Abstract
Sterile cultures of Lemna minor have been labeled with 32P1, and the ribosomal proteins have been examined for radioactivity. In relatively short term labeling a radioactive protein was found which ran as a single component in both urea/acetic acid and sodium lauryl sulfate gel electrophoresis. Acid hydrolysis of the labeled protein permitted the isolation of serine phosphate. After labeling to equilibrium with 32P1, calculation indicated only 0.6 to 0.75 atom of this protein phosphorus per ribosome.
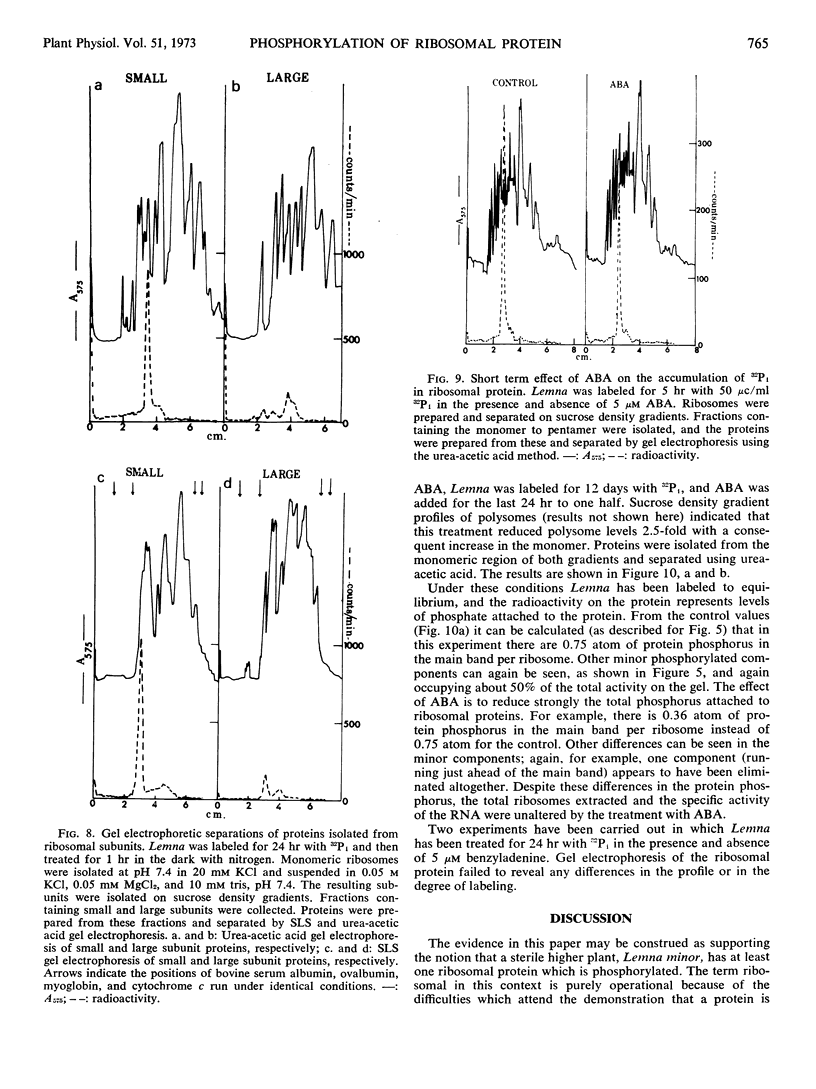
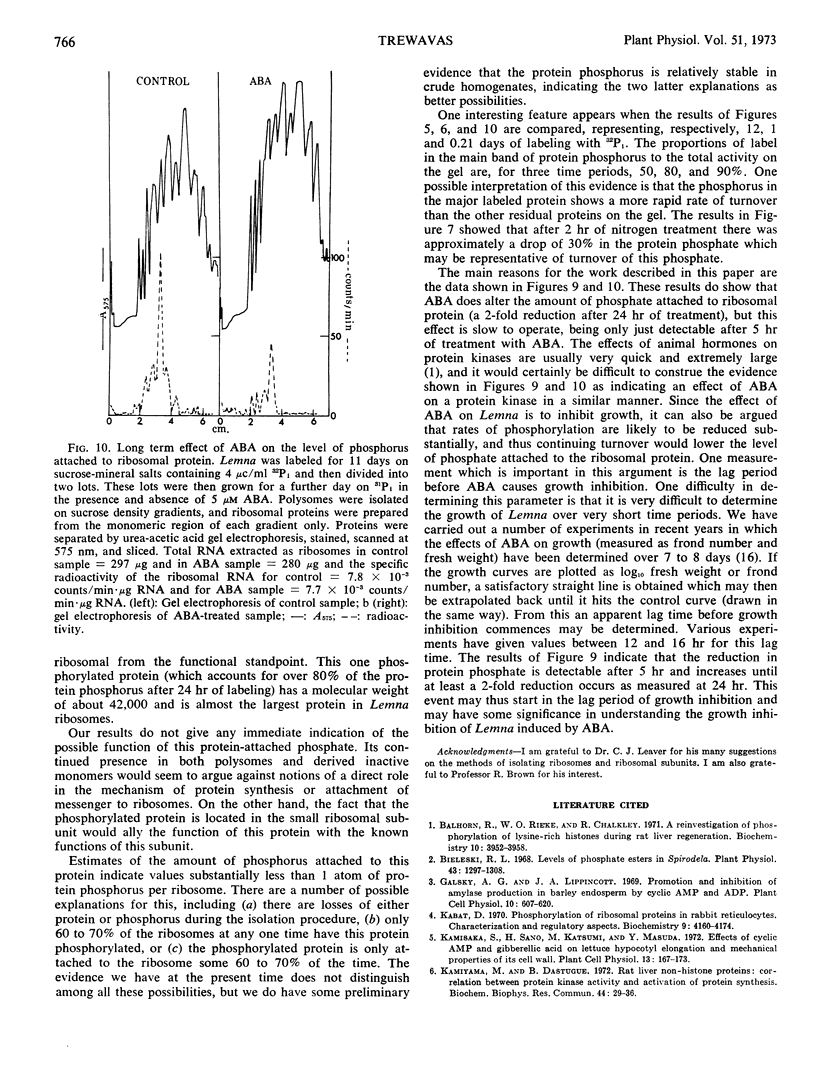
The phosphorylated protein is found in both polysomes and “derived” monomers and appears to be located in the ribosomal small subunit. Its apparent molecular weight is 42,000. Addition of growth-inhibiting concentrations of abscisic acid does not alter the apparent degree of labeling of this protein in 5 hours, but after 24 hours of treatment the total protein phosphorus was reduced from 0.75 atom of phosphorus per ribosome to 0.36 atom of phosphorus per ribosome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balhorn R., Rieke W. O., Chalkley R. Rapid electrophoretic analysis for histone phosphorylation. A reinvestigation of phosphorylation of lysine-rich histone during rat liver regeneration. Biochemistry. 1971 Oct 12;10(21):3952–3959. doi: 10.1021/bi00797a024. [DOI] [PubMed] [Google Scholar]
- Bieleski R. L. Levels of phosphate esters in spirodela. Plant Physiol. 1968 Aug;43(8):1297–1308. doi: 10.1104/pp.43.8.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kamiyama M., Dastugue B. Rat liver non-histone proteins: correlation between protein kinase activity and activation of RNA synthesis. Biochem Biophys Res Commun. 1971 Jul 2;44(1):29–36. doi: 10.1016/s0006-291x(71)80154-7. