Abstract
To determine the influence of exercise on pulmonary dose of inhaled pollutants, we compared biomarkers of inhaled ozone (O3) dose and toxic effect between exercise levels in humans, and between humans and rats. Resting human subjects were exposed to labeled O3 (18O3, 0.4 ppm, for 2 hours) and alveolar O3 dose measured as the concentration of excess 18O in cells and extracellular material of nasal, bronchial, and bronchoalveolar lavage fluid (BALF). We related O3 dose to effects (changes in BALF protein, LDH, IL-6, and antioxidant substances) measurable in the BALF. A parallel study of resting subjects examined lung function (FEV1) changes following O3. Subjects exposed while resting had 18O concentrations in BALF cells that were 1/5th of those of exercising subjects and directly proportional to the amount of O3 breathed during exposure. Quantitative measures of alveolar O3 dose and toxicity that were observed previously in exercising subjects were greatly reduced or non-observable in O3 exposed resting subjects. Resting rats and resting humans were found to have a similar alveolar O3 dose.
Keywords: ozone, inhalation toxicology, exercise, animal human extrapolation
Introduction
Ozone (O3) pollution of ambient air is a significant public health problem worldwide, and adds to the pollutant burden of particulate matter and volatile organics. Mandatory emission controls on automobiles and other pollution sources have been reasonably effective in limiting the accumulation of O3 in urban areas. However, the expense of O3 regulations and the continued refinement of low-dose health effects of O3 have kept it in the scientific and regulatory spotlight. Considerable attention is given to the U.S. National Ambient Air Quality standard for O3, which is presently 0.075 ppm averaged over 8 hours. Justification for this standard derives from controlled O3 exposures of exercising human subjects with support from human epidemiology and laboratory animal toxicology.1
In this study we explore the influence of physical exercise in humans on the resultant alveolar dose and effect of inhaled O3. To date, almost all clinical studies of O3 effects in humans have been performed while subjects exercised during O3 exposure. Here, we quantify the dose of O3 to the lung alveoli during resting O3 exposure, and compare this dose to that achieved during exercise. We also compare the human O3 dose to that of similarly exposed resting rats.
Physical exercise during exposure increases the alveolar O3 dose by switching the air flow to the mouth, where it is scrubbed less efficiently, and by increasing the amount of O3 that enters the lung due to increased minute ventilation (Ve) and tidal volume. Early human clinical studies showed enhanced physiological effects of O3 if subjects exercised during exposure.2 Since exercise is a part of everyday life, the inclusion of exercise with O3 exposure has been employed in almost all human clinical studies of O3.3 For technical reasons, exercise has not been employed in most animal inhalation studies.
Ozone has become a prototype for the study of chemically reactive air pollutants. Although O3 appears to react at the air-liquid interface of the entire respiratory tract, the target sites of greatest interest toxicologically appear to be the terminal airways and alveolar region. Terminal airways receive a proportionally higher dose of O3 because of their small surface area and lack of mucus covering.4 Alveolar epithelium is in close proximity to the blood, and it is believed that transport of O3 reaction products to blood might contribute to enhancement of atherosclerotic plaque formation.5
Studies with inhaled oxygen-18 labeled ozone (18O3) have shown that O3 reacts chemically with constituents of airway lining fluid, leaving behind oxygen atoms bound to cellular and extracellular material.6–8 Consistent with its known chemistry, O3 has a broad spectrum of reactivity with most biomolecules it interacts with. We showed previously that the concentration of 18O labeled products in 18O3 reactions in BALF cellular and extracellular constituents were related to O3 induced toxic effects including increased BALF protein concentrations and neutrophil counts.6 These results were observed for both humans and rats; however, resting rats had a much smaller accumulation of 18O and a corresponding lack of O3 effects on BALF protein and neutrophil count, unless the 18O3 exposure concentration was increased 5-fold to 2 ppm. The possibility that numerous studies of O3 exposed laboratory rats might actually underestimate human dose and effect has been difficult to explain because rats have been assumed to breathe more air than humans and therefore should receive a higher alveolar O3 dose. We show here that resting human subjects achieve a much lower alveolar O3 dose than exercising subjects and that this dose is comparable to that of resting rats. The resting subjects also show fewer detectable O3 induced cellular, biochemical, and physiological (FEV1) effects than exercising subjects.
Methods
Experimental design and recruitment of subjects
Two experiments involving resting exposure to O3 by human subjects and measurements made during or immediately after exposure are reported. The first was a 2 hour exposure to 18O3 by face mask, followed by nasal, bronchial, and bronchoalveolar lavage. The second was a 2 hour chamber exposure to unlabeled O3 in which 68 subjects were exposed to four different O3 concentrations, and then examined physiologically for a change in FEV1.
Study protocols for both experiments were approved by the Institutional Review Board at the University of North Carolina Medical School in Chapel Hill and the EPA; informed consent was obtained from all subjects before their participation in the study. Table 1 shows the physical characteristics and age of the subjects in the two experiments. Paid volunteers were selected on the basis of being healthy, non-smoking 18–35 years of age, and with no history of asthma or allergic rhinitis. They were predominantly students recruited from colleges in the Chapel Hill-Durham area of North Carolina. No attempt was made to catalogue ambient pollution levels at the time of our controlled exposures because the subjects lived in a low-industry area with relatively low ambient pollution. Subjects were excluded if they had cold or flu-like symptoms during the previous 6 weeks.
Table 1.
Characteristics of male subjects exposed while resting to air or O3 in the two studies reported here.
