Background: Cell behavior is affected by changes in extracellular matrix stiffness during disease progression.
Results: Fibronectin matrix assembly is inhibited on soft substrates but can be restored by manipulating cell-fibronectin binding or by partially unfolding substrate fibronectin.
Conclusion: On soft substrates, cells are deficient in integrin-fibronectin bond strength and therefore cannot induce fibronectin conformational changes required for assembly.
Significance: Rigidity-dependent changes in fibronectin conformation provide a novel mechanism for mechanotransduction.
Keywords: Extracellular Matrix, Fibroblast, Fibronectin, Integrin, Mechanotransduction, Protein Assembly
Abstract
Cells sense and respond to the mechanical properties of their microenvironment. We investigated whether these properties affect the ability of cells to assemble a fibrillar fibronectin (FN) matrix. Analysis of matrix assembled by cells grown on FN-coated polyacrylamide gels of varying stiffnesses showed that rigid substrates stimulate FN matrix assembly and activation of focal adhesion kinase (FAK) compared with the level of assembly and FAK signaling on softer substrates. Stimulating integrins with Mn2+ treatment increased FN assembly on softer gels, suggesting that integrin binding is deficient on soft substrates. Guanidine hydrochloride-induced extension of the substrate-bound FN rescued assembly on soft substrates to a degree similar to that of Mn2+ treatment and increased activation of FAK along with the initiation of assembly at FN matrix assembly sites. In contrast, increasing actin-mediated cell contractility did not rescue FN matrix assembly on soft substrates. Thus, rigidity-dependent FN matrix assembly is determined by extracellular events, namely the engagement of FN by cells and the induction of FN conformational changes. Extensibility of FN in response to substrate stiffness may serve as a mechanosensing mechanism whereby cells use pericellular FN to probe the stiffness of their environment.
Introduction
The extracellular matrix (ECM)2 is a network of proteins that encompasses cells within a tissue and provides them with information about their environment (1). It has long been known that the molecular composition of the pericellular environment regulates cell behaviors, but the mechanical properties also mediate cell responses. Cells sense and respond to differences in substrate rigidity, which has been shown to influence behaviors such as growth (2), morphology (3, 4), migration (4, 5), and differentiation (6–8), although details of the mechanochemical mechanisms have not been fully elucidated.
In multicellular organisms, cells function within dynamic tissues whose mechanical properties can change during development (9) or with the progression of diseases, including cancer (10), fibrosis (11), and atherosclerosis (12). Diseases often result in the stiffening of tissue with tumor formation and tissue fibrosis markedly increasing tissue stiffness. For example, healthy adipose tissue ranges from 0.2 to 2 kPa, but as a breast tumor develops, the surrounding tissues increase in stiffness to 4–12 kPa (10, 13, 14). An increase in tissue stiffness can also correlate with the degree of disease progression. For example, Yin et al. (11) reported a correlation between the stiffness of liver tissue and the progression of fibrosis, starting with measurements of 2 kPa for normal liver up to 12 kPa for the most advanced stages of fibrosis.
Diseases that lead to an increase in tissue stiffness are often also characterized by an increase in the deposition of ECM proteins. The protein fibronectin (FN) is a major component of the ECM, and an excessive and disordered FN matrix is present in fibrotic diseases (15) and hypertrophic scars (16). FN matrix assembly is mediated by integrin receptors and regulated by intracellular signals, cytoskeletal organization, and availability of FN (17). In an early study, Halliday and Tomasek (18) reported that cells form an FN matrix on tensioned collagen gels attached to plastic but not on relaxed, free floating gels, indicating an effect of substrate mechanical properties on FN matrix assembly. However, little is known about how the mechanical properties of the pericellular environment affect FN matrix assembly and what mechanisms are involved.
Substrate stiffness could affect FN matrix assembly at different points during the cell-mediated process. Assembly begins when FN dimers bind to α5β1 integrin receptors on the cell surface (17, 19). The cytoplasmic domains of the integrins associate with the cytoskeleton, enabling cells to transmit force to the extracellular environment. FN-integrin binding leads to an increase in contractility that allows cells to stretch their FN ligands from a compact to an extended form, unmasking cryptic FN-binding sites along the length of the molecule. Exposure of these FN-binding sites promotes intermolecular interactions and formation of fibrils that are initially soluble in the detergent deoxycholate (DOC) but are gradually and irreversibly converted into a stable, DOC-insoluble form that comprises the mature ECM. Whether cells increase FN fibril formation in response to sensing a rigid pericellular environment is an important question especially because FN assembly precedes and often seeds assembly of other ECM proteins such as collagen (20).
