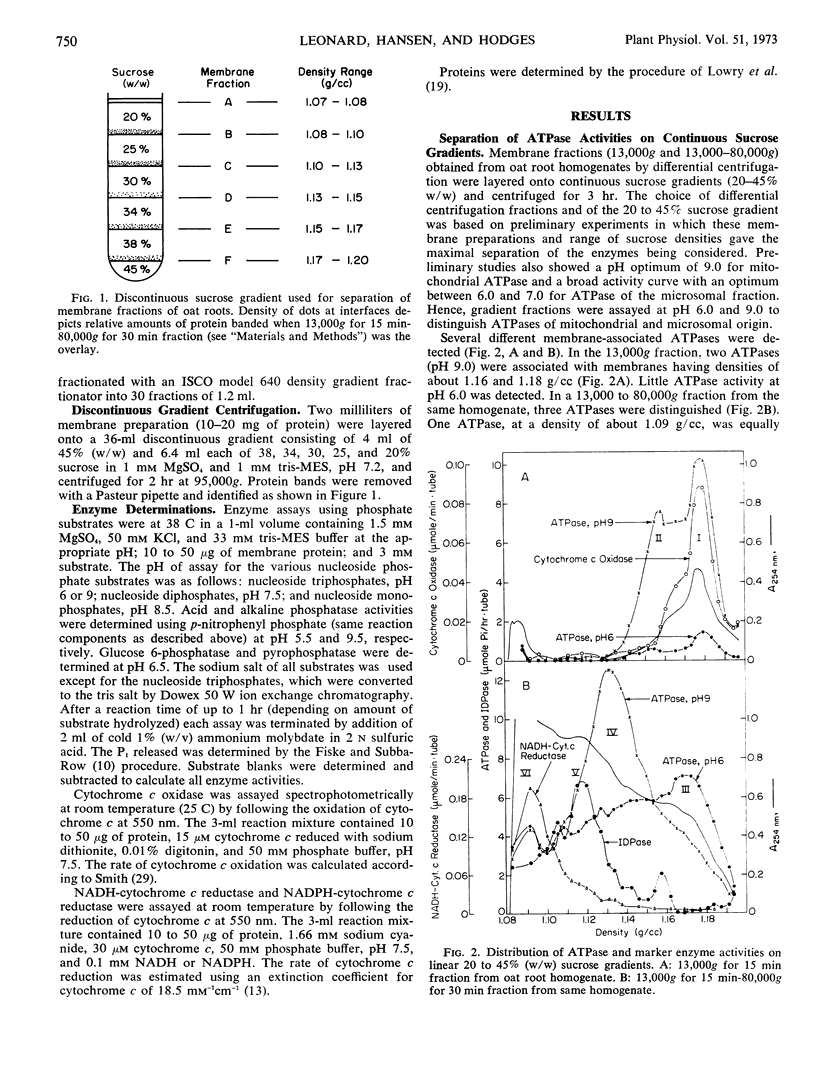
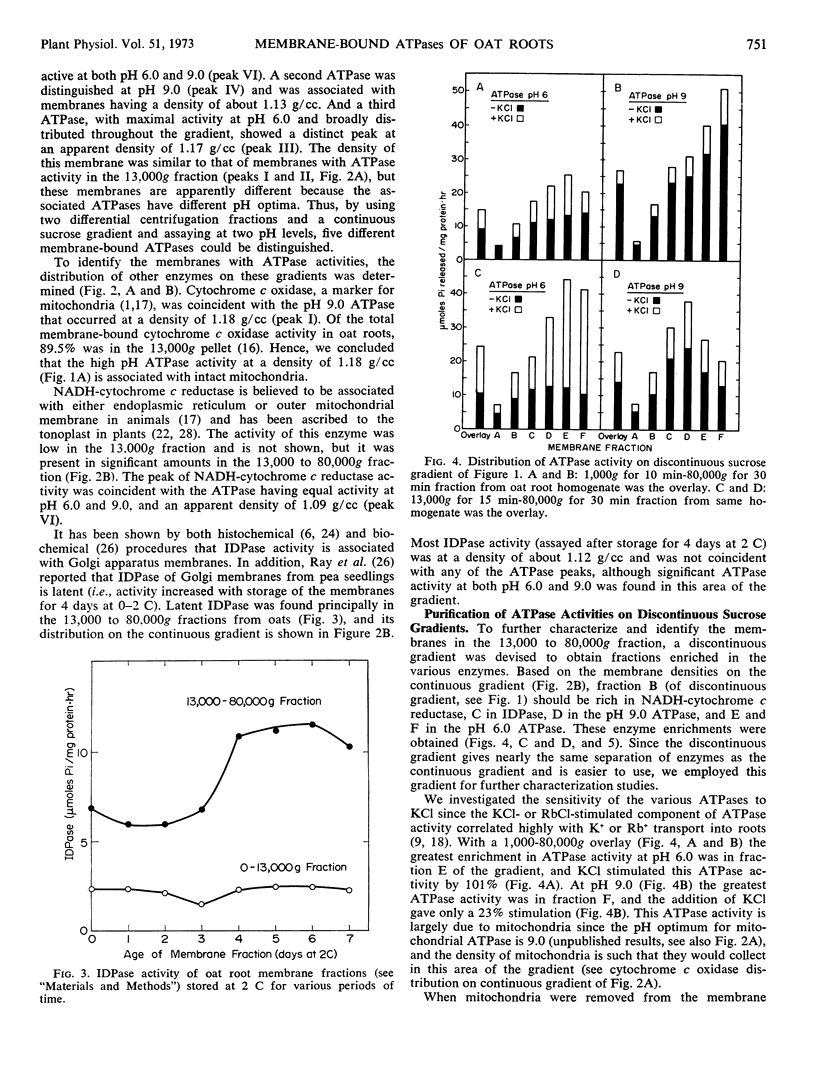
A membrane fraction sedimenting at low centrifugal force (13,000g) contained two ATPase activities at pH 9.0. One membrane ATPase was coincident with cytochrome c oxidase activity and had a density of 1.18 grams per cubic centimeter. This membrane system was identified as mitochondria. The other pH 9.0 ATPase in this fraction occurred at a density of 1.16 grams per cubic centimeter. The identity of this membrane is unknown.
