Abstract
Symptomatic medial compartment knee osteoarthritis (OA) is the leading cause of musculoskeletal pain and disability in adults. Therapies intended to unload the medial knee compartment have yielded unsatisfactory results due to low patient compliance with conservative treatments and high complication rates with surgical options. There is no widely available joint-unloading treatment for medial knee OA that offers clinically important symptom alleviation, low complication risk, and high patient acceptance. The KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) is a first-of-its-kind, implantable, extra-articular, extra-capsular prosthesis intended to alleviate knee OA-related symptoms by reducing medial knee compartment loading while overcoming the limitations of traditional joint-unloading therapies. Preclinical and clinical studies have demonstrated excellent prosthesis durability, substantial reductions in medial compartment and total joint loads, and clinically important improvements in OA-related pain and function. The purpose of this report is to describe the KineSpring System, including implant characteristics, principles of operation, indications for use, patient selection criteria, surgical technique, postoperative care, preclinical testing, and clinical experience. The KineSpring System has potential to bridge the gap between ineffective conservative treatments and irreversible surgical interventions for medial compartment knee OA.
Keywords: KineSpring, knee, medial, osteoarthritis, prosthesis
Introduction
Knee osteoarthritis (OA) is the leading cause of musculoskeletal pain and disability in the US.1–3 During ambulation, the medial knee compartment endures over two-thirds of the load across the knee joint.4 Consequently, symptomatic knee OA affects the medial knee compartment with a higher frequency compared to the patellofemoral and lateral compartments.5 Although the etiology of medial knee OA is multifactorial, chronic aberrant and excessive knee joint loading are major risk factors.6,7 The peak knee adduction moment, an indicator of maximum medial joint load endured during gait, is higher in patients with knee OA compared to healthy adults,8 and this excess joint loading, when applied chronically, is associated with greater OA-related pain severity and faster disease progression.9–11
Conversely, efforts to unload the medial compartment may improve knee OA symptoms and halt disease progression by providing a local mechanical environment that encourages articular cartilage healing.6 Lateral wedge insoles, valgus knee braces, and body weight loss are commonly prescribed conservative treatments that reduce joint loading forces at the medial knee compartment. However, the long-term effectiveness of these conservative approaches for alleviation of OA symptoms is poor, largely due to patient compliance challenges.12,13 High tibial valgus osteotomy reliably reduces medial knee joint loading,14,15 but the invasive surgical procedure is associated with considerable bone removal and reshaping, significant surgical risks, a protracted recovery period, and deteriorating clinical outcomes over the long term.16 Although the premise of chronically unloading the medial knee compartment in knee OA is fundamentally sound,6,7 there are no such treatments that are widely available and that offer the prospect of clinically important symptom alleviation, low complication risk, and high patient acceptance.
The KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) is an implantable extra-articular, extra-capsular prosthesis intended to alleviate knee OA-related symptoms by reducing medial knee compartment loading while overcoming the limitations of traditional joint-unloading therapies. The purpose of this report is to describe the KineSpring System, including implant characteristics, principles of operation, indications for use, patient selection criteria, surgical technique, postoperative care, and clinical experience.