| Age, yr | Height, cm | Weight, kg | |
|---|---|---|---|
| 18O3lavage study, 8 subjects | |||
| Mean | 26.4 | 183 | 87.9 |
| SE | 1.2 | 2 | 3.4 |
| Physiology study, 68 subjects | |||
| Mean | 25.2 | 181 | 81.7 |
| SE | 0.4 | 1 | 1.5 |
Resting 18O3 exposure study #1
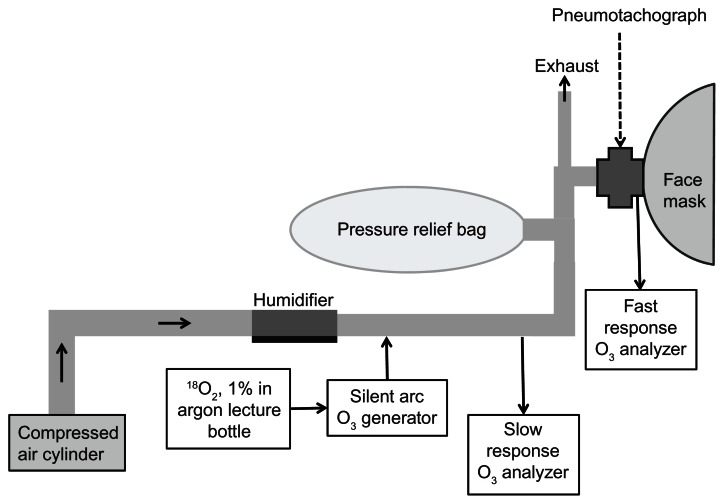
Eight male subjects were enrolled in the first study; they ranged in age from 21–32 years and in weight from 70–103 kg (Table 1). They were exposed on two separate occasions separated by at least 2 weeks. Ozone exposures reported here were performed during September to early December; they were compared to exercising subjects in a published study which were exposed in the same laboratory during July and August three years earlier. Subjects were asked to avoid exposure to environmental tobacco smoke or to other irritating substances such as paint fumes, and to avoid taking vitamin C or E supplements or NSAIDS for at least 48 hours prior to each exposure. Exposures took place during the morning and subjects ate no food after midnight the day prior to their exposure. Subjects breathed 18O3 through a face mask (to conserve 18O2) while resting in a seated position. No attempt was made to control or target the resting level of breathing in the subjects. As shown in Figure 1, subjects breathed into a silicone face mask that had been modified by blocking the air intake filtration ports and installing PTFE tubing to the front of the mask. Flow rates of breathing air were measured by a pneumotachograph (Hans Rudolph, Kansas City, MO, model 4700) that transmitted the signal via a preamplifier to a computer. A rapid response O3 analyzer (Monitor Labs model 8410 chemiluminescent O3 analyzer, flow rate 300 mL/min) measured inspired and expired O3 concentrations during each breath at randomly selected sampling times during the exposure. The air supply for the face mask came from a compressed cylinder that was humidified to 50% before flowing past the face mask at a rate of 60 L/min. A 60 L Teflon pressure relief bag equalized the air pressures during the breathing cycle. 18O3 was generated as previously described8 by passing a mixture of 1% 18O2 (99% purity, Isotec, Miamisburg, OH) in argon through a small electric arc O3 generator taken from a NO/NO2 air monitor (Bendix, Lewisburg, WV, modified to 3–10 mL/min flow rate). The efficiency of conversion of O2 to O3 in this system was 2%–4%. Oxygen-18 labeled ozone concentration in the breathing air was monitored by a slow response (Dasibi) monitor (flow rate 500 mL/min) and maintained to 0.4 ppm ± 2.0% by manually controlling the flow of the 18O2/argon mixture through the arc generator. We showed previously that the small enrichment in 18O2 in breathing air, which occurs due to inefficient 18O3 generation from 18O2, results in an insignificant enrichment of 18O in the tissues.
Figure 1.
Schematic of face mask exposure of human subjects to 18O3 with measurement of breathing airflow and O3 uptake.
Notes: Subjects breathed into a modified silicone face mask. Inspired and expired flow rates and times as well as breath-by-breath O3 removal from breathing air were measured for brief intervals during a 2 hour exposure to 18O3 at a concentration of 0.4 ppm. 18O3 concentration was maintained manually by adjusting the flow rate of an 18O2-argon mixture through a silent arc O3 generator (rate was 3–10 mL/min). Cylinder air maintained at 50% relative humidity flowed past the face mask at a rate of 60 L/min.
Nasal, bronchial, and bronchoalveolar lavage fluid collection
Nasal lavage (NL) was performed within one hour post exposure; five consecutive 0.2 mL sprays of sterile saline were injected into each nostril, then expelled into a small cup. This procedure was repeated 7 times, making the total saline instilled equal to 14 mL.
The bronchial lavage (BL) procedure consisted of one 20 mL instillation which was withdrawn prior to the BALF collection from the same lobe and consisted of 4 subsequent washes of 50 mL volume. The bronchial lavage followed by BALF collection was done on the middle lobe and was then repeated in the lingula. Thus, the total instilled saline for BL was 40 mL and the total for BALF was 400 mL. Clinical details of the BAL procedure have been previously described.9
Preparation of lavage fluids and blood for analysis
The first two aliquots of BALF were combined, and they along with the BL and NL fluid were centrifuged at 400 g for 10 minutes to pellet the cells. BALF surfactant fraction was obtained by centrifuging the cell-free supernatant of the combined lavage fluids at 27,000 × g for 30 min (4 °C). BALF, BL and NL supernatants were brought to 3% perchloric acid (PCA) by adding 60% acid. All PCA samples were centrifuged at 20,000 g for 20 minutes at 5 °C to pellet the protein. The PCA pellets were re-suspended in 0.25 N NaOH and analyzed for protein using the Coomassie blue binding method9 and using bovine serum albumin as a standard. Cells from all BALF washes were combined and re-suspended in RPMI and then counted. One million cells were then pelleted and suspended into 0.3 mL of 3% PCA.
Samples for 18O determination were lyophilized and individual analyses containing 0.3–0.8 mg of protein were weighed into silver cups for oxygen-18 analysis.
Venous blood was drawn from subjects prior to exposure and within an hour after exposure. A one mL sample of the heparinized blood was centrifuged to separate the red cells from the plasma and lyophilized. Oxygen-18 labeled ozone determination was made on both the plasma and the red cell fractions of the dried blood.
Analysis for BALF cytokines, LDH, and BALF cell phagocytosis
Methods have been published previously11 for most of the cytokines, LDH, and BALF cell phagocytosis assays. ELISA techniques were used for assay of elastase,9 interleukin-8 (kit from R & D, Minneapolis, MN) and tissue plasminogen activator (tPA, Enzyme Research Labs, South Bend, IN).
18O analysis of blood and lavaged constituents
Isotope ratio mass spectrometry was used to measure amounts of excess 18O in lyophilized samples of lavage fluids and in the red blood cell and plasma fraction of the dried venous blood, per published methods.6 Natural abundance 18O values from the air-exposed subjects were subtracted from the values measured in the 18O3 exposed subjects to obtain the excess 18O due to the 18O3 exposure. Hereafter, we will dispense with the distinction of ‘excess 18O’ and simply refer to it as ‘18O.’ We have shown in previous studies that the lyophilization procedure traps the portion of 18O3 reaction products that form adducts with tissue molecules.
Antioxidant analyses
Supernatants originating from PCA homogenizations were assayed by HPLC-EC for uric acid and ascorbic acid, per previously published methods.12 Total glutathione (GSx, consisting of the sum of GSH and GSSG) was analyzed by enzymatic recycling.13 BALF cells and supernatants were also analyzed for alpha tocopherol concentrations according to a published method.14
Resting ozone exposure: study #2, physiology
Subjects were exposed in whole-body inhalation chambers according to methods outlined previously,15 but instead of exercising, they were exposed while resting in a seated position. They breathed nasally as they normally would under resting conditions. The sequence of exposures was randomized and neither volunteers nor investigators were informed of the exposure; each individual experienced only one exposure, whether to air or to a given concentration of O3. FEV1 was measured three times in all subjects as follows: (1) prior to exposure, (2) at the intermediate time of 1 hour, and (3) at the end of the 2-hour of exposure of the same subject. Each FEV1 measurement was done in triplicate with the largest value of the three measurements reported for that subject. The baseline FEV1 values measured pre-exposure to air or O3 averaged 4.48 ± 0.082 L (N = 68). FEV1 percent change was determined using the following formula: [(pre-O3 − post-O3)/pre-O3] × 100.