In the present study, we used polyacrylamide gels of different stiffnesses to determine the effects of substrate rigidity on fibroblast assembly of FN matrix. Measurements of DOC-insoluble matrix and analyses of fibril formation were used to identify the steps of assembly that vary with gel stiffness. We observed that FN matrix assembly is up-regulated on rigid substrates and propose that this is primarily due to a deficiency in cell-mediated FN conformational changes on softer substrates. These findings establish an extracellular mechanism for stiffness-dependent regulation of FN matrix assembly.
EXPERIMENTAL PROCEDURES
Cell Culture, Fibronectin, and Antibodies
NIH 3T3 fibroblasts were cultured in DMEM and 10% bovine calf serum (Hyclone). Plasma FN was purified from fresh frozen rat plasma or spent human plasma by gelatin-Sepharose affinity chromatography (21). The recombinant 70-kDa fragment of FN was generated using the baculovirus insect cell expression system (22). Fibronectin and 70-kDa were biotinylated with sulfo-NHS-biotin (N-hydroxy sulfosuccinimidyl biotin) according to the manufacturer's instructions (Pierce). The following anti-FN antibodies were used in this study: rat-specific anti-FN monoclonal antibody IC3 (23) and polyclonal antiserum R457 against the N-terminal 70-kDa fragment of rat FN (22). Anti-GAPDH (14C10) antibody was purchased from Cell Signaling Technology. Antibodies against mouse collagen type I and total focal adhesion kinase (FAK) were purchased from Millipore. Alexa Fluor 488-conjugated goat anti-mouse IgG, Alexa Fluor 488-conjugated streptavidin, and anti-FAK (Tyr(P)-397) phosphospecific antibody were purchased from Invitrogen.
Preparation of Polyacrylamide Substrates
Polyacrylamide substrates were made as described previously (4, 24). Briefly, polyacrylamide gels were polymerized on 12-mm aminosilanized glass coverslips. To modulate the stiffness of the gels, the acrylamide (GE Healthcare) to cross-linker N,N′-methylene bisacrylamide (GE Healthcare) ratios were varied as described in Moshayedi et al. (24) for 0.3-, 31-, and 90-kPa gels as follows: 5%/0.07% for soft (0.9 kPa/1.6 kPa), 7.5%/0.06% for intermediate (3.6 kPa/4.4 kPa), and 7.5%/0.2% for rigid (9.4 kPa/7.6 kPa). Human plasma fibronectin in 50 mm EPPS, pH 8.5, 150 mm NaCl (Sigma-Aldrich) was covalently attached to the gel surfaces using a photoactivated bifunctional cross-linker, sulfo-SANPAH (N-sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate; Pierce) (25). In some gel preparations, FN was pretreated with guanidine hydrochloride (GdnHCl) prior to cross-linking to the gel surface. FN was diluted into a solution of 1 m GdnHCl in 50 mm EPPS, pH 8.5, 150 mm NaCl and then cross-linked to the gels as above.
Rheometry Measurements
Rheological measurements of the gels were performed on a Physica MCR 501 rheometer (Anton Paar, Austria). Measurements were done with a parallel plate (10 mm) at 25 °C in an environmental chamber to keep the gels hydrated. The complex shear modulus was determined using an oscillatory shear strain (0.01–0.1% strain, 0.75 Hz), and the values were adjusted to account for sample thickness. The Young's modulus (E) was calculated from the measured storage modulus (G′) using E = 2G′(1 + v) assuming that Poisson's ratio of polyacrylamide is 0.48 (Table 1) (26).
TABLE 1.
