Abstract
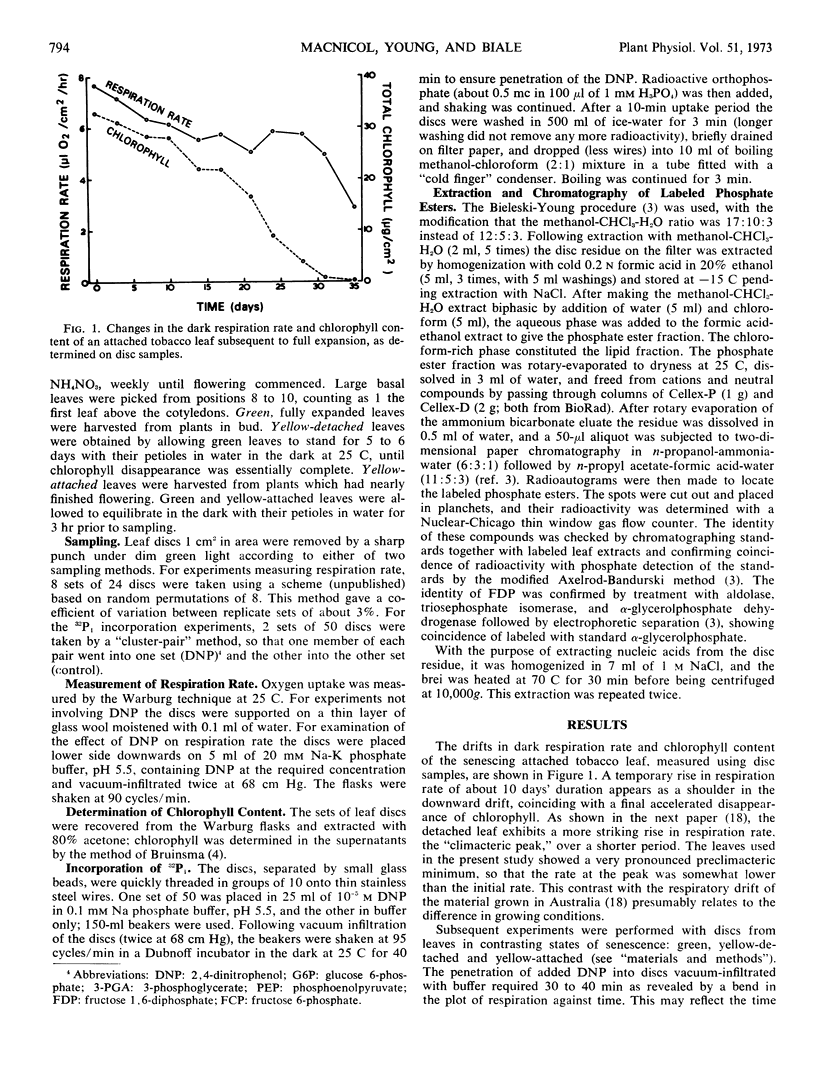
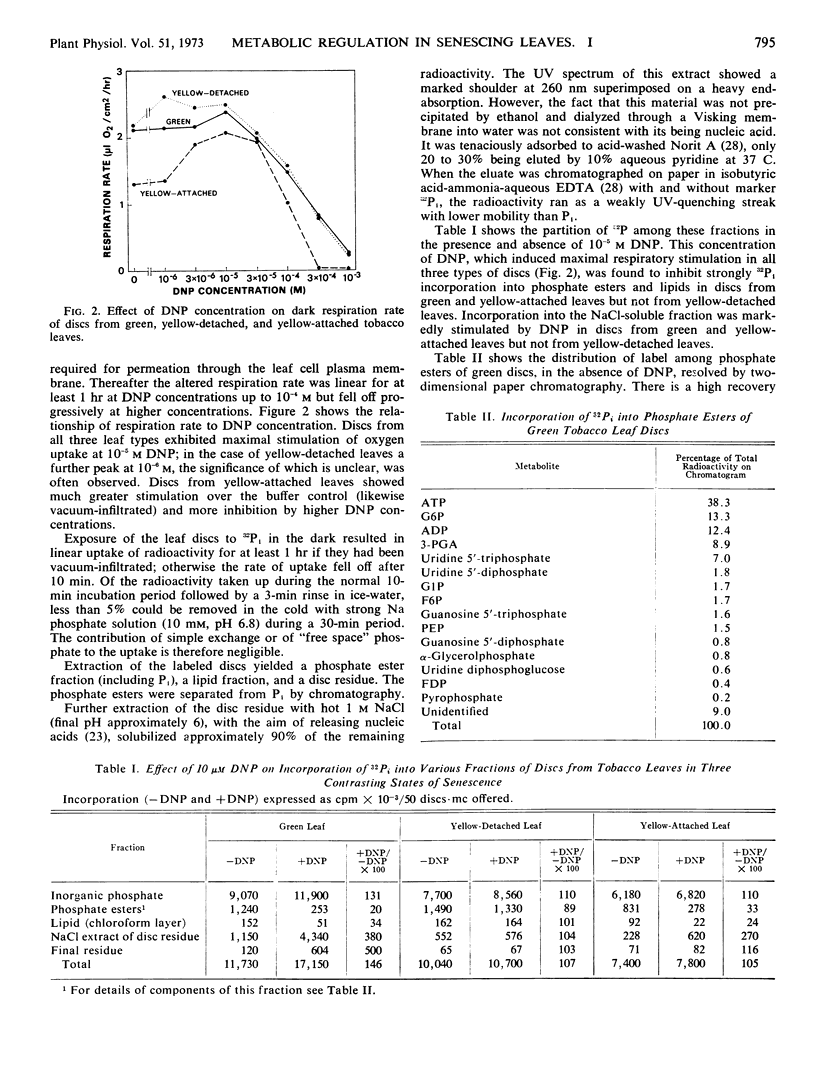
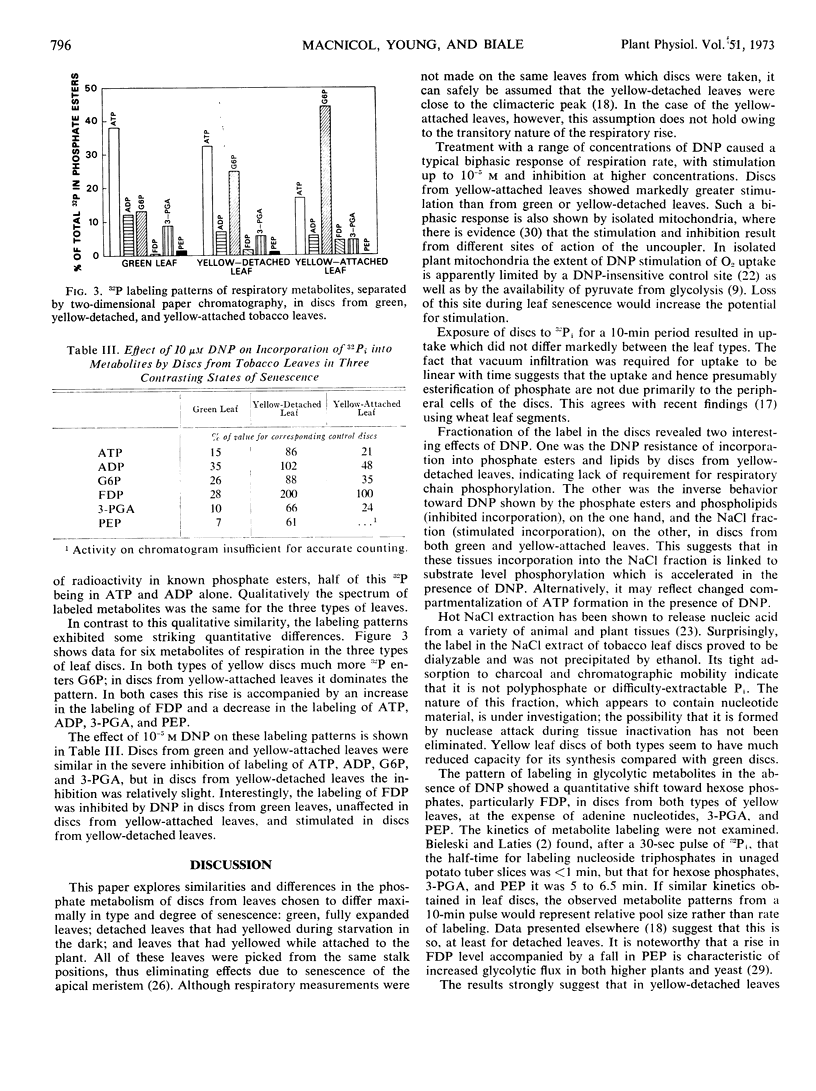
Changes in phosphate metabolism were explored in discs from tobacco (Nicotiana tabacum) leaves of three contrasting types: green leaves which were fully expanded and attached to the plant, leaves which had yellowed following excision and dark starvation, and leaves which had yellowed while attached to the plant. 2,4-Dinitrophenol at 10−5m stimulated the respiration rate of discs from green and yellow-detached leaves only slightly, but markedly stimulated that of discs from yellow-attached leaves. Following a 10-minute uptake period the incorporation of 32P-orthophosphate into phosphate esters and lipids of discs from yellow-detached leaves was resistant to 2,4-dinitrophenol, whereas in discs from green and yellow-attached leaves it was inhibited by 2,4-dinitrophenol. Incorporation into a salt-soluble fraction containing unidentified nucleotide material showed converse behavior in that it was stimulated by 2,4-dinitrophenol in discs from green and yellow-attached leaves; in discs from yellow-detached leaves it was resistant to 2,4-dinitrophenol. In discs from yellow-detached and yellow-attached leaves there was a shift in the labeling pattern of phosphate esters toward increased label in hexose phosphates at the expense of adenine nucleotides, 3-phosphoglycerate, and