Statistics
Two-tailed pairwise comparisons were made of data from air versus O3 exposures with P ≤ 0.05 assigned significance. Comparisons of the resting data with previously published exercising O3 exposure data are exploratory, with no corrections made for multiple comparisons. The FEV1 study employed three methods: (1) linear regression of the FEV1 changes against the O3 exposure concentration to determine whether the slope of the regression line differed from zero, (2) ANOVA followed by Dunnett’s test, and (3) Williams test for non-parametric data.16
Results
Breathing and ozone uptake measurements
Breathing frequency, tidal volume, and percentage of 18O3 uptake from breathing air during exposure of the resting subjects to air or 0.4 ppm 18O3, is shown in Table 2. Oxygen-18 labeled ozone exposure (compared to air) produced no significant change in the Ve or inspired or expired airflow measurements. The percentage uptake of 18O3 from breathing air was 79.9%; it was in close agreement among the 5 subjects examined. We compared these measurements to our previous study of exercising subjects (see Table 3). The Ve of our resting subjects was lower (8.3 L/min) than the resting Ve of subjects we reported from our earlier intermittent exercise regimen (13.5 L/min) in which subjects alternated 15 minute periods of rest and exercise.6 Comparing the volume of air breathed during the 2 hour exposure to 0.4 ppm 18O3 in the earlier intermittent exercise study with the volume of air breathed in the present study showed a 4.7-fold higher volume with exercise than with resting exposure (Table 3).
Table 2.
Breathing measurements and percentage O3 uptake in 8 resting subjects exposed by face mask to 18O3.
| Breaths measured | Inspired breaths | Expired breath | Total breath time, sec | Ve, L/min | O3 uptake, % | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Volume, L | Time, sec | Time, sec | |||||
| Air | |||||||
| Mean | 235 | 0.59 | 1.6 | 2.8 | 4.4 | 8.11 | |
| SE | 3 | 0.13 | 0.1 | 0.3 | 0.3 | 0.48 | |
| N | 8 | 8 | 8 | 8 | 8 | 8 | |
| O3 | |||||||
| Mean | 135 | 0.66 | 1.7 | 3.0 | 4.6 | 8.66 | 79.8 |
| SE | 3 | 0.15 | 0.2 | 0.5 | 0.5 | 0.47 | 1.8 |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
Table 3.
Comparison of ventilation and air volumes breathed per exposure in resting and exercising subjects exposed to 0.4 ppm 18O3 for 2 hr.
| Body weight, kg | N | Tidal Vol., L | Freq, breaths/min | Minute ventilation, L/min | Mean total air breathed, L/exposure | Reference | |
|---|---|---|---|---|---|---|---|
| Resting exposure (120 min) | 87.9 ± 3.4 | 8 | 0.59 ± 0.13 | 13.7 ± 0.9 | 8.3 ± 0.4 | 998 | Present study Hatch et al, 1994 |
| Resting periods (60 min total) | 76.2 ± 2.5 | 8 | 13.5 ± 0.1 | 810 | |||
| Exercising periods (60 min total) | 64.6 ± 3.2 | 3876 | |||||
|
|
|||||||
| Total | 4676 | ||||||
|
| |||||||
| Ratio: exercise/resting | 4.7 | ||||||
Note: Values are mean ± S.E.
Excess 18O in BALF supernatants and cells
The 18O accumulated by BALF cells, BALF supernatant, and NL of resting subjects exposed to 18O3 is shown in Table 4. Results reported previously for subjects exposed identically but with concurrent intermittent exercise are included for comparison. The concentration of 18O in BALF cells was 5.1-fold greater with exercising exposure than with resting exposure. The BALF extracellular fraction showed concentrations 2-fold higher than following resting exposure. The dried material of NL fluid was about twice as concentrated after resting exposure as it was after exercising exposure, suggesting that mouth breathing during exercise drew exposure away from the nose. The variability of the 18O data appeared to be about the same for resting as for exercising exposures. For comparison to rats, Table 4 also shows the previously reported 18O accumulated in BALF cells and fluids of F344 rats exposed while at rest to the same 0.4 ppm 2 hour exposure regimen. BALF cells from resting rats and resting humans accumulated about the same concentration of 18O, while the BALF surfactant of the rats incorporated less than half the concentration found in the resting humans. Blood plasma and pelleted red blood cells did not show a detectable 18O increase due to 18O3 exposure, similar to results observed previously in exercising subjects (data not shown).6
Table 4.
18O concentration in bronchoalveolar lavage and nasal lavage of human subjects exposed for 2 hours to 0.4 ppm 18O3: intermittent exercise versus rest during exposure.
| Bronchoalveolar lavage | Nasal lavage | ||
|---|---|---|---|
|
| |||
| Cell pellet | Surfactant | ||
| Excess oxygen-18, ug/g dry weight | |||
| Resting | 5.6 ± 1.7 (6) | 26.4 ± 2.4 (3) | 377 ± 62 (5) |
| Exercise (1) | 28.4 ± 5.5 (8) | 51.6 ± 7.9 (8) | 192 ± 58 (8) |
| Exercise/resting | 5.07 | 1.95 | 0.51 |
| Resting F344 rat (1) | 7.5 ± 1.6 (6) | 10.9 ± 1.4 (8) | NM |
Notes: Shaded values are newly reported here. (1) From Hatch et al, 1994. All enrichments in 18O are significantly elevated above baseline. Means ± standard error are given for (N) subjects or rats.
Abbreviation: NM, not measured.
BALF fluid changes in cellular and biochemical markers
We measured a slight but significant 19% decrease in total cells recovered in BALF fluid, as well as a slight increase (0.9% to 1.3%) in PMNs recovered in resting subjects exposed to 18O3 (Table 5). None of the other cellular changes were significant. Data from seven different cytokines and other biochemical indicators in BALF supernatant indicated no significant change due to 18O3 exposure (Table 1 supplementary). Table 2 (supplementary) shows that BALF supernatant protein as well as ascorbate, urate, and total glutathione (GSx) were not significantly altered by the resting O3 exposure.
Table 5.
BAL cell numbers and differential following resting exposure of 8 human subjects to O3.
| BAL vol recovered, ml | Total cells × 10e6 | % cells | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Macs | PMNs | Lymphos | Monos | Epith | Eos | |||
| Air | ||||||||
| Mean | 229.9 | 43.6 | 87.3% | 0.9% | 8.9% | 1.5% | 0.3% | 0.3% |
| SE | 10.9 | 6.0 | 2.9% | 0.3% | 2.8% | 0.4% | 0.1% | 0.1% |
| O3 | ||||||||
| Mean | 240.3 | 35.4 | 90.8% | 1.3% | 6.6% | 1.2% | 0.4% | 0.1% |
| SE | 9.3 | 5.8 | 1.8% | 0.2% | 1.7% | 0.5% | 0.2% | 0.1% |
| O3/air | 1.05 | 0.81 | 1.04 | 1.52 | 0.75 | 0.76 | 1.32 | 0.46 |
| P value, O3 vs. air | 0.04 | 0.02 | ||||||
Table 6 shows that serum-opsonized Candida albicans was engulfed by ~20% fewer phagocytes in BALF from 0.4 ppm O3 exposed resting subjects. This effect was not observed for other types of opsonization due to greater variability of responses. Phagocytosis expressed as Candida particles per cell was not affected by resting exposure to O3.