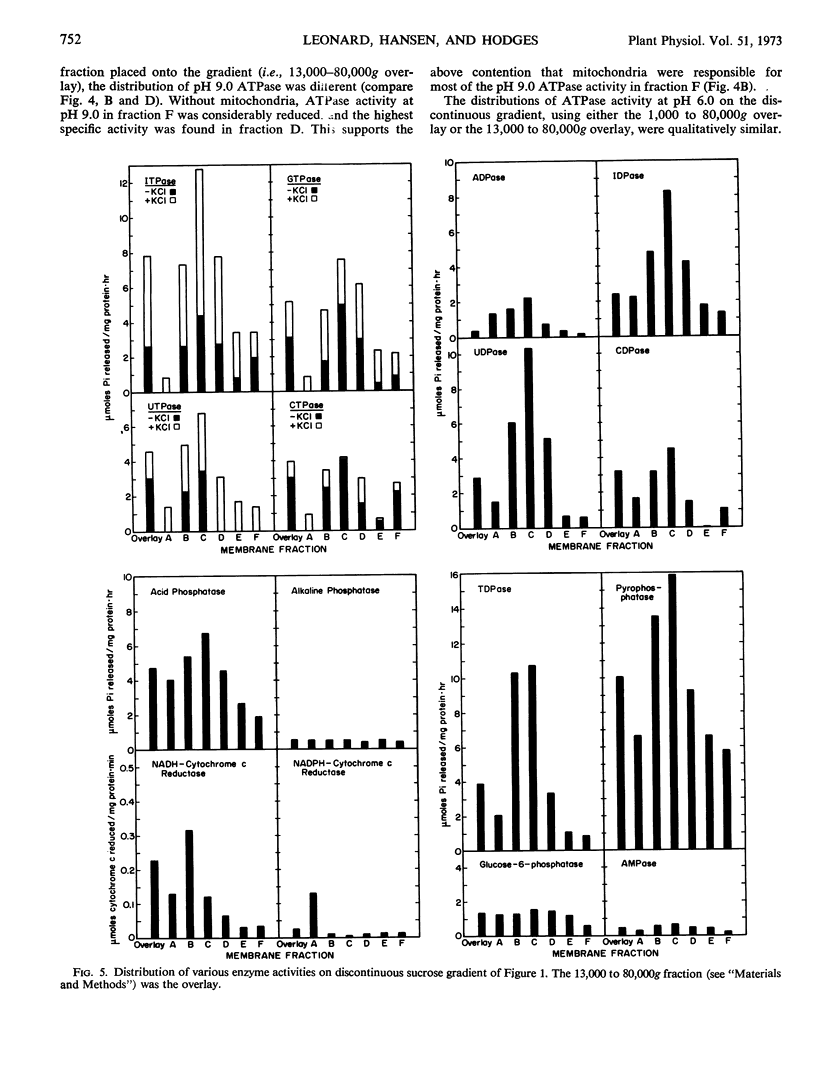
Three additional ATPases were in a membrane fraction sedimenting at high centrifugal forces (13,000-80,000g). One membrane ATPase coincided with NADH-cytochrome c reductase activity, had a density of about 1.09 grams per cubic centimeter, and was equally active at pH 6.0 and 9.0. A second membrane ATPase of the 13,000 to 80,000g fraction had a density of 1.13 grams per cubic centimeter and was more active at pH 9.0 than at pH 6.0. A third membrane ATPase had greater activity at pH 6.0 than at pH 9.0, and the membrane had an apparent density of 1.17 grams per cubic centimeter on the sucrose gradient. This ATPase was especially sensitive to KCI. The identity of the membranes which contain ATPases is discussed in relation to the distribution of other enzymes on the gradient.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Fisher J. D., Hansen D., Hodges T. K. Correlation between ion fluxes and ion-stimulated adenosine triphosphatase activity of plant roots. Plant Physiol. 1970 Dec;46(6):812–814. doi: 10.1104/pp.46.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS H. G., DE DUVE C. Le système hexose-phosphatasique. II. Répartition de l'activité glucose-6-phosphatasique dans les tissus. Bull Soc Chim Biol (Paris) 1950;32(1-2):20–29. [PubMed] [Google Scholar]
- Hall J. L. Cytochemical localization of ATP-ase activity in plant root cells. J Microsc. 1971 Jun;93(3):219–225. doi: 10.1111/j.1365-2818.1971.tb02284.x. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Active and passive transport of potassium in cells of excised pea epicotyls. Plant Physiol. 1970 Feb;45(2):133–138. doi: 10.1104/pp.45.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGLAND T. E., HACKETT D. P. The intracellular localization of some oxidative activities in etiolated pea stems. Biochim Biophys Acta. 1961 Dec 23;54:577–580. doi: 10.1016/0006-3002(61)90099-3. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]