Young's moduli of polyacrylamide gels
The storage moduli (G′) were measured for three polyacrylamide gel substrates using rheology, and the averages of at least two tests on separate gels are shown. The average storage moduli (G′ in pascals (Pa)) were used to calculate the corresponding Young's moduli (E in kPa). Values are the mean ± S.E. for at least two experiments.
| Gel | G′ | E |
|---|---|---|
| Pa | kPa | |
| Soft | 544 ± 17 | 1.6 ± 0.1 |
| Intermediate | 1464 ± 216 | 4.3 ± 0.6 |
| Rigid | 2511 ± 128 | 7.4 ± 0.4 |
Immunofluorescence of Matrix and Matrix Assembly Sites
For analysis of matrix, cells were plated onto FN-conjugated polyacrylamide gels in a 24-well dish at a density of 4 × 105 cells/well in complete medium. In most experiments, cells were allowed to attach and spread for 2 h after which 10 μg/ml rat plasma FN was added to the culture medium. 10 μm lysophosphatidic acid (LPA), 5 nm calyculin A, or 1 mm MnCl2 was also added at this time. After an additional 4 or 10 h, cells were washed, fixed with 4% paraformaldehyde, and stained with either IC3 rat-specific anti-FN ascites at 1:1000 dilution, R457 anti-FN antiserum at 1:100 dilution, or IC3 plus anti-collagen I antibody at 1:40 dilution in 2% ovalbumin in PBS followed by secondary antibodies. Matrix was visualized using a Nikon Eclipse Ti microscope equipped with a Hamamatsu ORCA R2 camera. Images were acquired and normalized using iVision software (BioVision Technologies). Exposures that capture matrix fibrils do not detect diffuse background staining of FN that is cross-linked to the gel surface. Fields were randomly chosen using DAPI staining to visualize cell nuclei. A similar procedure was used for analysis of matrix assembly sites except cells were plated at 5 × 104 cells/well, and after 2 h, 20 μg/ml biotinylated 70-kDa plus 10 μg/ml rat plasma FN were added. Bound 70-kDa was detected after fixation with Alexa Fluor 488-streptavidin (Invitrogen) used at 1 μg/ml. Images of bound 70-kDa were normalized using iVision software, and average intensities per pixel for matrix assembly sites were measured using ImageJ. Cells were outlined, and the area and average intensity per pixel were measured. Only cells within the area range of 450 square pixels (the smallest cell on substrates cross-linked with GdnHCl-treated FN) to 1412 square pixels (the largest cell on substrates with untreated FN) were used.
DOC Lysis and Immunoblotting
Cells were plated onto polyacrylamide gels as described for matrix immunofluorescence. Matrix was solubilized using 200 μl of DOC lysis buffer (2% DOC, 20 mm Tris-HCl, pH 8.8, 2 mm EDTA, protease inhibitor mixture (Roche Applied Science) (23). After centrifugation, the DOC-insoluble pellet was solubilized in 60 μl of 1% SDS, 20 mm Tris-HCl, pH 8.8, 2 mm EDTA, protease inhibitors. Total protein concentrations were determined for the DOC-soluble samples using a BCA assay (Pierce), and equal amounts of total DOC-soluble protein or the equivalent proportions of DOC-insoluble samples were analyzed by SDS-PAGE using 5% polyacrylamide gels and Precision Plus Protein Standards (Bio-Rad). Samples were immunoblotted with IC3 ascites diluted 1:10,000. DOC-soluble samples were immunoblotted in parallel with antibodies against GAPDH to ensure equal sample loading. Immunoblots were developed with SuperSignal West Pico chemiluminescent substrate (Pierce). Band intensities were measured with Quantity One software (Bio-Rad). Each blot was exposed for at least three different times, and the band intensities were quantified from the exposures that yielded signals within the linear range. Quantities for each band were normalized to GAPDH and then to either the 12-h soft band intensity or the 6-h rigid untreated band intensity before being averaged over multiple experiments. For FAK analysis, cells were lysed after 2 h with modified radioimmunoprecipitation assay lysis buffer (50 mm HEPES, pH 7.5, 1.5 mm MgCl2, 1% Triton X-100, 150 mm NaCl, 0.1% SDS, 1% DOC, 1 mm EGTA, 1 mm Na3VO4, 10 mm Na4P2O7, protease inhibitor mixture). Whole cell lysates were analyzed as above except that 10% polyacrylamide gels were used, and samples were immunoblotted with either anti-FAK (total) or anti-FAK (Tyr(P)-397) antibody diluted 1:1000.