The KineSpring knee implant system
Prosthesis characteristics
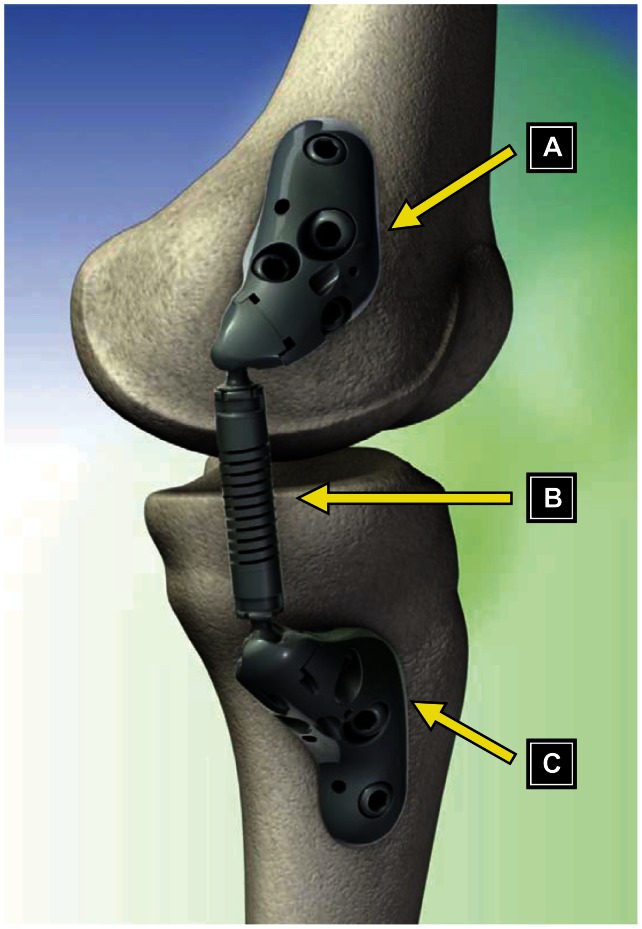
The KineSpring System is an implantable, joint-unloading prosthesis that is designed to fill the void between ineffective conservative treatments and invasive, irreversible surgical procedures. The KineSpring System consists of titanium alloy low-contact femoral and tibial bases and a cobalt chrome alloy absorber that reduces the load carried by the diseased medial compartment of the knee joint during the stance phase of gait (Figure 1). The low-contact femoral and tibial bases are affixed to the bone with compression and locking screws. The bases are designed with three undersurface stand-offs that allow the bases to contact the bone at discrete locations without requiring elevation or removal of the periosteum. If desired, the surgeon can choose to elevate the periosteum prior to fixing the bases, but this periosteal manipulation is not required for effective fixation with this design. The single-spring absorber is designed to compress and absorb up to 29 lb of joint load during knee extension and to lengthen and become passive during knee flexion.17
Figure 1.
Components of the KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA). (A) Femoral base, (B) absorber unit, (C) tibial base.
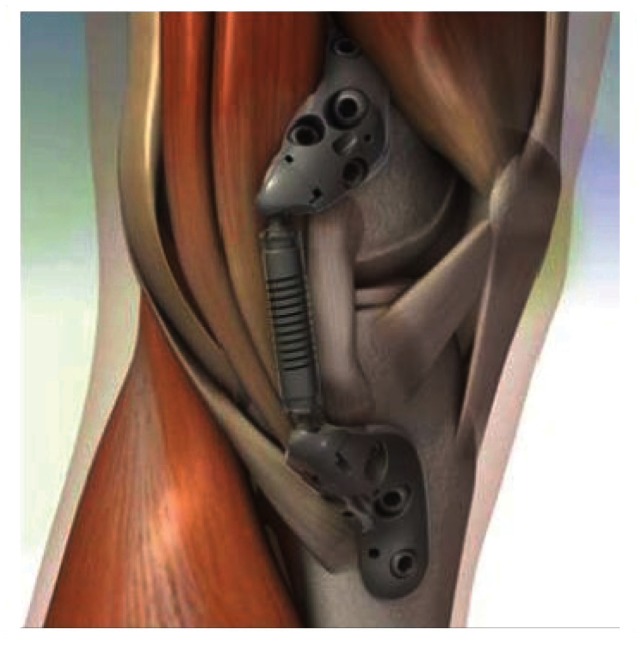
The KineSpring System is both extra-articular and extra-capsular (Figure 2). Implantation of the device is achieved without resection of bone, muscle, or ligaments and without violation of the joint capsule. The load absorber resides in the subcutaneous tissue on the medial aspect of the knee and is positioned superficial to the medial collateral ligament.
Figure 2.
Schematic of the KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) in relation to key anatomical structures.
Notes: The KineSpring bases are implanted on the anteromedial aspects of the distal femur and proximal tibia, respectively. The spring resides superficial to the medial collateral ligament with no penetration of the joint capsule or bone resection required.
Device implantation without bone resection or joint invasion provides a surgical procedure that is easily reversible if future device explant is required. This characteristic contrasts with high tibial osteotomy or arthroplasty, both of which are invasive surgical procedures that cause permanent and significant anatomical modifications.