Table 6.
Bronchoalveolar lavage cell phagocytosis of Candida albicans particles following resting exposure to O3.
| Air | O3 | Air | O3 | Air | O3 | |
|---|---|---|---|---|---|---|
| Percentage of macrophages that phagocytized particles | ||||||
| Mean | 23.7 | 17.9 | 55.7 | 55.2 | 69.6 | 55.4 |
| SE | 4.4 | 6.6 | 14.8 | 14.8 | 29.8 | 15.2 |
| N | 7.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 |
| O3/air | 0.76 | 0.99 | 0.80* | |||
| Number of Candida particles per cell | ||||||
| Mean | 1.4 | 1.0 | 6.5 | 6.6 | 3.5 | 3.7 |
| SE | 0.2 | 0.2 | 0.4 | 0.6 | 0.4 | 0.6 |
| N | 7 | 7 | 7 | 7 | 6 | 7 |
| O3/air | 0.69 | 1.02 | 1.05 | |||
Note:
P = 0.04 by 2-tail t-test of means.
FEV1 changes in resting subjects exposed to O3
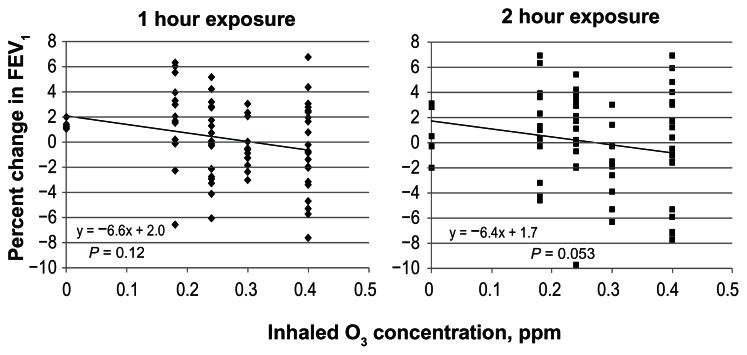
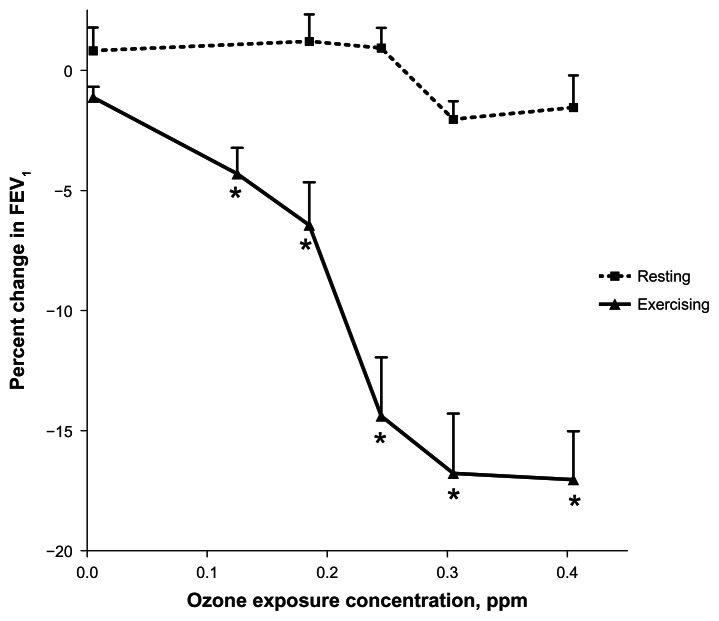
A scatter plot of FEV1 changes observed in each individual subject exposed to air or to 4 different concentrations of O3 is shown in Figure 2. FEV1 was assessed at an interim 1 hour point, at the completion of the 2 hour resting inhalation of air, and at four different concentrations of O3. A linear regression of all data for each time period was performed and the slopes of the regression tested for significance against a zero slope. A slope of approximately −6.5% per ppm O3 was observed for both measurement at 1 and 2 hours. Linear regression indicated that the slope was not significantly different from zero at one hour and marginally significant after 2 hours of O3 exposure (P = 0.053). This significant result was dependent on inclusion of an outlier (judged by Grubb’s test) at 0.25 ppm. Further experiments could possibly unmask effects observed at 0.4 ppm exposure where the subjects appeared to separate into two groups: responders and non-responders. Figure 3 shows a comparison of the resting data obtained against our earlier published study involving exercising subjects (intermittent 15 minutes on and 15 minutes off to Ve ~ 65 L/minute maximum).17 Non-parametric Williams test reported previously on the exercising study indicated that an O3 concentration of 0.12 ppm represented the lowest dose that was significantly different from control. Application of the same test to the resting O3 exposure yielded no significant effect for any O3 concentration. Tests at individual exposure concentrations indicated borderline significance (0.3 ppm, p = 0.049) but only if not corrected for multiple comparisons. Similarly, ANOVA followed by Dunnett’s test correcting for multiple comparisons yielded no significance for all O3 concentrations in resting subjects.
Figure 2.
The percentage change in FEV1 in individual resting subjects exposed to four concentrations of O3 and to air plotted against the O3 inhaled concentration.
Notes: FEV1 was measured pre-exposure, and after 1 and 2 hours of exposure in the same subjects. The regression trend lines had a similar slope. The 2 hour O3 exposure line appeared to have a slope be significantly different from zero slope (P = 0.053).
Figure 3.
Comparison of O3-induced FEV1 changes (mean ± S.E.) observed after 2 hours of exposure to various concentrations of O3 while at rest or while exercising intermittently (15 minute intervals) at a level of 65 L/min Ve.
Note: Asterisks indicate the lowest concentration of O3 exposure at which a significant change from air exposed occurred (by Williams test for nonparametric data).16
Comparison of O3 effect markers: resting versus exercising
A side-by-side comparison summary of O3 effects observed during resting versus exercising exposures is presented in Table 7. The change in the mean values observed for O3 exposure compared to air exposure is represented as either an O3 minus Air value or an O3 to Air ratio if that was more appropriate. The FEV1 percent change and the fold increase in neutrophils was about 5-fold greater during exercise than during resting O3 exposure. The mean decrement in BALF cell recovery appeared to be similar following exercising and resting exposures. BALF protein was increased 2 fold with exercising exposure to O3 and was unchanged with resting exposure to O3.
Table 7.
Comparison between resting and exercising effects and dose 1–2 hours following 0.4 ppm, 2 hr O3 exposure.
| Measurement | Resting exposure | Exercising exposure | Exercise/rest | Reference for exercising exposure |
|---|---|---|---|---|
| O3minus air | ||||
| FEV1 % decrement | ~2 | 10.3* | 5.0 | 1 |
| BALF cell 18O | 5.6* | 28.4* | 5.1 | 1 |
| BAL surfactant 18O | 26.4* | 51.6* | 2.0 | 1 |
| Nasal lavage 18O | 377* | 192* | 0.5 | 1 |
| O3/air ratio | ||||
| BALF cells recovered, % decrement | 19* | 28* | 1.5 | 1 |
| BALF neutrophils, fold increase | 1.52* | 7.6* | 5.0 | 1,2 |
| BALF protein, fold increase | 0.99 | 1.9* | 2.0 | 1,2 |
| BALF cell phagocytosis (serum opsonized), % decrement | 20* | 23 | 1.2 | 2 |
| BALF cell phagocytosis (IgG opsonized ), % decrement | 1 | 26* | 26 | 2 |
| BALF cell phagocytosis (unopsonized), % decrement | 24 | 45* | 1.9 | 2 |
| BALF IL6, fold increase | 1.1 | 7.3* | 6.9 | 2 |
| BALF LDH, fold increase | 1.2 | 1.5* | 1.3 | 2 |
| BALF a1-AT, fold increase | 1.1 | 1.7* | 1.5 | 2 |
| BALF C3a fold increase | 2.3 | 1.4 | 0.6 | 2 |
Notes:
Significant effect of O3 compared to respective air exposed.