RESULTS
Preparation and Characterization of Polyacrylamide Gels
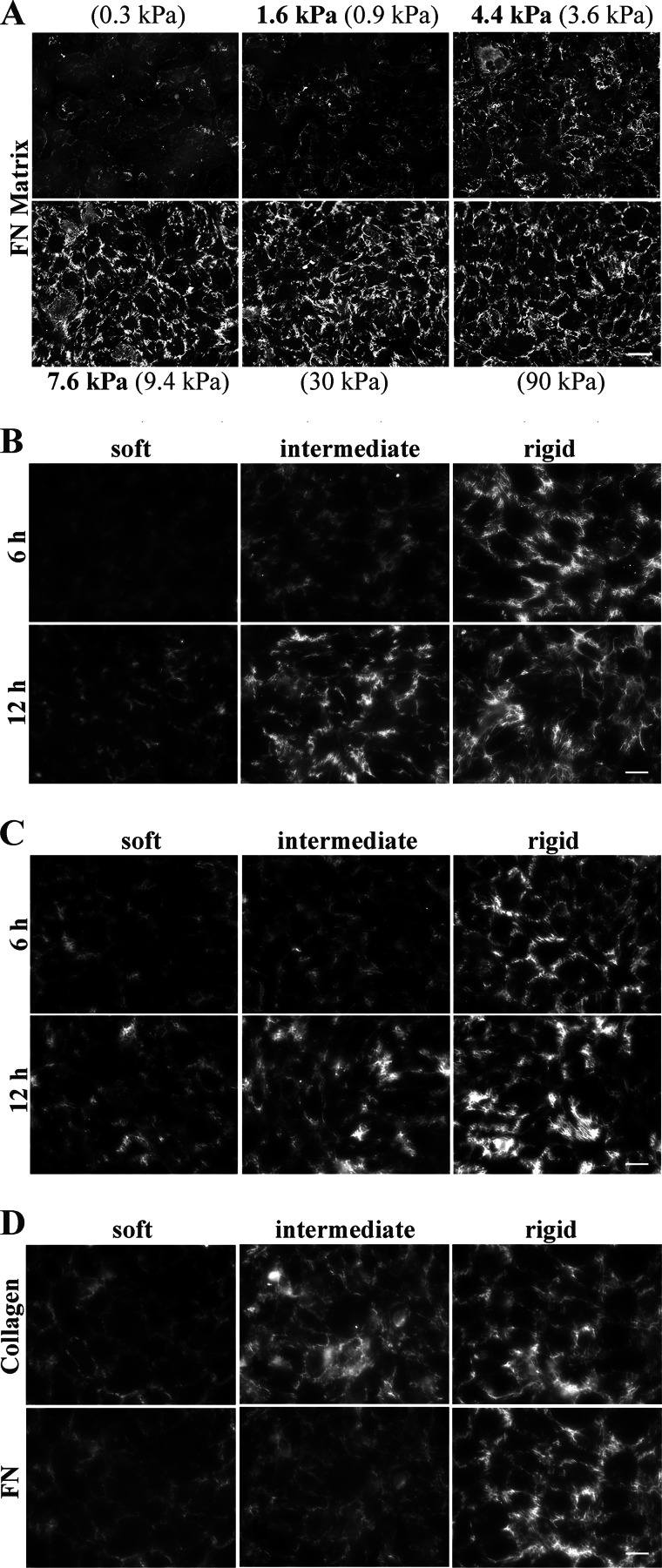
To study the effect of substrate stiffness on FN matrix assembly, we measured assembly on polyacrylamide gel substrates of varying stiffnesses. Polyacrylamide gels were prepared according to the procedure of Moshayedi et al. (24) using recipes for gels with Young's moduli (E) of 0.3, 0.9, 3.6, 9.4, 31, and 90 kPa. The Young's moduli for our preparations of the 0.9-, 3.6-, and 9.4-kPa gels calculated from rheological measurements were 1.6, 4.3, and 7.4 kPa, respectively (Table 1). Using an ELISA to detect biotinylated FN cross-linked to gel surfaces, we found that higher levels of FN were cross-linked to stiffer substrates. Therefore, FN concentrations used for cross-linking were adjusted to yield equal amounts of surface-bound FN on all gel stiffnesses (data not shown). Detection of FN matrix by immunofluorescence showed equivalent amounts of matrix on 9.4-kPa and stiffer gels and essentially no matrix on the softest 0.3-kPa gel (Fig. 1A). Gels prepared with recipes for 0.9, 3.6, and 9.4 kPa elicited different responses in matrix assembly and coincide with stiffnesses of healthy or diseased tissues, so these three stiffnesses were used for subsequent experiments. Hereafter, these substrates will be referred to as soft (E = 1.6 kPa), intermediate (E = 4.3 kPa), and rigid (E = 7.4 kPa) based on the rheological measurements in Table 1.