Principles of operation
The KineSpring System absorbs a maximum load of 29 lb during full knee extension and reduces chronic medial compartment loading without imparting additional forces on the lateral compartment (Figures 3 and 4). This magnitude of unloading is comparable to the magnitude of knee adduction moment reduction that has been shown to improve function and alleviate knee pain in OA patients.18 The kinematics of this device accommodate the natural motions of the knee joint by using two ball-and-socket joints. The device accommodates the wide range of normal physiological knee motion with the capability of > 60° internal–external rotation, 50° of varus–valgus angulation, and 155° of flexion–extension.
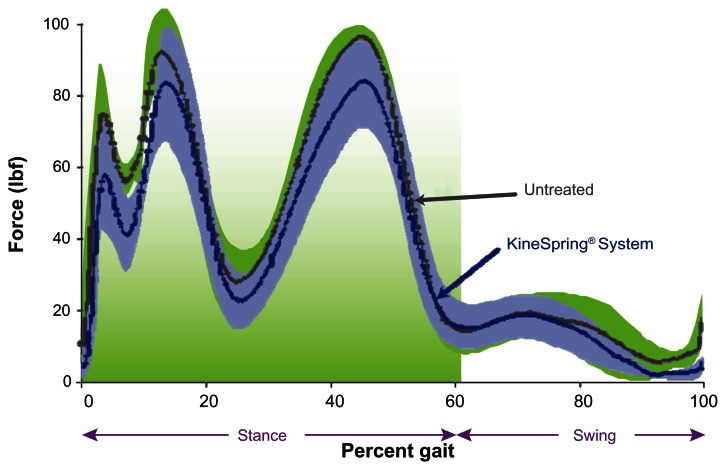
Figure 3.
Medial knee joint loading throughout simulated gait cycle in cadaver knees.
Notes: Plot of medial compartment loading during the gait cycle showing reductions with the KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) (green) implanted relative to the untreated knee (blue). Significant differences are noted at heel strike (3% of gait), initial loading (13% of gait), and toe-off (45% of gait).
Figure 4.
Lateral knee joint force throughout simulated gait cycle in cadaver knees.
Notes: Plot of lateral compartment loading during the gait cycle showing no substantial difference between the KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) (green) and the untreated knee (blue).
Intended use
The KineSpring System is intended to treat the symptoms of pain and decreased function secondary to OA of the medial compartment of the knee. Contraindications for this device include: (1) active, local infection or previous intra-articular infection; (2) neuropathic (Charcot) joint; (3) rheumatoid arthritis of the knee; (4) joint instability in the affected knee; (5) moderate-to-severe osteoporosis; (6) symptomatic lateral or patellofemoral OA in the affected knee; (7) varus alignment > 10° in the affected knee; (8) hyperextension > 10°; (9) severe deformity leading to impaired fixation or improper positioning of the implant; and (10) metal allergy or hypersensitivity. The ideal candidates for the KineSpring System are patients with isolated symptomatic medial compartment OA of mild-to-moderate severity (Kellgren–Lawrence grade I to III). Unlike surgical knee OA treatments such as arthroplasty and high tibial valgus osteotomy, age, sex, and obesity have no influence on clinical outcomes with the KineSpring System.19
Surgical technique
The procedure is performed with the patient under general anesthesia and in the supine position with the operative leg extended and slightly raised. A lateral fluoroscopic view is obtained and the hip/thigh is manipulated to ensure a true lateral position. Each base is inserted through 5–8 cm incisions. Initially, the femoral base is placed subvastus at the medial distal femoral cortex and affixed with a compression screw and three locking screws. Next, a tissue tunnel is created between each incision using blunt dissection to accommodate the absorber unit. The tibial base is attached to the absorber, inserted through the tissue tunnel, and attached to the fixed femoral base. The tibial base is then placed anterior to the pes anserinus insertion at the medial proximal tibial cortex and affixed with screws as previously described. System function is verified prior to absorber activation. The wound is then closed in a standard fashion.
Postoperative care
The typical postoperative recovery after implant with the KineSpring System consists of three phases. Phase I activities are focused on adequate wound healing. This 2-week period consists of regular wound control, pain medication (eg, femoral nerve block or analgesics) as needed, frequent elevation, ice, and lymph drainage to minimize edema. The typical hospital stay after KineSpring System implant is 1 to 5 days.
