Abstract
Rotavirus nonstructural protein 4 (NSP4) is a protein with pleiotropic properties. It functions in rotavirus morphogenesis, pathogenesis, and is the first described viral enterotoxin. Since many bacterial toxins function as potent mucosal adjuvants, we evaluated whether baculovirus-expressed recombinant simian rotavirus SA11 NSP4 possesses adjuvant activity by co-administering NSP4 with keyhole limpethemocyanin (KLH), tetanus toxoid (TT) or ovalbumin (OVA) as model antigens in mice. Following intranasal immunization, NSP4 significantly enhanced both systemic and mucosal immune responses to model immunogens, as compared to the control group, in an antigen-specific manner. Both full-length and a cleavage product of SA11 NSP4 had adjuvant activity, localizing this activity to the C-terminus of the protein. NSP4 forms from virulent and avirulent porcine rotavirus OSU strain, and SA11 NSP4 localized withina 2/6 -virus like particle (VLP) also exhibited adjuvant effects. These studies suggest that the rotavirus enterotoxin NSP4 can function as an adjuvant to enhance immune responses for a co-administered antigen.
Introduction
A wide range of infectious pathogens come into contact with the host at mucosal surfaces. Conventional parenteral vaccines are generally ineffective at eliciting mucosal immunity [1–3]. Recent efforts have focused on the development of mucosal vaccines in an attempt to combat invading pathogens at the site of contact by efficiently inducing both mucosal and systemic immune responses. However, one major drawback is the intrinsic low immunogenicity of many protein antigens when administered mucosally. Therefore, the need for mucosal adjuvants is pivotal for development of effective and safe mucosal vaccines.
The most widely studied mucosal adjuvants are the cholera toxin (CT) from Vibrio cholerae, and its close relative, the heat-labile enterotoxin (LT) from Escherichia coli. Aside from their functioning as enterotoxins, both CT and LT have been shown to function as potent adjuvants via binding to the ganglioside GM1-receptor, which results in cellular activation, expression of surface molecules and cytokine production [4]. However, intranasal delivery of these bacterial enterotoxins may induce neurotoxic effects [5, 6]. Mutant forms of cholera (mCT) and heat-labile toxin (mLT), which lack toxicity while retaining adjuvanticity, have been described [7]. The development of a safe, non-toxic mucosal adjuvant that can be delivered intranasally would be an attractive alternative to bacterial toxins.
The first described viral enterotoxin is the rotavirus non-structural protein 4 (NSP4). NSP4 is capable of inducing dose- and age-dependent diarrhea in neonatal mice without causing histological alterations [8]. A cleavage product, NSP4(112-175), found in the supernatant of rotavirus-infected cell cultures [9] can cause Ca++ mobilization in vitro and induce dose- and age-dependent diarrhea in vivo, just like the full-length protein. Since bacterial toxins, such as CT and LT, are well established to function as potent mucosal adjuvants, we asked if NSP4 also possesses adjuvant activity. In this study we tested the viral enterotoxin NSP4 from several virus strains for adjuvant activity in mice following intranasal administration of classical model protein antigens and evaluated the mucosal and systemic antibody responses.
Materials and Methods
Animals
Six to eight week old inbred BALB/c female mice were obtained from Charles River Laboratories (Wilmington, MA). All animals were housed in microisolator cages throughout the study period as previously described [10, 11].
Expression and Purification of Recombinant NSP4
Recombinant NSP4 including the full length and cleavage product (112-175) from simian SA11 C13, full length NSP4 from virulent and attenuated porcine rotavirus strains OSU-v and OSU-a were purified from Spodoptera frugiperda (Sf9) insect cells infected with baculovirus recombinants as described previously [9, 12, 13]. Briefly, NSP4-encoding rotavirus gene 10 sequences were cloned in the TOPO TA vector (Invitrogen Life Technologies, Chicago, IL) and subcloned into the baculovirus transfer vector pFastBAC1 (Invitrogen). Recombinant baculoviruses expressing NSP4 were generated as described by the manufacturer, and recombinant virus stocks were plaque purified. NSP4 was first semi-purified by fast protein liquid chromatography using a quaternary methylamine anion exchange column pre-equilibrated with buffer (20 mM Glycine-HCl, pH 8.1). The NSP4-rich fractions were pooled and further purified using an agarose immunoaffinity column onto which purified anti-NSP4 (114-135) rabbit IgG had been immobilized [8]. The bound NSP4 was eluted with 0.1 M Tris-HCl buffer at pH 2.8. The eluate was dialyzed against 50 mM NH4HCO3, lyophilized, and stored at 4°C. Prior to use, NSP4 proteins were reconstituted in PBS.
Expression and Purification of NSP4-containing 2/6 Virus-Like Particle (VLP)
Rotavirus 2/6 virus like particles were expressed using complementary DNA sequences (cDNA) for simian rotavirus SAl1 gene segment 2, which codes VP2, and gene segment 6, which codes VP6 were made from mRNA and sub-cloned into pCRII TOPO TA vectors (Invitrogen). The rotavirus genes were inserted into a baculovirus transfer vector capable of co-expressing up to four different proteins (see below). The plasmid, pBAC4X (Novagen, San Diego, CA), contains two polyhedron promoters and two p10 promoters with the homologous promoters orientated in opposite directions, one of each in the left hand direction, and the others, in the right-hand direction. Each newly inserted sequence was subsequently confirmed by restriction digestion and the cloned gene was sequenced to confirm its integrity.