FIGURE 1.
FN matrix assembly is up-regulated on the rigid substrate. A, NIH 3T3 cells were plated on substrates ranging between 0.3 and 90 kPa, and at 6 h, NIH 3T3-derived FN matrix was detected by staining with anti-FN antiserum. Predicted stiffness values from Moshayedi et al. (24) are in parentheses; measured kPa values are in bold. Scale bar, 50 μm. B, NIH 3T3 cells were plated on soft, intermediate, or rigid substrates (1.6, 4.3, or 7.4 kPa, respectively) and after 6 or 12 h were fixed and stained with anti-FN antiserum to detect NIH 3T3-derived FN matrix. Scale bar, 25 μm. C, NIH 3T3 cells were allowed to spread on gels for 2 h; then rat plasma FN was added to the culture medium at 10 μg/ml; and after an additional 4 or 10 h, cells were fixed and stained with a rat-specific anti-FN antibody (IC3). Scale bar, 25 μm. D, NIH 3T3 cells were treated as in B and then fixed and stained with both anti-collagen I antibody and anti-FN IC3 antibody. Scale bar, 25 μm.
Fibronectin Matrix Assembly Is Increased on Stiffer Substrates
During FN matrix assembly, cells transform FN dimers into linear and branched arrangements of fibrils that are gradually and irreversibly converted into a mature DOC-insoluble form (27) through formation of strong protein-protein interactions (17, 28, 29). To determine whether substrate stiffness affects cell-mediated assembly of FN into a matrix, we monitored formation of FN matrix on soft, intermediate, and rigid substrates. NIH 3T3 cells were plated at a density that was sufficient to support matrix assembly; cells formed monolayers with similar cell shapes and levels of confluence on all three substrates. After 6 and 12 h, assembled matrix was examined by immunofluorescence of matrix fibrils and by quantification of stable DOC-insoluble matrix. More FN matrix fibrils were assembled on the rigid substrate (Fig. 1B, right) than on soft or intermediate substrates (Fig. 1B, left and center) at both time points. Cells on intermediate substrates also have more fibrils at 12 h than cells on the soft substrate (Fig. 1B). These results show that cells assemble FN matrix relative to the stiffness of their substrate.
Substrate stiffness might affect gene expression or protein secretion (30), which could contribute to differences in assembly; therefore, FN levels in the medium were normalized by addition of exogenous rat FN at 10 μg/ml. Assembly was analyzed at 6 and 12 h after plating. Exogenous FN in the matrix was detected with a rat FN-specific monoclonal antibody (Fig. 1C) and showed the same correlation between amount of matrix and substrate rigidity as observed for 3T3 cell-derived FN in Fig. 1B. On the rigid substrate, cells incorporated a higher level of rat FN, and fibrils between adjacent cells appear longer and denser than cells on soft and intermediate substrates at both time points (Fig. 1C). Cells on the intermediate substrate (Fig. 1C, center) also had more fibrils than cells on the soft substrate (Fig. 1C, left). In fact, very little staining is visible on the soft substrate at either time point (Fig. 1C, left). Immunofluorescence of total (3T3 plus exogenous rat) FN detected with a polyclonal anti-FN antiserum exhibited the same trend (data not shown). These results demonstrate that more FN matrix is assembled on stiffer substrates than on softer substrates. This progression is detected regardless of the source of FN or the presence or absence of exogenous FN. Thus, FN matrix assembly is enhanced by increasing rigidity of the substrate.
Type I collagen fibrils do not form in the absence of an FN matrix (20, 31, 32), and FN is believed to serve as a scaffold for collagen fibril formation (33). As shown in Fig. 1D, the level of deposition of type I collagen by NIH 3T3 cells corresponds with the amount of FN matrix and increases with substrate stiffness. Note the co-alignment of collagen and FN fibrils. Similar increases in collagen fibrils with stiffness were observed when cultures were stained with anti-type I collagen antibodies only (data not shown). Thus, as with FN assembly, collagen deposition is also enhanced on a rigid substrate. Stiffness-dependent FN matrix assembly may influence collagen deposition in vivo.