Phase II typically encompasses 3 to 6 weeks posttreatment and focuses on increasing range of motion and returning to activities of daily living. The use of crutches should be discontinued, as tolerated, and exercises to improve flexibility and strength are slowly introduced. Common rehabilitation exercises during this period include stationary cycling with no resistance, assisted single leg balancing, slow-to-normal-speed walking, isometric quadriceps strengthening, and functional core stabilization movements. Patients employed in sedentary occupations typically return to work between 2 and 4 weeks posttreatment.
Phase III continues beyond 6 weeks and emphasizes increasing strength, returning to manual occupations, and, potentially, returning to sport. Patients are encouraged to continue strengthening exercises and to begin sport-specific training in a gradual manner. General guidelines for returning to sport include waiting at least 4 to 6 weeks before swimming, cycling, or golfing; 2 to 3 months before jogging; 3 to 6 months before playing racquet sports; and at least 6 months before skiing. The typical timeframe for return to a physically demanding job is 6 to 8 weeks. Timelines are provided as guidelines only, and each patient will have a unique recovery pattern.
Preclinical testing
Cyclic and static loading
An in vitro study was completed to assess the mechanical durability of the KineSpring implant including direct testing of the fixation system under cyclic and static loads. Five tibial and femoral bases were fixed to composite sawbones. The test constructs were oriented to simulate 0° knee flexion and then mounted on Instron-type uniaxial test frames. During cyclic fatigue testing, the test constructs were maintained in a physiological environment and cyclic loading was sinusoidally applied at 10 Hz between 27 N and 267 N per cycle (exceeding the expected maximum cyclic duty load range of 0 to 178 N on the implant constructs due to the cyclic compression of the spring after implantation). Components were examined after 2 million, 5 million, and 10 million cycles of load application. Following loading to 10 million cycles, each construct was statically loaded to failure. All test specimens survived 10 million cycles of fatigue loading. Visual examination revealed no evidence of cracking or plastic deformation as a result of the cyclic fatigue loading. Examination of the components with special consideration for interfaces between the screws and bases, screws and bones, and bases and bones showed no discernible changes in the components. Static loading to failure established the construct strength as 4050 ± 209 N and the failure mode was uniformly seen to be fracture of the bone analog (with no breaking or cracking of any socket, base, or screw components).
Simulated use testing
Simulated-use testing of the KineSpring System was completed on an additional five implant constructs (bases, screws, and absorber modules) using a test system that allows cyclic application of rotations to simulate flexion and extension of the knee during gait. The implant constructs were fixed in the test system, and flexion–extension rotations between 0° and 68° ± 4° were applied at 2 Hz, which replicates the in vivo compression–relaxation cycle of the spring absorber when implanted. Constructs were maintained in a physiological environment during testing and testing was carried out to 15 million flexion–extension cycles. All test specimens survived 15 million cycles of simulated use flexion–extension motion and loading. Visual examination revealed no evidence of cracking or plastic deformation. Examination of the components with special consideration for interfaces between the screws and bases and springs and ball and socket joints showed no evidence of cracking or other failure through 15 million motion and loading cycles.
Soft tissue response
Soft tissue response to the articulating subcutaneous implant was studied in a chronic ovine model.20 Eleven sheep were implanted with ovine-specific KineSpring constructs, each consisting of bases secured to the medial femoral and tibial cortices with bone screws, and a joint-spanning load absorber. Tissue response was characterized by gross and microscopic pathology at 4, 12, 26, and 52 weeks. Macroscopically, early evidence of an acute inflammatory response was observed at 4 weeks, but resolved at subsequent time points. Skin incisions were completely healed with no evidence of irritation or ulceration by 26 weeks in all animals. Histological evidence at 4 weeks showed that the device was covered with a soft tissue membrane that was edematous, slightly inflamed, and had surface fibrin deposition. However, this inflammatory response resolved by 12 weeks. At 52 weeks, the histological results were characterized by the formation of a dense, mature fibrous tissue layer around the implant.