(i) Insertion of VP6 into the pBAC4X Baculovirus Transfer Plasmid ( pB4X/VP6)
The VP6 gene segment was PCR amplified from the full length clone pSP65/SA11-6 using the sense primer 5′-TCTAGAGGCCGGCCTTTTAAACG (XbaI restriction site underlined) and the antisense primer 5′-AGGCCTGGTGAATCCTCTCAC-3′ (StuI site underlined). Cohesive ends were generated by digesting the sequence with XbaI and StuI and the gene was inserted into XbaI/StuI linearized baculovirus transfer plasmid pBAC4X behind the left-hand polyhedron promoter.
(ii) Insertion of VP2 into a Cloning Vector (pVPΔ2)
A truncated form of the SA11 VP2 gene lacking the protease-sensitive region encoding amino acid residues from the N-terminus to residue 92 (VPΔ2) [14] was amplified using the sense primer 5′-ATGGGAGGCGGAGGCGCTAACAAAACTATCC-3′ and antisense 5′-TTAGGTCATATCTCCACAATGG-3′ and cloned into the TOPO TA pCRII plasmid (pVPΔ2).
(iii) Fusion of NSP4 to VP2 ( pNSP4-Δ2)
NSP4(112-175) was PCR-amplified using the 5′-ended primer 5′-CCATGGTTGACAAATTGAC-3′ (NcoI restriction site underlined) and 3′-ended primer 5′-GCTAGCTCCTCCTCCCATTGCTGCAGT-3′ (NheI site underlined). The amplified DNA fragment was digested with NcoI and NheI and ligated into NcoI/NheI linearized pVPΔ2 vector.
(iv) Consruction of Baculovirus Transfer Plasmid encoding SA11 VP6 and NSP4-VP2 (pB4X/NSP4-Δ2/6)
The pNSP4-Δ2 was digested with NotI and AvrII restriction enzymes to remove the gene encoding the fusion protein NSP4-Δ2 and inserted behind the second, right-hand, polyhedron promoter by ligation into pB4X/VP6 linearized by NotI and SpeI restriction enzymes.
A recombinant baculovirus encoding the three rotavirus recombinant proteins was generated as described by the manufacturer, and virus stocks were plaque purified. VLPs containing the SA11 rotavirus proteins VP6 and fusion protein NSP4-VP2 (NSP4-2/6 VLP) were purified using CsCl2 gradients and characterized as previously described [15]. The endotoxin level in each 2/6-VLP preparation was quantitated (<0.05 U/dose) using the Limulus amebocyte assay (Associates of Cape Cod, Inc., Woods Hole, Mass.). Electron microscopy was performed on each of the VLP preparations just prior to inoculation to confirm the integrity of the VLPs.
Inoculation of Animals
Groups of five BALB/c mice were used to test each antigen. All experiments included a group of mice co-administered 10 μg of the mucosal adjuvant, mutant Escherichia coli heat-labile enterotoxin [LT(R192G)]( mLT) as a immunostimulatory control [16]. The animals were anaesthetized by intraperitoneal administration of ketamine (3.75 mg/mouse), xylazine (0.19 mg/mouse), and acepromazine (0.037 mg/mouse)[10] before immunization. Two doses of intranasal immunization of 100 μg of KLH or OVA alone or with full length NSP4 (6μg) or the truncated NSP4(112-175) (10 or20 μg) were carried out three weeks apart. T etanus toxoidused for immunization was kindly provided by Dr. Jerry McGhee (University of Alabama, Birmingham)or from the Statens Serum Institute(Copenhagen, Denmark). Animalswere immunized intranasally with 10 μg of TT alone or co-administered with10 μg of either full-length NSP4 or NSP4 internalized in VLPs (NSP4-2/6 VLP ) three times, two weeks apart.
Sample Collection
Serum and fecal samples were collected before vaccination (0 DPI) and at 14 days post second or third immunization. Blood samples were collected by tail bleed for separation of serum. Fecal samples were collected with a fecal collection cage as previously described [17] and processed to make 20% (weight/volume) suspensions in stool diluent as described previously [11, 18]. All samples were stored at −80° until assayed.