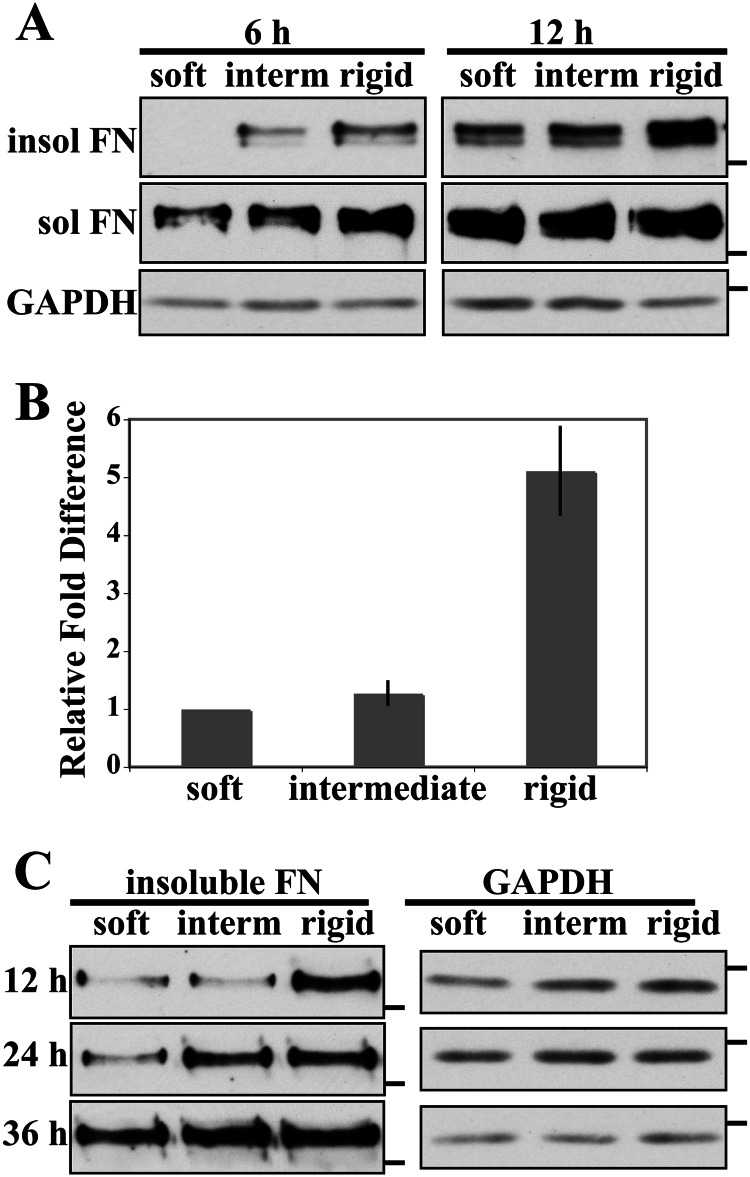
The amount of stable DOC-insoluble FN matrix was used to compare assembly on different stiffnesses. DOC-insoluble FN matrix was detectable in immunoblots of lysates from cells on the rigid substrate at 6 h and was significantly higher at 12 h (Fig. 2A). No FN was detected from cells on the soft gel, and very little was seen in lysates from cells on the intermediate stiffness at 6 h, although DOC-insoluble exogenous FN matrix was detected on the soft and intermediate substrates at 12 h (Fig. 2A). These results show that increased substrate rigidity up-regulates the assembly of stable FN matrix. DOC-soluble FN was present on all substrates at both time points, indicating that although cells are able to bind to FN, conversion of bound FN to DOC-insoluble fibrils is enhanced by substrate stiffness. Analyses of total FN in the DOC-insoluble material yielded similar results (data not shown).
FIGURE 2.