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissociation of N(2) Gas-induced Monomeric Ribosomes and Functioning of the Derived Subunits in Protein Synthesis in Pea. Plant Physiol. 1971 Nov;48(5):547–552. doi: 10.1104/pp.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier D., Santhanam K., Wagle S. R. Studies on the inhibition of amino acid incorporation into protein by isolated rat liver ribosomes by protein kinase. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1881–1886. doi: 10.1016/0006-291x(72)90065-4. [DOI] [PubMed] [Google Scholar]
- Narayanan A., Vermeersch J., Pradet A. Dosage enzymatique de l'acide adénosine 3',5'-monophosphate cyclique dans les semences de laitue, variété "Reine de mai". C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2406–2407. [PubMed] [Google Scholar]
- Pollard C. J. Influence of gibberellic acid on the incorporation of 8-14C adenine into adenosine 3',5'-cyclic phosphate in barley aleurone layers. Biochim Biophys Acta. 1970 Mar 24;201(3):511–512. doi: 10.1016/0304-4165(70)90176-5. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Shlatz L., Marinetti G. V. Protein kinase mediated phosphorylation of the rat liver plasma membrane. Biochem Biophys Res Commun. 1971 Oct 1;45(1):51–56. doi: 10.1016/0006-291x(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- Trewavas A. The Turnover of Nucleic Acids in Lemna minor. Plant Physiol. 1970 Jun;45(6):742–751. doi: 10.1104/pp.45.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood H. N., Lin M. C., Braun A. C. The inhibition of plant and animal adenosine 3':5'-cyclic monophosphate phosphodiesterases by a cell-division-promoting substance from tissues of higher plant species. Proc Natl Acad Sci U S A. 1972 Feb;69(2):403–406. doi: 10.1073/pnas.69.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]