Sample Analysis
ELISA to measure KLH-or OVA-specific serum antibody responses. All ELISAs were performed on 96-well polyvinyl chloride microtiter plates (Dynatech, McLean, VA). Plates were coated with 100 μl of KLH or OVA (10 μg/ml) in carbonate-bicarbonate buffer (pH 9.6) and incubated for 4 hours at room temperature. Non-specific protein binding sites were blocked with 5% BLOTTO. Following each step after the block, the plates were washed three times with 0.05% Tween 20 in PBS with an Ultrawasher Plus Platewasher (Dynatech). Serum samples from individual animals were serially diluted two-fold down the plate in 5% BLOTTO. Samples were then incubated for 2 hours at 37°C. Horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Sigma) diluted 1:7,500 in 2.5% BLOTTO was then added to all wells and incubated for 1 hour at room temperature. All reactions were detected using TMB Microwell ELISA substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). The substrate was allowed to react for 10 minutes at room temperature, and then the reaction was stopped by adding an equal volume of 1 M H3PO4. Optical densities (OD) at 450 nm were determined with a Spectra Max 190 Plate Reader (Molecular Devices, Inc., Palatine, IL). End point titer values were determined as the reciprocal of the highest dilution that had an absorbance value greater than or equal to 0.1 above the background value.
ELISA to measure KLH- and OVA-specific fecal antibody responses. Plates were coated with antigen as described above. Purified mouse IgA standard (Sigma Chemical Co.) was diluted in 1% BLOTTO-0.5% fetal bovine serum (FBS), added at an initial concentration of 0.5 μg/ml, and serially diluted two-fold down the plate. All plates were incubated for 4 hours at room temperature then blocked and washed as described above. Fecal samples (20% suspension) from individual animals were serially diluted two-fold down the plate with 1% BLOTTO-0.5% FBS and incubated for 2 hours at 37°C to assay for KLH-specific IgA. Horseradish peroxidase-conjugated goat anti-mouse IgA antibody (Sigma Chemical Co.) diluted 1:10,000 in 2.5% BLOTTO-0.5% FBS was then added to all wells and incubated for 1 hour at 37°C. All reactions were developed, stopped and detected as described above. The level of KLH-specific IgA was calculated from the linear portion of a standard curve determined as previously described [18]. Each fecal antibody response was expressed as the ratio of nanograms of KLH-specific IgA per microgram of total IgA.
ELISA to measure TT-specific serum and fecal antibody responses. Plates were coated with 10 μg/ml of TT overnight at room temperature. All reactions were detected, quantitated and analyzed as described above.
Data Analysis
End point titers of antigen-specific antibody responses were determined for each individual animal. The geometric mean titers (GMTs) were determined for each group of mice. Standard errors were calculated for log-transformed titers. Statistical analyses were performed with SPSS version 10.0 for Windows (SPSS, Inc., Chicago, Illinois). Antibody titers or levels of antibodies between groups were compared by using the Kruskal-Wallis test followed by the Mann-Whitney U rank sum test.
Results
Adjuvant activity of full length NSP4 from SA11
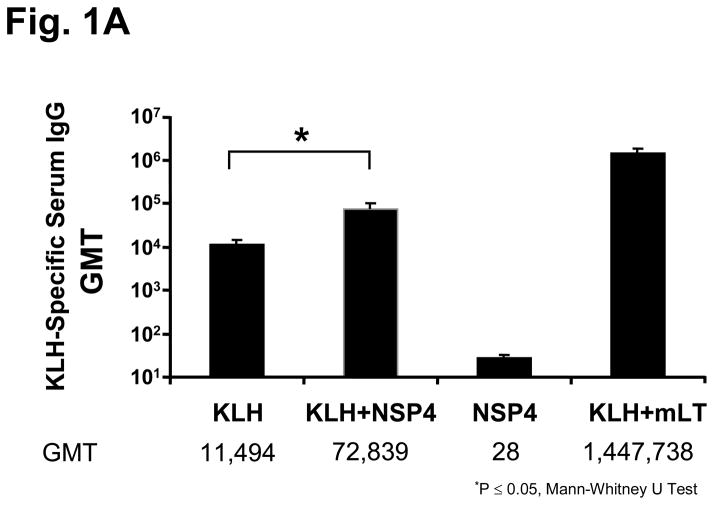
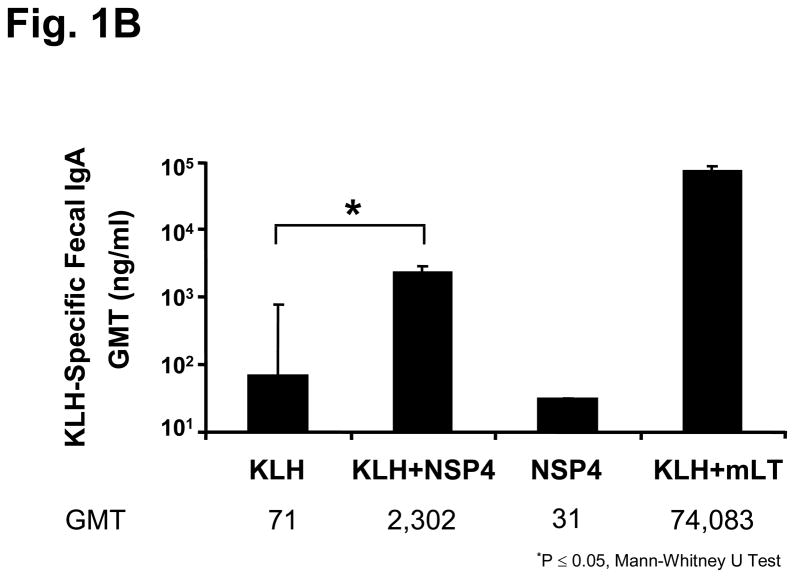
Animals immunized with 100 μg of KLH and either a 6 or 20 μg dose of full-length NSP4 as an adjuvant. Both doses of NSP4 exhibited a statistically significant (p= 0.04 Mann-Whitney U Test) 6-fold increase in KLH-specific serum IgG titers (GMT=72,839) compared to the group of mice receiving KLH alone (GMT=11,494) (Figure 1a) and so the lower dose was chosen for future experiments. In addition, those animals also showed significantly higher (p=0.05 Mann-Whitney U Test) (>30-fold increase) KLH-specific fecal IgA antibody responses (GMT=2,302 ng/ml) compared to the antigen alone group (GMT=71 ng/ml) (Figure 1b). Serum IgG and fecal IgA specific antibody levels decreased approximately 20 fold and 30 fold, respectively, when mice were inoculated with KLH co-administered with NSP4 compared to mLT (GMT; IgG=1,447,738; IgA=74,083 ng/ml).