Hatch et al, 1994;
Devlin et al, 1996.
Discussion
The goal of this study was to measure O3 dose and effect in such a way that would improve the basis for extrapolating O3 dose and effect between exercise levels in humans, as well as between rats and humans. New data presented here include: (1) fractional removal of O3 from breathing air, (2) 18O3 dose measurements made in nasal lavage fluid and BALF, (3) O3 induced cellular and biochemical effects measurements in the same BALF, and (4) pulmonary function (FEV1) measurements made in a parallel group of resting subjects. These data provide a basis for extrapolating alveolar O3 dose between resting and exercising humans, and between resting rats and resting humans.
Fractional removal of ozone from breathing air
Past studies have examined the fractional removal of O3 from breathing air to arrive at estimates of O3 dose to the respiratory tract. Exercising subjects have been reported to remove a smaller fraction of O3 from the breathing air than resting subjects;18 however, when that fraction is multiplied by the increased volume of air breathed during exercise, the O3 retained in the lung is definitely increased by exercise.19 Our result of ~79% of removal of O3 from breathing air agrees with the 73%–76% removal measured at the face of subjects breathing at rest in a previous study.20 The fractional uptake of O3 from breathing air by the whole body, or by the nasal or thoracic regions, has been measured previously either by a facial exposure similar to ours or by placement of catheters into the posterior pharynx. Our result is lower than the ~88% uptake measured by integration of breath by breath O3 concentrations at the posterior pharynx, which was reported in previous studies21,22 that cited as possible reasons for their higher percentage uptake a larger tidal volume in their subjects compared to the Wiester study. Our resting subjects had a tidal volume (0.59 L) similar to that reported by Wiester et al (0.63–0.64 L) and lower than that reported by Gerrity et al (0.75–0.83 L).
18O3 dose measurements in lavaged fluids
We have demonstrated here that human subjects exposed while at rest to 18O3 accumulate 18O in BALF cells and surfactant material in lower concentrations than exercising subjects. The 18O label that remains in the tissue after lyophilization appears to be the result of oxygen addition reactions of 18O3 with biomolecules. Accumulation of 18O in BALF cells and surfactant material suggests that 18O3 penetrates into the alveolar region of the lung during resting exposure. BALF cells and surfactant reside at the air-liquid interface and appear (from histological evidence) to have an alveolar origin.23 The fact that lung parenchyma following lavage contains very little 18O following 18O3 exposure, as seen in a study involving rhesus monkeys,4 suggests that the reaction of 18O3 is concentrated at the air-liquid interface. This finding is in agreement with physicochemical modeling predictions that suggest that O3, because of its high chemical reactivity, does not penetrate far into the surface fluid or epithelial cells.24 We have not yet been able to detect excess 18O in human blood following either resting or exercising exposure. This inability is probably due to the difficulty in detecting the label after such a large dilution into the large systemic volume of blood.
Ozone-induced cellular and biochemical effects measurements
Our ability to measure both O3 dose and effect in the same BALF cells and fluids makes it possible to determine the relationship between dose and effect in the same subject. Results suggest that the sensitivity for detecting excess 18O is greater than the sensitivity for detecting many of the biological effects in BALF at early post exposure times. Our present finding that resting exposure to O3 produced few statistically significant biological effects in BALF highlights the low-dose nature of alveolar O3 exposure, even at the relatively high inhaled O3 concentration of 0.4 ppm. We detected small but significant decreases in BALF cell recovery and neutrophil counts following resting exposure (Table 5). Other indicators previously measured in BALF during exercise were not detectable here after resting exposure. Many of the O3 effect markers examined here immediately post exposure would have been greater if measured 16–24 hours post exposure.11 In agreement with our lack of cellular effects following resting O3 exposure, a previous report showed a lack of effect of resting O3 exposure on BALF cell DNA single strand breaks, as opposed to a positive effect if O3 exposure occurred during exercise.25
Airway antioxidants participate in the reactions of inhaled O3, and measurement of changes in antioxidants can provide insight on where O3 reacts. Our results showed only a non-significant lowering of NL ascorbate by O3 at zero hour post O3 exposure (Table 2, supplementary). Two published studies measured antioxidants under a less vigorous exercise regimen targeting 20 L/min per m2 body surface area rather than the present 35 L/min per m2 with exposure to 0.2 ppm of O3 for 2 hours. The first found significant increases in dehydroascorbic acid in bronchial lavage and BALF six hours post exposure.26 The second found insignificant changes in NL fluid antioxidants at zero and six hours post exposure and 26%–100% elevations in BALF and BL concentrations of GSx, ascorbate, and uric acid at six hours post exposure.27 Thus, although previous studies do not exactly match our exposure scenario, they do confirm the difference in response during rest and exercise.
Ozone induced pulmonary function (FEV1) changes
Our regression of FEV1 changes versus four resting concentrations of O3 up to 0.4 ppm showed a slope that appears to be different from zero (Fig. 2). Previously published reports that looked at FEV1 changes immediately following resting 2 hour O3 exposures and which found no significant decrements at O3 concentrations lower than 0.5 ppm were probably due to the smaller number of subjects examined.28,29 In addition to a lower delivered O3 dose in resting exposures, the inability to detect significant alterations in FEV1 may be due to higher variability of response incident to a less targeted control of breathing during rest than is possible during exercising exposures.
Extrapolation between exercise levels in humans
We found that the fold change in BALF cell 18O3 reaction product concentration roughly correlates with the average Ve between different exercise levels; this lends support to the use of Ve as a factor in extrapolating pulmonary dose of O3 between different levels of physical activity. ‘Effective dose’ was first defined as the product of concentration, Ve, and exposure time by Silverman et al30 and has often been used as a default assumption since. A recent meta-analysis of 23 published human exposure studies showed strong associations between total BALF protein and neutrophilia responses, and O3 dose defined as the product of exposure concentration, ventilation, and time.31
The increase in Ve which accompanies exercise is due to increases in both breathing frequency and tidal volume, and it would therefore be valuable to define the relative contribution of each. Our earlier 18O3 exposure study6 did not measure tidal volume or frequency; however, a study which did measure these parameters under similar conditions suggests that the exercise periods saw a 3.8-fold increase in tidal volume and a 2.3-fold increase in breathing frequency.17 This study also employed male subjects of similar age (22.5 ± 3.1 year), weight (76.2 ± 7.5 kg), and Ve (66.2 ± 7.6 L/min) as our previous study (see Table 3). They reported tidal volumes of 2.2 L and breathing frequencies averaging 31 breaths/minute during the 15 minute intermittent exercising periods
The 18O3 dose is a measurement closer to the pulmonary target site for O3 than previous estimates of O3 dose, which were obtained by measurement of removal of the gas from breathing air as it passed through the nasal or thoracic regions.21,22 Our resting and exercising 18O dose measurements of BALF cells can be used to create a two-point regression line from which to make a crude extrapolation to higher exercise levels. Although human controlled exposure studies to date have had a reasonable level of activity for normal people, they do not reach the Ve levels or the duration that might be experienced by the sizable population that now participates in marathons and other high Ve activities. It would not be uncommon for people participating in such activities to achieve a 4-fold higher average Ve (to 120 L/min) for a 2-fold longer time (4 hour) than has yet been investigated in human clinical studies. There is a need for further research at low O3 concentrations during continuous high exercise levels. There is also a need for a further expansion of the sample size and time points measured post exposure.