Intra-articular load testing
To determine the effect of the KineSpring System on intra-articular loads, a gait-simulation study was performed on six cadaver knees tested in each of two configurations: (1) without the implant (untreated) and (2) with the implant (treated).17 Gait was simulated with each of the cadaver knees mounted in a cadaver-based kinematic test system for investigating knee biomechanics.21 Medial and lateral femorotibial pressures were measured throughout testing using thin-film dynamic pressure sensors placed inframeniscally. Simulated gait cycles were performed at 0.05 Hz. A comparison of the loads carried within the joint in the treated and untreated cadaver knees showed a substantial and significant effect of the KineSpring System. Femorotibial forces in the medial compartment of the knee throughout stance phase were reduced by 31 ± 11 lb (P = 0.002) when the device was implanted. The reductions in peak medial forces were greatest around heel strike (29 ± 18 lb, P = 0.01) and around toe-off (44 ± 20 lb, P = 0.008).
In addition, the total joint load (the sum of medial and lateral forces) was also significantly reduced in the treated knees. Reductions in total joint load during stance averaged 22 ± 9 lb (P = 0.002). Larger reductions in total joint load were observed at foot flat (mid-stance) (24 ± 18 lb, P = 0.019) and around toe-off (31 ± 13 lb, P = 0.005). These reductions in medial and total intra-articular loads were within the clinically effective ranges of other joint-unloading therapies.18 The treated knees showed no substantial difference in lateral compartment load compared to the untreated knees.
Clinical experience
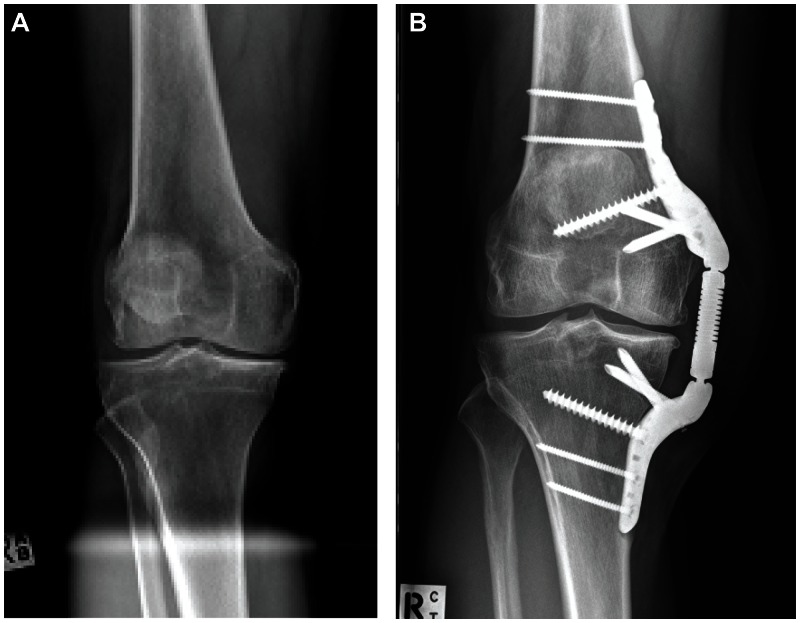
The initial clinical experience with the KineSpring System is promising. Composite data from three clinical trials (OASYS, ACTRN12608000451303; OAKS, ACTRN12609001068257; COAST, ISRCTN63048529)22–24 in 99 patients with 17 months mean follow-up suggest excellent safety and effectiveness. All devices were successfully implanted and activated with no intraoperative complications. Statistically significant mean improvements of 56%, 50%, and 38% were observed for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain, Function, and Stiffness scores, respectively (all P < 0.001). WOMAC clinical success rates were 77.8% for pain, 77.8% for function, and 68.7% for stiffness. The worldwide experience with the current generation KineSpring System has yielded excellent safety and durability outcomes with only 12 (8%) patients undergoing device removal during follow-up for soft tissue impingement (6), return of OA symptoms (4), or deep infection (2). Only one patient in this cohort was converted to arthroplasty after removal of the KineSpring device. Typical pretreatment and follow-up radiographs from this worldwide experience are shown in Figure 5.
Figure 5.
(A) Preoperative anteroposterior radiograph showing pronounced osteoarthritis of the medial compartment. (B) The KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA, USA) at 2 years post-implant.
Note: Joint narrowing is less pronounced on the medial aspect on posttreatment imaging.