Figure 1.
KLH-specific serum and fecal antibody response profiles of mice intranasally inoculated with KLH and SA11 NSP4. Animals (n=5) were administered two times, three weeks apart, with KLH alone, KLH + NSP4, NSP4 alone, or KLH + mLT as the immunostimulatory control. Samples were collected 14 days post second inoculation and assayed by ELISA. Panel (a) shows the KLH-specific serum IgG results in Geometric Mean Titers (GMT) and panel (b) shows the level of KLH-specific fecal IgA responses expressed as the ratio of nanograms of KLH-specific IgA per microgram of total IgA among various immunized groups of mice.
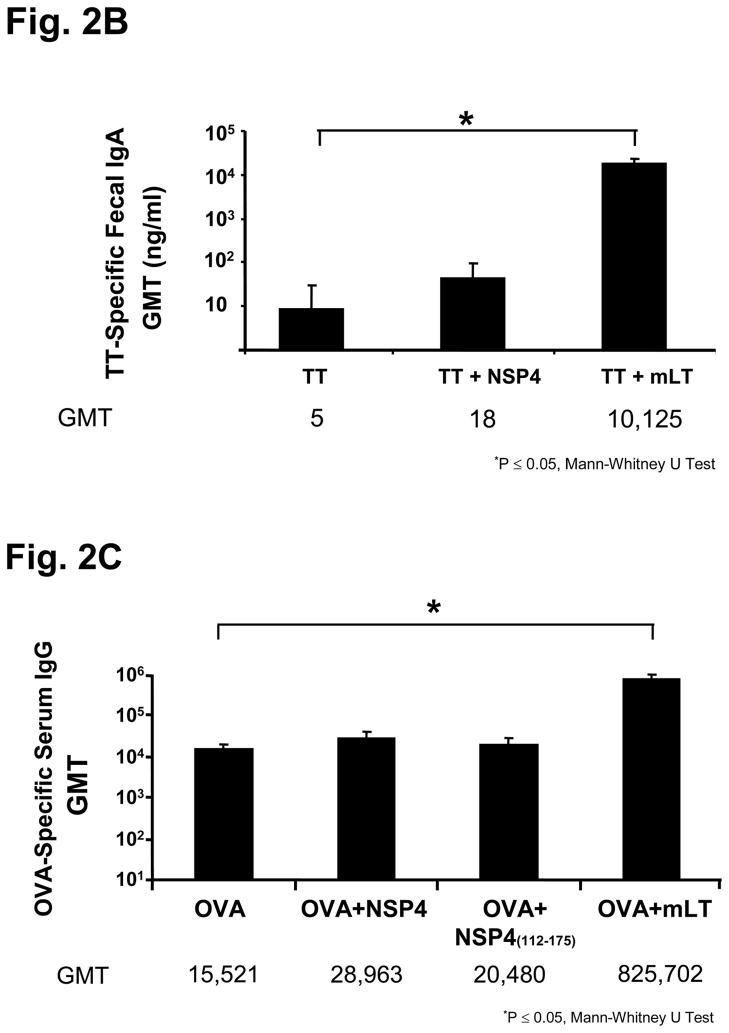
When full-length NSP4 was given with TT (10 μg), it enhanced serum TT-specific total immunoglobulin (GMT=11,143) responses (17-fold increase) to a greater extent than to those seen with KLH, when compared to the antigen alone group (Figure 2a). However, in contrast to the enhanced fecal antibody responses observed when KLH was given as the antigen, there was no significant increase (p>0.05, Mann-Whitney U Test) of TT-specific fecal antibody response in the group of animals that received NSP4 and TT as compared to TT alone (Figure 2b).