Analysis of DOC-insoluble FN matrix. A, NIH 3T3 cells were allowed to spread on gels for 2 h, then rat plasma FN was added at 10 μg/ml, and cells were lysed in DOC buffer 6 and 12 h after plating. Rat FN in DOC-soluble (sol) and DOC-insoluble (insol) fractions was detected with IC3 monoclonal antibody. The 6- and 12-h blots are matched ECL exposures except for DOC-insoluble FN for which the 6-h samples were exposed for 60 s and the 12-h samples were exposed for 45 s to prevent overexposure. B, quantification of rat FN in DOC-insoluble matrix at 12 h. The band intensities were measured in the linear range, normalized to GAPDH, and then normalized to the 12-h soft sample (mean ± S.E. (error bars), n = 3). C, NIH 3T3 cell were treated as in A, but cells were lysed 12, 24, and 36 h after plating. DOC-insoluble rat FN was detected with IC3 antibody. In A and C, molecular mass markers of 250 kDa for FN blots and 37 kDa for GAPDH blots are indicated by dashes. interm, intermediate.
DOC-insoluble FN band intensities from 12-h cell lysates were quantified as described under “Experimental Procedures.” Average -fold differences in Fig. 2B show that the level of matrix assembly on the rigid substrate is about 5-fold higher than matrix on soft and intermediate substrates. Cells on the soft and intermediate substrates assembled similar amounts of FN matrix at 12 h as determined by quantification of rat FN matrix (Fig. 2B) and total FN (not shown). Essentially no DOC-insoluble material was detected on the soft substrate, and the level on the intermediate substrate was very low. These low levels precluded determination of band intensities at the 6-h time point. These results show that substrate stiffness affects cell-mediated FN matrix assembly with rigid substrates enhancing assembly and softer substrates limiting the amount of FN matrix assembled.
To determine whether substrate stiffness inhibits or delays FN matrix assembly, we analyzed the kinetics of assembly by measuring DOC-insoluble FN over time. As before, after 12 h, more DOC-insoluble FN was present on the rigid substrate than on softer substrates (Fig. 2C). DOC-insoluble FN on the intermediate and rigid substrates appeared equivalent at 24 h. However, cells on the soft substrate required at least 36 h to accumulate DOC-insoluble FN at levels approaching those of cells on the rigid substrate. Thus, softer substrates cause a significant delay in cell-mediated FN matrix assembly, and the rate of assembly increases with stiffness.
Increased Cell Contractility Does Not Rescue FN Matrix Assembly on Soft Substrates
To investigate the mechanism responsible for the stimulation of FN matrix assembly on the rigid substrate, we first focused on cell contractility. FN matrix assembly requires an intact actin cytoskeleton (34–36), and stimulation of actin-mediated cell contractility by treatment with the phospholipid LPA, which activates Rho GTPase (37), increases FN matrix assembly by confluent cells on rigid glass or plastic substrates (38). Inhibition of cell contractility reduces FN fibril formation and FN binding to cell monolayers (35). To determine the potential involvement of cell contractility in stiffness-dependent FN matrix assembly, we examined whether stimulating contractility with LPA could induce cells on the soft substrate to assemble matrix at levels similar to those of cells on the rigid substrate. Cells plated on soft, intermediate, and rigid substrates were allowed to spread for 2 h before the addition of exogenous FN and 10 μm LPA. Six hours after plating, the cells were either fixed and stained for exogenous FN or lysed in DOC buffer for analysis of DOC-insoluble matrix by immunoblotting. Addition of LPA led to a noticeable increase in fibrils on the rigid substrate but had little if any effect on the amount of fibrils on the soft and intermediate substrates (Fig. 3A). Similar results were obtained when cells were treated with calyculin A, an inhibitor of myosin light chain phosphatase that also increases cell contractility (Fig. 3B).
FIGURE 3.
LPA and calyculin A do not increase FN matrix assembly on soft substrates. A, NIH 3T3 cells were allowed to spread on gels for 2 h at which time rat FN at 10 μg/ml and 10 μm LPA were added. At 6 h after plating, cells were fixed and stained for rat FN using IC3 antibody. Scale bar, 50 μm. B, cells were plated as in A, but 5 nm calyculin A (Caly A) was added instead of LPA. Scale bar, 50 μm. C, cells treated as in A or B were lysed after 6 h, and DOC-insoluble FN was analyzed by immunoblotting with IC3 antibody. Molecular mass markers of 250 kDa for FN blots and 37 kDa for GAPDH blots are indicated by dashes. interm, intermediate.