Two clinical trials are currently underway to further evaluate the safety and effectiveness of the KineSpring System. The GOAL study (NCT01610505)25 is a prospective, nonrandomized, controlled postmarket study comparing outcomes of 225 patients treated with the KineSpring System or high tibial valgus osteotomy. The first patient was enrolled in June 2012 and enrollment is expected to continue through 2013. A single-arm FDA-approved Investigational Device Exemption study ([SOAR] NCT01738165)26 with 30 patients began enrollment in December 2012. Patient enrollment is anticipated to continue through mid-2013; the primary outcome will be evaluated at 2 years, and patients will be followed for 5 years posttreatment.
Discussion
Knee OA affects 18 million people in the US alone27 and is the most common cause of impaired mobility in people aged over 65 years.28 The prevalence of OA is anticipated to exponentially increase over the next several decades.29 There is currently a large unmet therapeutic need in knee OA patients who are unresponsive to conservative care but are unwilling to undergo invasive surgery such as high tibial valgus osteotomy or knee arthroplasty. Additionally, the economic burden associated with patients in this treatment gap may rise to 24 billion USD by 2025.13 The current trajectory is such that the demand for musculoskeletal health care services will overwhelm the supply of physicians in the near future30,31 and, therefore, it is imperative to explore alternative efficacious treatments to this devastating disease.
Standard knee OA treatments are based on one of several potential mechanisms of action, including: pain palliation (eg, acetaminophen, nonsteroidal anti-inflammatory drugs, intra-articular corticosteroid injections); joint unloading (eg, body weight loss, valgus brace, lateral heel wedge, high tibial valgus osteotomy); cartilage repair (eg, arthroscopic debridement); viscosupplementation (eg, intra-articular hyaluronic acid); or joint replacement (eg, unicompartmental or total knee arthroplasty). The overall inability of conservative therapies to effectively manage knee OA symptoms is not surprising, since standard joint-unloading therapies are not well tolerated13 and analgesic medications actually increase medial joint-loading forces, despite short-term pain relief.32–34 Consequently, the adverse mechanical environment at the knee remains and disease progression continues.6,7
The ideal knee OA treatment would sufficiently unload the medial knee compartment to assuage symptoms without imparting excessive loads at the patellofemoral or lateral compartments. Such a therapy would also have an excellent safety profile, wide patient acceptance, and offer the option of complete reversibility should the need arise. The potential clinical and economic ramifications of a therapy that ameliorates OA-related symptoms and that may delay or even obviate the need for arthroplasty are tremendous.13 Evidence to date suggests that the characteristics of the KineSpring System align well with those of the ideal knee OA treatment. Long-term outcomes from ongoing clinical trials with the KineSpring System are eagerly awaited.
Acknowledgment
We thank Mr Randy Asher for graphical assistance.
Footnotes
Disclosure
LEM and JEB received financial support from Moximed, Inc, Hayward, CA, USA. The authors report no other conflicts of interest in this work.
References
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 4.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 5.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1823–1829. doi: 10.1007/s00167-011-1403-6. [DOI] [PubMed] [Google Scholar]
- 7.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24. xv. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Baliunas AJ, Hurwitz DE, Ryals AB, et al. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(7):573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 9.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91( Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57(7):1254–1260. doi: 10.1002/art.22991. [DOI] [PubMed] [Google Scholar]
- 11.Sharma L, Song J, Dunlop D, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. doi: 10.3109/03091902.2010.535592. [DOI] [PubMed] [Google Scholar]