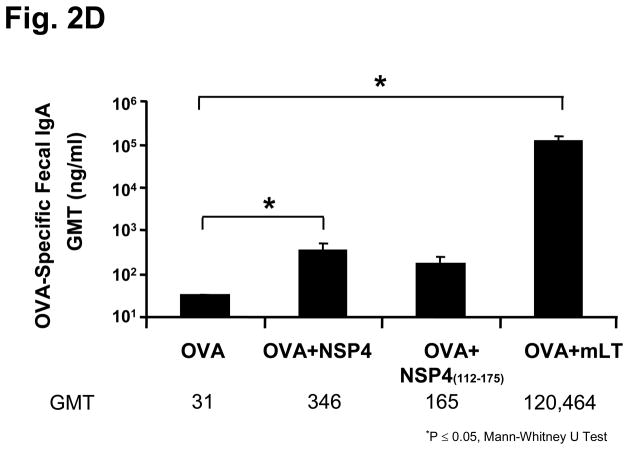
Figure 2.
Serum and fecal antibody profiles of mice intranasally inoculated with SA11 NSP4 and either tetanus toxoid (TT) or ovalbumin (OVA). Animals (n=5) were administered with model antigen alone, co-administered with full-length NSP4 or with mLT as an immunostimulatory control. Samples were collected 14 days post final inoculation and assayed by ELISA. Panel (a) shows the KLH-specific serum IgG and total immunoglobulin results in Geometric Mean Titers (GMT), panel (b) shows the level of KLH-specific fecal IgA responses, panel (c) shows OVA-specific serum IgG levels and panel (d) shows OVA-specific IgA fecal levels among the various immunized groups of mice.
In contrast to the observations with KLH and TT, NSP4 did not enhance serum antibody responses to OVA (GMT=28,963) compared to the antigen alone (GMT=15,521) group (Figure 2c). However, a significantly higher level (11-fold increase; (p=0.02 Mann-Whitney U) of OVA-specific fecal IgA (GMT=346 ng/ml) was detected when NSP4 was co-administered when compared with the OVA alone (GMT=31 ng/ml) control (Figure 2d).
The adjuvant domain of NSP4 is located in the C-terminus of the protein
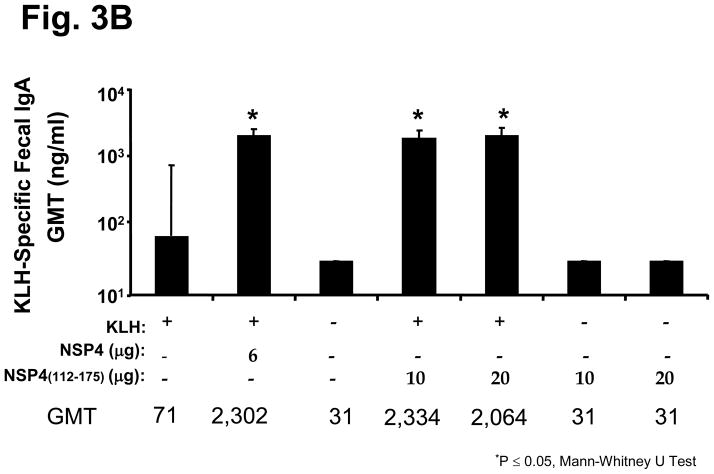
The adjuvant activity of the cleavage product NSP4(112-175) was tested using KLH. Similar to full-length NSP4, either 10 μg or 20 μg of the cleavage product NSP4(112-175) enhanced KLH-specific serum IgG (5-fold) and fecal IgA (30 fold) (Figure 3a and 3b) to levels higher than those observed in mice that received KLH alone (p<0.05 Mann-Whitney U). As both doses induced equivalent antibody titers we chose the lowest dose to perform the subsequent experiments. These data indicate that the adjuvant domain of this protein is located in the C-terminus of NSP4 and that 10 μg of the cleavage product is optimal to elicit this effect.
Figure 3.
KLH-specific serum and fecal antibody response profiles of mice intranasally inoculated with KLH and SA11 NSP4 cleavage product. Full-length NSP4 as well as the cleavage product NSP4(112-175) were given intranasally two times three weeks apart and KLH-specific serum IgG expressed as GMT (a) and fecal IgA expressed as ratio of ng of KLH-specific IgA per μg of total IgA (b) were evaluated in all immunized groups of animals 14 days after the second inoculation.
An avirulent NSP4 can also function as an adjuvant
To test whether NSP4 from other rotavirus strains besides the simian SA11 Cl3 NSP4 can also function as adjuvants, we tested the adjuvant activity of NSP4 from both a virulent and tissue culture-attenuated pair of porcine rotavirus strains, OSU-v and OSU-a, respectively. As shown in Figure 4, both OSU-a (GMT =14,703) and OSU-v (GMT =14,703) NSP4 induced an enhanced (8-fold increase) TT-specific serum, but not fecal, antibody response compared to the group receiving TT antigen alone. In addition, the levels of antibody induced by OSU-a and OSU-v NSP4 were similar to that induced by SA11, Cl3 NSP4.
Figure 4.