Extrapolation between rats and humans
We report here and in our previous study6 that a direct comparison between rat and human alveolar O3 dose can be achieved by comparing the 18O content in BALF cells obtained from humans and rats similarly exposed to 18O3. In our previous study, rats had to be exposed to 2.0 ppm 18O3 in order to achieve a BALF cell dose similar to exercising humans exposed to 0.4 ppm. It is apparent from the present study that the exercise level of the human subjects accounted for their higher BALF O3 dose. The finding that human resting BALF 18O dose approximates that of the resting rat BALF 18O dose is unexpected because rats are known to have a higher ratio of body surface area/body volume and breathe more air; they should therefore experience a higher O3 dose than humans. Allometric relationships predict that a resting rat lung would be exposed to 2.8 times the volume of inhaled air per wet lung weight than a resting human lung (see Appendix 2). We offered previously as an explanation for lower than expected dose to the rat lung the fact that rats are nocturnal and are therefore exposed during their dormant period (our daytime). Other reasons might include the following: (1) an approximately 8-fold higher BALF ascorbate concentration in rats compared to humans,32 as ascorbate appears to quench O3 reactions in the lung and therefore serves as a shield to BALF cells, causing them to retain less 18O3;7,8 (2) the ability of rats to lower body temperature and Ve during O3 exposure;33 (3) a higher nasopharyngeal removal of O3 in rats;34 and (4) a lower whole-body percent retention of O3 from breathing air in rats.21,35,36 A complete discussion of these differences is beyond the scope of the present paper; however, it is apparent that moderate exercise in humans is able to increase alveolar O3 dose to levels much higher than that seen in similarly exposed resting rats. We therefore confirm with quantitative evidence the important contribution of physical exercise to the alveolar dose of O3, and suggest a similar effect of exercise on the alveolar dose of other chemically reactive gases with properties similar to O3.
Conclusion
Results confirm that exercise contributes greatly to both the dose and effect of O3 measured by indicators in BALF. Quantification of 18O3 reaction products in BALF cells has provided a basis for extrapolation of acute O3 dose between resting and exercising exposures. The comparison between resting and exercising O3 effects, along with the dose measurements in each type of exposure provide an improved understanding of low-dose O3 effects. Results confirm the use of Ve as a factor in the extrapolation of inhaled dose of O3 at different levels of physical activity, and suggest that higher and more continuous activity levels will yield significant effects at even lower ambient levels of O3. The similarity of alveolar O3 dose and effect between resting human and resting rats strengthens the extrapolation of rat inhalation data to humans.
Supplementary Data
Appendix 1
The approximate Ve of marathon runners was reported by Mahler37 to be 86.2% of maximal ventilatory volume (MVV). MVV was reported to be 180 and 176 L/min in trained runners and control subjects, respectively. Thus, the Ve of trained marathon runners can be estimated as 0.86*180 = 155 L/min. Since our earlier human clinical study6 employed alternating 15 minute periods of rest (Ve = 13.5 L/min) during half of the 2 hour exposure time, the average Ve for the two hours of O3 exposure would have been 39 L/min (see Table 3). It appears that the exercise induced Ve of marathon runners could attain the level of 3.97 (155/39) times higher than our earlier ‘exercising’ human subjects and sustain that level for over twice the time. Less trained runners would experience a lower exposure level because they do not sustain the high Ve possible in the trained athletes; however, in a race event they would run for a longer time. The main difference between trained and untrained runners appears to be that the trained runners are able to sustain a Ve/MVV ratio that is 24% higher than untrained runners. They also consume oxygen at a 55% higher rate and for a longer time.37
Appendix 2
The relationship between body weight and Ve across species has been reported as 379 M^0.8, where M = body weight in kg and Ve is in milliliters.38 The same author reports that the wet lung weight in grams varies by the relationship 11.3 M^0.99. Substituting values for a 0.3 kg rat and a 70 kg human yields the following: (11.342 mL/min)/758 g = 15.0 mL/min/g for human and 145/3.43 = 42.3 for the rat. Thus, a resting rat would be predicted to have an exposure 2.82-times higher than a resting human (42.3/15 = 2.82).
Table S1.
Bronchoalveolar lavage fluid cytokines and enzymes following resting exposure to 18O3.
| IL-6, pg/mL | IL-8, pg/mL | tPA, IU/mL | Elastase, uM/hr | C3a, ng/mL | a1-AT, ug/mL | LDH, U/mL | |
|---|---|---|---|---|---|---|---|
| Air | |||||||
| Mean | 2.6 | 14.0 | 112 | 46.4 | 189 | 1.80 | 3.79 |
| SE | 0.2 | 0.7 | 20 | 19.7 | 111 | 0.30 | 0.24 |
| O3 | |||||||
| Mean | 2.7 | 26.5 | 104 | 81.7 | 434 | 2.0 | 4.4 |
| SE | 0.3 | 12.5 | 13 | 45.3 | 171 | 0.35 | 0.56 |
| O3/air | 1.06 | 1.90 | 0.93 | 1.76 | 2.30 | 1.10 | 1.17 |
Notes: No significant changes due to ozone exposure were detected in any of the measurements (2 tailed paired t test). N = 7 subjects in all groups.
Table S2.
Protein and antioxidant changes in lavage fluids following resting exposure to 18O3.
| Protein, ug/mL | Ascorbate, uM | Urate, uM | GSH, uM | Alpha tocopherol, nM | |
|---|---|---|---|---|---|
| Nasal lavage fluid | |||||
| Air | |||||
| Mean | 686 | 6.00 | 59.2 | 3.50 | NM |
| SE | 163 | 2.54 | 7.6 | 1.56 | |
| O3 | |||||
| Mean | 727 | 3.82 | 46.9 | 3.75 | NM |
| SE | 244 | 1.38 | 8.3 | 0.84 | |
| O3/air | 1.06 | 0.64 | 0.79 | 1.07 | |
| Bronchial lavage fluid | |||||
| Air | |||||
| Mean | 36.6 | 0.35 | 0.35 | 0.48 | NM |
| SE | 3.4 | 0.05 | 0.04 | 0.04 | |
| O3 | |||||
| Mean | 41.7 | 0.46 | 0.77 | 0.66 | NM |
| SE | 4.6 | 0.12 | 0.33 | 0.12 | |
| O3/air | 1.14 | 1.31 | 2.17 | 1.37 | |
| Bronchoalveolar lavage fluid | |||||
| Air | |||||
| Mean | 117 | 0.46 | 1.16 | 0.70 | 5.1 |
| SE | 13 | 0.06 | 0.17 | 0.10 | 2.5 |
| O3 | |||||
| Mean | 116 | 0.54 | 1.23 | 0.74 | 2.8 |
| SE | 16 | 0.06 | 0.14 | 0.09 | 1.2 |
| O3/air ratio | 0.99 | 1.16 | 1.07 | 1.06 | 0.55 |
Abbreviation: NM, not measured.