- 13.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–892. doi: 10.1016/j.mehy.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Ogden S, Mukherjee DP, Keating ME, Ogden AL, Albright JA, McCall RE. Changes in load distribution in the knee after opening-wedge or closing-wedge high tibial osteotomy. J Arthroplasty. 2009;24(1):101–109. doi: 10.1016/j.arth.2008.01.303. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami H, Sugano N, Yonenobu K, et al. Change in the locus of dynamic loading axis on the knee joint after high tibial osteotomy. Gait Posture. 2005;21(3):271–278. doi: 10.1016/j.gaitpost.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Dowd GS, Somayaji HS, Uthukuri M. High tibial osteotomy for medial compartment osteoarthritis. Knee. 2006;13(2):87–92. doi: 10.1016/j.knee.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel SM, Clifford AG, Maloney WJ, O’Connell MK, Tornetta P., III Unloading the OA knee with a novel implant system. J Appl Biomech. doi: 10.1123/jab.29.6.647. Epub December 27, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D, Banks SA, Mitchell KH, D’Lima DD, Colwell CW, Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25(6):789–797. doi: 10.1002/jor.20379. [DOI] [PubMed] [Google Scholar]
- 19.London NJ, Smith J, Miller LE, Block JE. Mid-term outcomes and predictors of clinical success with the KineSpring®Knee Implant System. Clin Med Insights Arthritis Musculoskelet Disord. doi: 10.4137/CMAMD.S11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen MJ, Townsend KL, Bauer TW, Gabriel SM, O’Connell M, Clifford A. Evaluation of the safety of a novel knee load-bypassing device in a sheep model. J Bone Joint Surg Am. 2012;94(1):77–84. doi: 10.2106/JBJS.J.00918. [DOI] [PubMed] [Google Scholar]
- 21.Sutton LG, Werner FW, Haider H, Hamblin T, Clabeaux JJ. In vitro response of the natural cadaver knee to the loading profiles specified in a standard for knee implant wear testing. J Biomech. 2010;43(11):2203–2207. doi: 10.1016/j.jbiomech.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 22.Moximed, Inc. anzctr.org.au [website on the Internet] Camperdown, Australia: The Australian New Zealand Clinical Trials Registry; 2008. [Accessed March 7, 2013]. Safety and feasibility of a load bypass knee support system (LBKSS) for the treatment of osteoarthritis. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82977. ANZCTR Trial ID: ACTRN12608000451303. [Google Scholar]
- 23.Moximed, Inc. anzctr.org.au [website on the Internet] Camperdown, Australia: The Australian New Zealand Clinical Trials Registry; 2009. [Accessed March 7, 2013]. A multi-center, open-label, interventional study of patients with medial compartment knee osteoarthritis (OA) treated with the KineSpring®System. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320854. ANZCTR Trial ID: ACTRN12609001068257. [Google Scholar]
- 24.Moximed, Inc. controlled-trials.com [website on the Internet] London, UK: Current Controlled Trials Ltd; 2009. [Accessed March 7, 2013]. The treatment of medial compartmental knee osteoarthritis (OA) symptoms with the KineSpring™ Unicompartmental Knee Arthroplasty (UKA) System. Available from: http://www.controlled-trials.com/ISRCTN63048529. ISRCTN identifier: ISRCTN63048529. [Google Scholar]
- 25.Moximed, Inc. ClinicalTrials.gov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed March 7, 2013]. A study of the KineSpring System versus high tibial osteotomy surgery for the treatment of medial compartment knee osteoarthritis (GOAL) Available from: http://clinicaltrials.gov/show/NCT01610505. NLM identifier: NCT01610505. [Google Scholar]
- 26.Moximed, Inc. ClinicalTrialsgov [website on the Internet] Bethesda, MD: US National Library of Medicine; 2012. [Accessed March 7, 2013]. Pilot study of the KineSpring® System to evaluate symptom relief in patients with medial knee osteoarthritis (SOAR) Available from: http://clinicaltrials.gov/show/NCT01738165. NLM identifier: NCT01738165. [Google Scholar]
- 27.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 30.Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598–1605. doi: 10.2106/JBJS.H.00067. [DOI] [PubMed] [Google Scholar]
- 31.Knee surgery in arthroses and rheumatoid arthritis. Lakartidningen. 1980;77(22):2085–2118. [No authors listed] Swedish. [PubMed] [Google Scholar]
- 32.Briem K, Axe MJ, Snyder-Mackler L. Medial knee joint loading increases in those who respond to hyaluronan injection for medial knee osteoarthritis. J Orthop Res. 2009;27(11):1420–1425. doi: 10.1002/jor.20899. [DOI] [PubMed] [Google Scholar]
- 33.Henriksen M, Simonsen EB, Alkjaer T, et al. Increased joint loads during walking a consequence of pain relief in knee osteoarthritis. Knee. 2006;13(6):445–450. doi: 10.1016/j.knee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz DE, Sharma L, Andriacchi TP. Effect of knee pain on joint loading in patients with osteoarthritis. Curr Opin Rheumatol. 1999;11(5):422–426. doi: 10.1097/00002281-199909000-00017. [DOI] [PubMed] [Google Scholar]