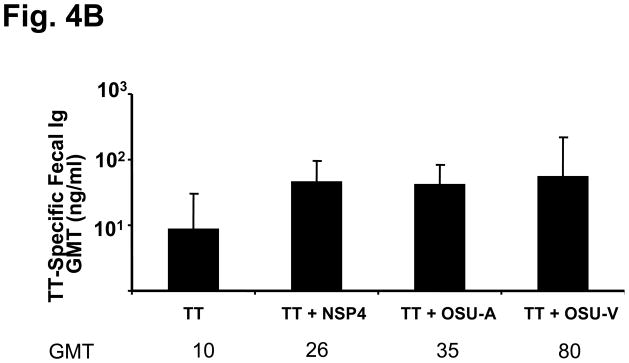
TT-specific serum and fecal antibody response profiles of mice intranasally co-administered TT with full length NSP4 from the rotavirus strains SA11, OSU-v and OSU-a. Animals (n=5) were given TT alone or co-administered with either of the full-length NSP4 forms three times two weeks apart. Samples were collected 14 days post final inoculation and assayed by ELISA. TT-specific serum total immunoglobulins expressed as GMT (a) and fecal IgA in ng specific IgA per μg total IgA (b).
NSP4 delivered by a rotavirus 2/6-VLP also has intranasal adjuvant effects
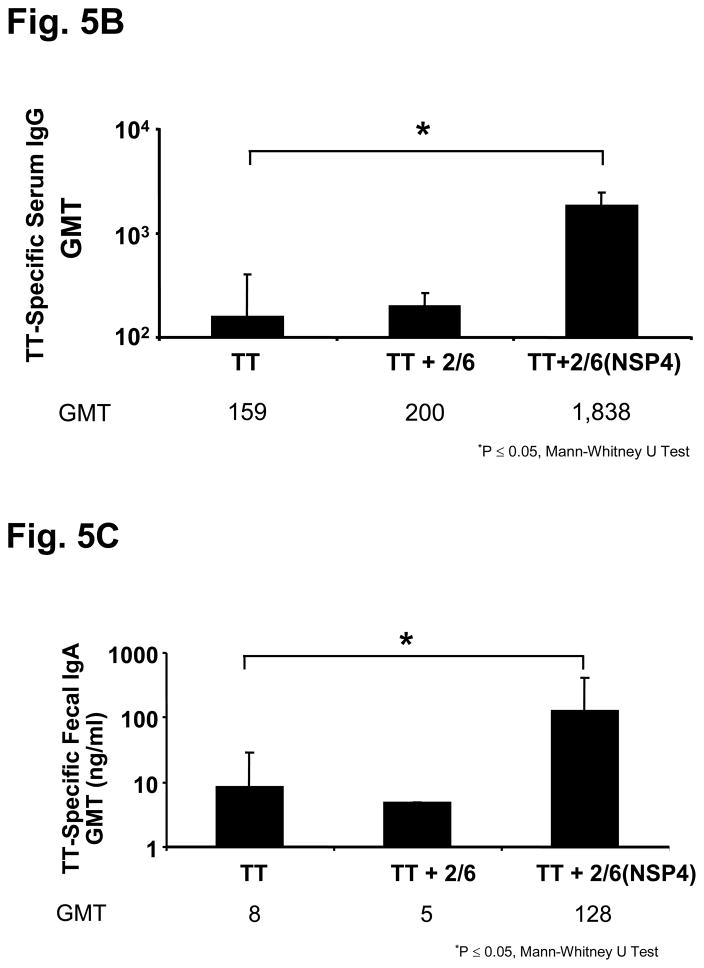
We next determined if NSP4, localized within a virus-like particle (VLP), retained adjuvant activity. NSP4(112-175) was genetically fused to the inner core protein VP2 and when co-expressed with VP6 in insect cells VLPs (NSP4-2/6) were produced which were morphological indistinct from 2/6 VLP (Figure 5a). Significantly increased (12-fold) TT-specific serum antibody was induced in the group of mice that received NSP4-2/6 intranasally with TT (GMT=1,838) compared to the TT alone group (GMT=159) (Figure 5b). In addition, despite the inability of the soluble NSP4 to enhance humoral response against TT, NSP4 internalized within 2/6-VLPs elicited significantly increased fecal IgA levels (p≤0.05) compared to the co-administered antigen (Figure 5c). This adjuvant effect was due to the presence of NSP4 since 2/6 VLPs given with TT did not increase antigen-specific antibody responses and the level of antibody was comparable to the group receiving TT alone.
Figure 5.
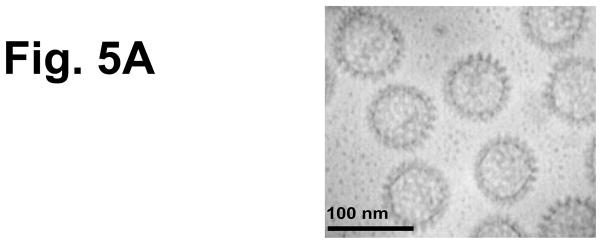
Characterization of 2/6-VLP containing NSP4 and TT-specific serum and fecal antibody response profiles of mice intranasally co-administered TT with rotavirus 2/6 VLP containing NSP4. To test another form of NSP4 obtained from a system other than the baculovirus-expressed and affinity-purified SA11 NSP4-containing 2/6 VLPs were used as immunogens. (a) Negative stain (1% uranyl acetate, pH4.5) electron micrographs of NSP4-2/6 VLP produced in insect Sf9 cells. Magnification bar equals 100nm. Animals (n=5) were given TT alone or co-administered with either 2/6 VLP alone or 2/6 VLP containing NSP4 three times two weeks apart. Samples were collected 14 days post final inoculation and assayed by ELISA TT-specific serum IgG (b) and fecal IgA (c) were evaluated for enhanced responses compared to the antigen alone group.