Acknowledgements
The authors thank MaryAnn Bassett for clerical assistance, Beth Owens and Dr. Janice Dye for internal review of the manuscript, and Judith Schmid for statistical assistance.
Footnotes
Author Contributions
Conceived and designed the experiments: GH and RD. Analysed the data: GH, JB, WM, ES, JS, RS, KC and RD. Wrote the first draft of the manuscript: GH; Contributed to the writing of the manuscript: GH, JM, JB, WM, ES, JS, RS, KC, RD. Agree with manuscript results and conclusions: GH, JM, JB, WM, ES, JS, RS, KC, RD. Jointly developed the structure and arguments for the paper: GH, WM, RD. Made critical revisions and approved final version: GH, JM, JB, WM, ES, JS, RS, KC, RD. All authors reviewed and approved of the final manuscript.
Competing Interests
All of the authors were employed by the United States Environmental Protection Agency at the time of the completion of the study (some are now retired). There are no conflicts of interest.
Disclaimer
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, United States Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This work was funded entirely by the United States Environmental Protection Agency.
References
- 1.US E P A. Air quality criteria for ozone and related photochemical oxidants: Integrated Science Assessment. Research Triangle Park, NC: 2013. pp. 118–122. EPA/600/R-05/004AF. URL: http://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=247492#Download. [Google Scholar]
- 2.McDonnell WF, Stewart PW, Smith MV, Pan WK, Pan J. Ozone-induced respiratory symptoms: exposure-response models and association with lung function. Eur Respir J. 1999;14:845–53. doi: 10.1034/j.1399-3003.1999.14d21.x. [DOI] [PubMed] [Google Scholar]
- 3.Mautz WJ. Exercising animal models in inhalation toxicology: interactions with ozone and formaldehyde. Environ Res. 2003;92(1):14–26. doi: 10.1016/s0013-9351(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 4.Plopper CG, Hatch GE, Wong V, et al. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose and glutathione depletion in rhesus monkeys. Am J Respir Cell Mol Biol. 1998;19:387–99. doi: 10.1165/ajrcmb.19.3.3183. [DOI] [PubMed] [Google Scholar]
- 5.Chuang GC, Yang Z, Westbrook DG, et al. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol. 2009;297(2):L209–16. doi: 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatch GE, Slade R, Harris LP, et al. Ozone dose and effect in humans and rats: A comparison using oxygen-18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med. 1994;150:676–83. doi: 10.1164/ajrccm.150.3.8087337. [DOI] [PubMed] [Google Scholar]
- 7.Kari F, Hatch G, Slade R, Crissman K, Simeonova PP, Luster M. Dietary restriction mitigates ozone-induced lung inflammation in rats: A role for endogenous antioxidants. Am J Respir Cell Mol Biol. 1997;17:740–7. doi: 10.1165/ajrcmb.17.6.2844. [DOI] [PubMed] [Google Scholar]
- 8.Gunnison AF, Hatch GE. O3-induced inflammation in prepregnant, pregnant, and lactating rats correlates with O3dose estimated by 18O. Am J Physiol. 1999;276:L332–40. doi: 10.1152/ajplung.1999.276.2.L332. [DOI] [PubMed] [Google Scholar]
- 9.Koren HS, Devlin RB, Graham DE, et al. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989 Feb;139(2):407–15. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Devlin RB, McDonnell WF, Becker S, et al. Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4 ppm ozone for 2 hr: A comparison of mediators found in bronchoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol Appl Pharm. 1996;138:176–85. doi: 10.1006/taap.1996.0111. [DOI] [PubMed] [Google Scholar]
- 12.Kutnink MA, Skala JH, Sauberlich HE, Omaye ST. Simultaneous determination of ascorbic-acid, isoascorbic acid (erythorbic acid) and uricacid in human-plasma by high-performance liquid-chromatography with amperometric detection. J Liq Chromatogr. 1985;8(1):31–46. [Google Scholar]
- 13.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–55. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 14.Vandewoude M, Claeys M, De Leeuw I. Determination of alpha-tocopherol in human plasma by high-performance liquid chromatography with electrochemical detection. J Liq Chromatogr. 1984;311(1):176–82. doi: 10.1016/s0378-4347(00)84706-4. [DOI] [PubMed] [Google Scholar]
- 15.Seal E, McDonnell WF, House DE, et al. The pulmonary response of white and black adults to six concentrations of ozone. Am Rev Respir Dis. 1993;147(4):804–10. doi: 10.1164/ajrccm/147.4.804. [DOI] [PubMed] [Google Scholar]
- 16.Shirley E. Nonparametric equivalent of Williams test for contrasting increasing dose levels of a treatment. Biometrics. 1977;33(2):386–9. [PubMed] [Google Scholar]
- 17.McDonnell WF, Horstman DH, Hazucha MJ, et al. Pulmonary effects of ozone exposure during exercise: dose-response characteristics. J Appl Physiol. 1983;54(5):1345–52. doi: 10.1152/jappl.1983.54.5.1345. [DOI] [PubMed] [Google Scholar]
- 18.Hu SC, Benjebria A, Ultman JS. Longitudinal distribution of ozone absorption in the lung: effects of respiratory flow. J Appl Physiol. 1994;77(2):574–83. doi: 10.1152/jappl.1994.77.2.574. [DOI] [PubMed] [Google Scholar]
- 19.United States Environmental Protection Agency. Air Qualilty Criteria for Ozone and Related Photochemical Oxidants. Integrated Science Assessment. 2006. http://www.epa.gov/ncea/isa/
- 20.Wiester MJ, Stevens MA, Menache MG, McKee JL, Jr, Gerrity TR. Ozone uptake in healthy adult males during quiet breathing. Fund Appl Toxicol. 1996;29(1):102–9. doi: 10.1006/faat.1996.0011. [DOI] [PubMed] [Google Scholar]