phosphoenolpyruvate. It is concluded that incorporation into phosphate esters in discs from yellow-detached leaves is by substrate level phosphorylation coupled to enhanced aerobic glycolysis. In discs from yellow-attached leaves, on the other hand, incorporation depends on oxidation phosphorylation, and it is suggested that the shift in labeling pattern is caused by senescence-induced changes in activity of glycolytic enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L., Laties G. G. Turnover Rates of Phosphate Esters in Fresh and Aged Slices of Potato Tuber Tissue. Plant Physiol. 1963 Sep;38(5):586–594. doi: 10.1104/pp.38.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D. J., Pate J. S. Ageing in the whole plant. Symp Soc Exp Biol. 1967;21:559–599. [PubMed] [Google Scholar]
- Givan C. V., Torrey J. G. Respiratory Response of Acer pseudoplatanus Cells to Pyruvate and 2,4-Dinitrophenol. Plant Physiol. 1968 Apr;43(4):635–640. doi: 10.1104/pp.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C. Senescence in Plant Development: The death of plants or plant parts may be of positive ecological or physiological value. Science. 1961 Dec 1;134(3492):1727–1732. doi: 10.1126/science.134.3492.1727. [DOI] [PubMed] [Google Scholar]
- Macdonald I. R., Macklon A. E. Anion absorption by etiolated wheat leaves after vacuum infiltration. Plant Physiol. 1972 Mar;49(3):303–306. doi: 10.1104/pp.49.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Metabolic Regulation in the Senescing Tobacco Leaf: II. Changes in Glycolytic Metabolite Levels in the Detached Leaf. Plant Physiol. 1973 Apr;51(4):798–801. doi: 10.1104/pp.51.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Simon E. W. Types of leaf senescence. Symp Soc Exp Biol. 1967;21:215–230. [PubMed] [Google Scholar]
- Solomos T. Observations on yeast pyruvate kinase activity in vivo. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1076–1083. doi: 10.1016/0006-291x(70)90904-6. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Selwyn M. J. Effects of ring substituents on the activity of phenols as inhibitors and uncouplers of mitochondrial respiration. Eur J Biochem. 1971 Aug 25;21(4):565–574. doi: 10.1111/j.1432-1033.1971.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Wyen N. V., Erdei S., Farkas G. L. Isolation from Avena leaf tissues of a nuclease with the same type of specificity towards RNA and DNA. Accumulation of the enzyme during leaf senescence. Biochim Biophys Acta. 1971 Mar 25;232(3):472–483. doi: 10.1016/0005-2787(71)90601-0. [DOI] [PubMed] [Google Scholar]
- YEMM E. W. Respiration of barley plants; protein catabolism and the formation of amides in starving leaves. Proc R Soc Lond B Biol Sci. 1950 Jan 10;136(885):632–649. doi: 10.1098/rspb.1950.0012. [DOI] [PubMed] [Google Scholar]