Discussion
In this study we demonstrated the mucosal adjuvant activity of rotavirus non-structural protein NSP4 using model antigens. Full-length NSP4 from the SA11 rotavirus strain as well as a cleavage product NSP4(112-175) were able to function as intranasal adjuvants and enhanced both serum and mucosal antibody responses specific to the co-administered antigen. In addition, an attenuated NSP4 from an avirulent porcine OSU-a rotavirus as well as NSP4 delivered inside a rotavirus VLP can efficiently enhance antigen-specific antibody responses. The adjuvant property of NSP4 varied depending upon the co-administered antigen suggesting that the outcome of adjuvanticity is affected by the nature of the antigen tested.
In this study, porcine OSU-a NSP4 induced comparable antibody levels to SA11 or OSU-v NSP4 when tested as adjuvants. The virulent porcine NSP4 OSU-v and attenuated OSU-a were cloned from a pair of porcine rotavirus strains. OSU-v induces severe diarrhea in piglets and neonatal mice; however, serial passage in tissue culture resulted in an attenuated strain, called OSU-a, with significantly reduced pathogenicity [19]. SA11 NSP4 and OSU-v NSP4 exogenously administered to human colonic adenocarcinoma HT29 cells induce a significant mobilization (10-fold increase) in intracellular calcium ([Ca2+i]) compared to OSU-a. Although further studies will be needed to fully understand the mechanism of adjuvancity of these proteins, the fact that all three forms of NSP4 (SA11, OSU-v and OSU-a) possess similar adjuvant activities suggests that this activity is independent of the diarrhea-inducing or calcium mobilization abilities of these proteins. Future studies should also test the adjuvant activity potency of NSP4 from other rotavirus strains.
The mechanism by which NSP4 exerts its adjuvant function remains to be determined. Although the viral enterotoxin NSP4 causes diarrhea in rodents like the well-characterized bacterial enterotoxins, LT and CT, the mechanisms of pathogenesis and host age restrictions are different. Therefore, we anticipate that the mechanism by which NSP4 exerts its adjuvant effect is likely to be different from LT or CT. NSP4 does not induce detectable elevations in intracellular cAMP (unpublished data), which has been shown to be necessary for bacterial toxins to function as mucosal adjuvants [20]. Another possible explanation maybe due to the direct effect NSP4 exerts on tight junctions similar to the zonula occludens toxin (ZOT) which also possesses adjuvant function [21, 22]. Consequently NSP4 can decrease membrane permeability [23] and such interruptions of the tight junction can impact mucosal permeability, integrity and overall function of the epithelium. Another possible mechanism could be related to the recent discovery that the α1β1 and α2β1 integrins are receptors for full-length SA11, OSU-a/–v NSP4 and NSP4(112-175) [24]. Ligand-binding to integrin receptors can trigger an intracellular signal transduction pathway resulting in transcription factor activation with subsequent downstream attenuation of the immune system. As these integrins play a role in modulating the immune system [25–27] it will be interesting to determine if NSP4 exerts its adjuvant effect through binding to these receptors.
Even though other mucosal adjuvants have been explored extensively in the past, to date, none have been approved for human use to be given by mucosal routes. Our studies showed mLT induced significantly higher fecal and serum antibody titers when co-administered with TT and KLH model antigens, a result that confirms previous studies with this bacterial enterotoxin or with CT as well as its mutants in extensive testing in various animal models in combination with a variety of viral, bacterial, and parasitic pathogens; however, safety issues still remain a concern for these bacterial enterotoxin adjuvants [5]. Therefore, an effective, safe and practical mucosal adjuvant remains to be identified and characterized for the development of mucosal vaccines. Since NSP4 does not bind to GM1 receptors like CT or LT [13] it may not possess neurotoxic side effects., However future preclinical, safety trials will need to be undertaken to ensure NSP4 does not enter the brain or possess other toxicity.
Furthermore, we observed differences in adjuvant response depending upon the nature of the co-administered antigen. The presence of NSP4 induced a stronger immune response to the co-administered antigen compared to the immune response elicited by administering the same antigen alone. This finding correlates with the fact that inclusion of specific adjuvants in vaccine preparations can modify the presentation modality of antigens to the immune system and/or improve the induction of the immune response over that induced by the same antigen given alone [28].