- 21.Gerrity TR, McDonnell WF, House DE. The relationship between delivered ozone dose and functional responses in humans. Toxicol Appl Pharm. 1994;124(2):275–83. doi: 10.1006/taap.1994.1033. [DOI] [PubMed] [Google Scholar]
- 22.Gerrity TR, Biscardi F, Strong A, Garlington AR, Brown JS, Bromberg PA. Bronchoscopic determination of ozone uptake in humans. J Appl Physiol. 1995;79(3):852–60. doi: 10.1152/jappl.1995.79.3.852. [DOI] [PubMed] [Google Scholar]
- 23.Pinkerton KE, Gehr P, Crapo JD. Architecture and cellular composition of the air-blood barrier. In: Parent RA, editor. Comparative Biology of the Normal Lung. Vol. 1. CRC Press; 1991. pp. 121–8. [Google Scholar]
- 24.Pryor WA. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radical Bio Med. 1992;12(1):83–8. doi: 10.1016/0891-5849(92)90060-t. [DOI] [PubMed] [Google Scholar]
- 25.Lee JG, Madden MC, Hatch G, et al. Ozone-induced DNA single strand breaks in human and guinea pig lung cells in vivo. Inhal Toxicol. 1997;9:811–28. [Google Scholar]
- 26.Mudway IS, Behndig AF, Helleday R, et al. Vitamin supplementation does not protect against symptoms in ozone-responsive subjects. Free Radical Bio Med. 2006;40(10):1702–12. doi: 10.1016/j.freeradbiomed.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 27.Behndig AF, Blomberg A, Helleday R, Duggan ST, Kelly FJ, Mudway IS. Antioxidant responses to acute ozone challenge in the healthy human airway. Inhal Toxicol. 2009;21(11):933–42. doi: 10.1080/08958370802603789. [DOI] [PubMed] [Google Scholar]
- 28.Folinsbee LJ, Silverman F, Shephard RJ. Exercise responses following ozone exposure. J Appl Physiol. 1975;38(6):996–1001. doi: 10.1152/jappl.1975.38.6.996. [DOI] [PubMed] [Google Scholar]
- 29.Horvath SM, Gliner JA, Matsen-Twisdale JA. Pulmonary function and maximum exercise responses following acute ozone exposure. Aviat Space Environ Med. 1979;50:901–5. [PubMed] [Google Scholar]
- 30.Silverman F, Folinsbee LJ, Barnard J, Shephard RJ. Pulmonary function changes in ozone-interaction of concentration and ventilation. J Appl Physiol. 1976;41(6):859–64. doi: 10.1152/jappl.1976.41.6.859. [DOI] [PubMed] [Google Scholar]
- 31.Mudway IS, Kelly FJ. An investigation of inhaled ozone dose and the magnitude of airway inflammation in healthy adults. Am J Respir Crit Care Med. 2004;169:1089–95. doi: 10.1164/rccm.200309-1325PP. [DOI] [PubMed] [Google Scholar]
- 32.Slade R, Crissman K, Norwood J, Hatch G. Comparison of antioxidant substances in bronchoalveolar lavage cells and fluid from humans, guinea pigs, and rats. Exp Lung Res. 1993;19(4):469–84. doi: 10.3109/01902149309064358. [DOI] [PubMed] [Google Scholar]
- 33.Watkinson WP, Gordon CJ. Caveats regarding the use of the laboratory rat as a model for acute toxicological studies—modulation of the toxid response via physiological and behavioral mechanisms. Toxicology. 1993;81(1):15–31. doi: 10.1016/0300-483x(93)90153-j. [DOI] [PubMed] [Google Scholar]
- 34.Hatch G, Wiester MJ, Overton JH, Aissa M. Respiratory tract dosimetry of 18-O-labeled ozone in rats: implications for a rat-human extrapolation of ozone dose. In: Tea Schneider., editor. Atmospheric Ozone Research and its Policy Implications. Amsterdam: Elsevier Science Publishers, BV; 1989. [Google Scholar]
- 35.Wiester MJ, Tepper JS, King ME, Menache MG, Costa DL. Comparative study of ozone (O3) uptake in three strains of rats and in the guinea pig. Toxicol Appl Pharm. 1988;96(1):140–6. doi: 10.1016/0041-008x(88)90256-6. [DOI] [PubMed] [Google Scholar]
- 36.Wiester MJ, Stevens MA, Menache MG, McKee JL, Gerrity TR. Ozone uptake in healthy adult males during quiet breathing. Fund Appl Toxicol. 1996;29(1):102–9. doi: 10.1006/faat.1996.0011. [DOI] [PubMed] [Google Scholar]
- 37.Mahler DA, Moritz ED, Loke J. Ventilatory responses at rest and during exercise in marathon runners. J Appl Physiol. 1982;52(2):388–92. doi: 10.1152/jappl.1982.52.2.388. [DOI] [PubMed] [Google Scholar]
- 38.Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22:453–60. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Bronchoalveolar lavage fluid cytokines and enzymes following resting exposure to 18O3.
| IL-6, pg/mL | IL-8, pg/mL | tPA, IU/mL | Elastase, uM/hr | C3a, ng/mL | a1-AT, ug/mL | LDH, U/mL | |
|---|---|---|---|---|---|---|---|
| Air | |||||||
| Mean | 2.6 | 14.0 | 112 | 46.4 | 189 | 1.80 | 3.79 |
| SE | 0.2 | 0.7 | 20 | 19.7 | 111 | 0.30 | 0.24 |
| O3 | |||||||
| Mean | 2.7 | 26.5 | 104 | 81.7 | 434 | 2.0 | 4.4 |
| SE | 0.3 | 12.5 | 13 | 45.3 | 171 | 0.35 | 0.56 |
| O3/air | 1.06 | 1.90 | 0.93 | 1.76 | 2.30 | 1.10 | 1.17 |
Notes: No significant changes due to ozone exposure were detected in any of the measurements (2 tailed paired t test). N = 7 subjects in all groups.
Table S2.
Protein and antioxidant changes in lavage fluids following resting exposure to 18O3.
| Protein, ug/mL | Ascorbate, uM | Urate, uM | GSH, uM | Alpha tocopherol, nM | |
|---|---|---|---|---|---|
| Nasal lavage fluid | |||||
| Air | |||||
| Mean | 686 | 6.00 | 59.2 | 3.50 | NM |
| SE | 163 | 2.54 | 7.6 | 1.56 | |
| O3 | |||||
| Mean | 727 | 3.82 | 46.9 | 3.75 | NM |
| SE | 244 | 1.38 | 8.3 | 0.84 | |
| O3/air | 1.06 | 0.64 | 0.79 | 1.07 | |
| Bronchial lavage fluid | |||||
| Air | |||||
| Mean | 36.6 | 0.35 | 0.35 | 0.48 | NM |
| SE | 3.4 | 0.05 | 0.04 | 0.04 | |
| O3 | |||||
| Mean | 41.7 | 0.46 | 0.77 | 0.66 | NM |
| SE | 4.6 | 0.12 | 0.33 | 0.12 | |
| O3/air | 1.14 | 1.31 | 2.17 | 1.37 | |
| Bronchoalveolar lavage fluid | |||||
| Air | |||||
| Mean | 117 | 0.46 | 1.16 | 0.70 | 5.1 |
| SE | 13 | 0.06 | 0.17 | 0.10 | 2.5 |
| O3 | |||||
| Mean | 116 | 0.54 | 1.23 | 0.74 | 2.8 |
| SE | 16 | 0.06 | 0.14 | 0.09 | 1.2 |
| O3/air ratio | 0.99 | 1.16 | 1.07 | 1.06 | 0.55 |
Abbreviation: NM, not measured.