Virus-like particles (VLP) as an alternative vaccine strategy is an important area in the field of rotavirus vaccinology. In this study we explored the ability of NSP4 to act as an adjuvant for non-replicating rotavirus virus-like particle (VLP) vaccines developed in our laboratory. We found that NSP4 retained its adjuvant properties even when administered within a NSP4-2/6 VLP. The observed adjuvant effect of NSP4-2/6 was due to the presence of NSP4 since 2/6 VLPs given with antigen did not increase antigen-specific antibody responses. The addition of NSP4 to 2/6 VLPs could increase the adjuvanticity and immunogenicity of rotaviral vaccines and may alleviate the need for co-administered adjuvants.
Future experiments will examine any adjuvant effect NSP4 exerts on the cellular arm of the immune system against co-administered antigen, elucidate the mechanism by which NSP4 functions as an adjuvant and also determine if NSP4 also possesses adjuvant properties when administered by alternative routes.
Acknowledgments
This work was supported by funding from the U.S. Public Health Service, The Enteric Pathogens Research Unit, NIAID contract N01-A165299 and from the National Institutes of Health (grants DK30144, DK56338, AI080656), and E.C. was funded by a pediatric gastroenterology training fellowship (grant T32 DK07664) from the National Institutes of Health.
We thank Dr. Jerry R. McGhee for providing the tetanus toxoid and Dr. John D. Clements for providing the mutant LT (LT-R192G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasegawa H, et al. Development of mucosal adjuvants for intranasal vaccine for H5N1 influenza viruses. Ther Clin Risk Manag. 2009;5(1):125–32. doi: 10.2147/tcrm.s3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanley M, Gissmann L, Nardelli-Haefliger D. Immunobiology of human papillomavirus infection and vaccination - implications for second generation vaccines. Vaccine. 2008;26(Suppl 10):K62–7. doi: 10.1016/j.vaccine.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Lehner T, et al. A rational basis for mucosal vaccination against HIV infection. Immunol Rev. 1999;170:183–96. doi: 10.1111/j.1600-065x.1999.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzler AC, Burke JM, Wetzler LM. Induction of cell signaling events by the cholera toxin B subunit in antigen-presenting cells. Infect Immun. 2007;75(6):3150–9. doi: 10.1128/IAI.00581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ginkel FW, et al. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 6.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N Engl J Med. 2004;350(9):860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 7.Hagiwara Y, et al. A second generation of double mutant cholera toxin adjuvants: enhanced immunity without intracellular trafficking. J Immunol. 2006;177(5):3045–54. doi: 10.4049/jimmunol.177.5.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball JM, et al. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272(5258):101–4. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, et al. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J Virol. 2000;74(24):11663–70. doi: 10.1128/jvi.74.24.11663-11670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero RA, et al. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J Virol. 2001;75(20):9713–22. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neal CM, et al. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71(11):8707–17. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, et al. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J Virol. 1998;72(5):3666–72. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, et al. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1997;94(8):3960–5. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng CQ, et al. The N terminus of rotavirus VP2 is necessary for encapsidation of VP1 and VP3. J Virol. 1998;72(1):201–8. doi: 10.1128/jvi.72.1.201-208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolotti-Ciarlet A, et al. Immunogenicity and protective efficacy of rotavirus 2/6-virus-like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine. 2003;21(25–26):3885–900. doi: 10.1016/s0264-410x(03)00308-6. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63(5):1617–23. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball JM, et al. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J Virol. 1998;72(2):1345–53. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco MA, Greenberg HB. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69(12):7800–6. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohl EH, Theil KW, Saif LJ. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984;19(2):105–11. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 1999;18(1–2):38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 21.Marinaro M, Fasano A, De Magistris MT. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71(4):1897–902. doi: 10.1128/IAI.71.4.1897-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinaro M, et al. Zonula occludens toxin is a powerful mucosal adjuvant for intranasally delivered antigens. Infect Immun. 1999;67(3):1287–91. doi: 10.1128/iai.67.3.1287-1291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tafazoli F, et al. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J Virol. 2001;75(3):1540–6. doi: 10.1128/JVI.75.3.1540-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo NS, et al. Inaugural article: integrins alpha1beta1 and alpha2beta1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci U S A. 2008;105(26):8811–8. doi: 10.1073/pnas.0803934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietl J, et al. Uterine granular lymphocytes are activated natural killer cells expressing VLA-1. Immunol Today. 1992;13(6):236. doi: 10.1016/0167-5699(92)90161-Y. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Villar JJ, et al. Functional analysis of alpha 1 beta 1 integrin in human natural killer cells. Eur J Immunol. 1996;26(9):2023–9. doi: 10.1002/eji.1830260909. [DOI] [PubMed] [Google Scholar]
- 27.Rubio MA, et al. Monocyte activation: rapid induction of alpha 1/beta 1 (VLA-1) integrin expression by lipopolysaccharide and interferon-gamma. Eur J Immunol. 1995;25(9):2701–5. doi: 10.1002/eji.1830250945. [DOI] [PubMed] [Google Scholar]
- 28.Buonaguro FM, Tornesello ML, Buonaguro L. Virus-like particle vaccines and adjuvants: the HPV paradigm. Expert Rev Vaccines. 2009;8(10):1379–98. doi: 10.1586/erv.09.81. [DOI] [PubMed] [Google Scholar]