Analysis of DOC-insoluble FN confirmed the immunofluorescence results. Although the amount of FN in the DOC-insoluble material was increased on the rigid substrates with LPA or calyculin A treatment, no DOC-insoluble FN was detected on the soft or intermediate substrates regardless of treatment (Fig. 3C). Quantification of the relative amounts of DOC-insoluble FN revealed that cells on the rigid substrate showed on average a 3.5- and a 6.7-fold increase in DOC-insoluble FN matrix with LPA and calyculin A, respectively. These results show that stimulation of cell contractility with either of these two treatments is effective at increasing FN matrix on the rigid substrate. In contrast, modulation of Rho GTPase or myosin light chain phosphatase activity did not stimulate FN matrix assembly on a soft substrate to the levels observed on a rigid substrate. Taken together, these results indicate that the difference in FN matrix assembly between the soft and rigid substrates is not due to a deficit in cell contractility on soft substrate.
Integrin Stimulation Rescues FN Matrix Assembly on Soft Substrates
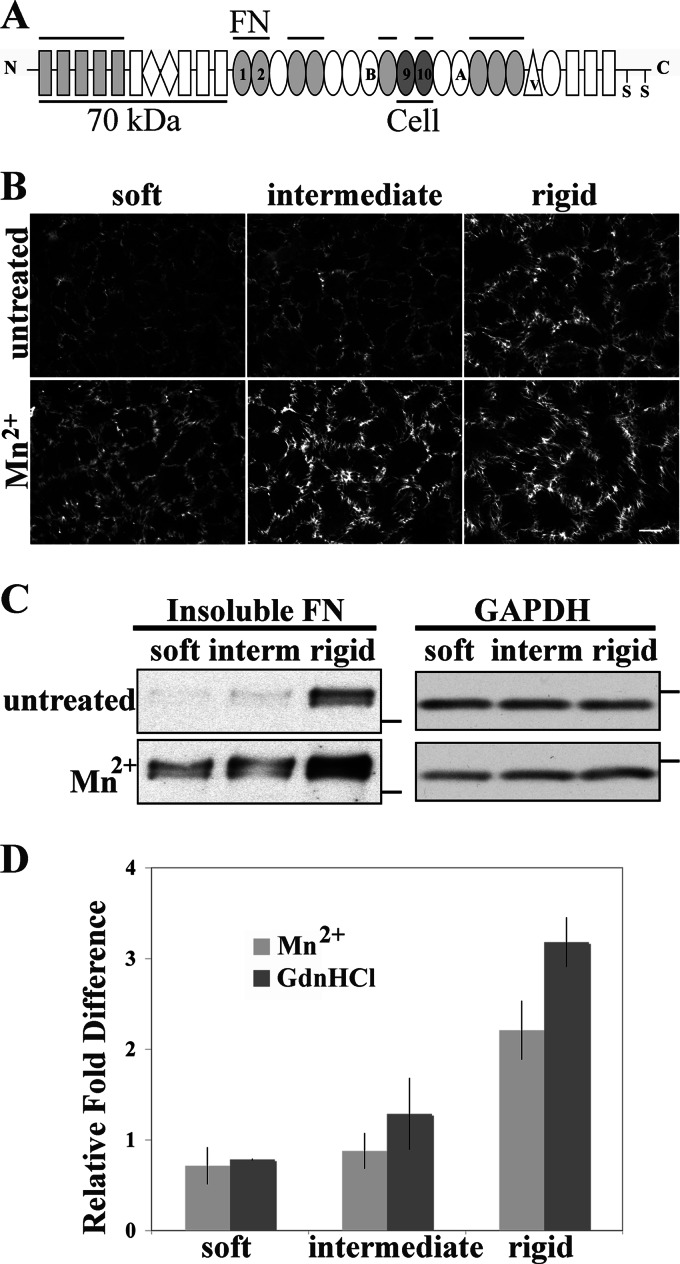
Cells assemble FN matrix primarily through the use of α5β1 integrin receptors (17). No change in α5 integrin mRNA expression was observed on our substrates (data not shown), leading us to focus on differences in integrin-FN interactions. The strength of integrin binding can be manipulated by activating the integrins with divalent cations such as Mn2+ to increase ligand binding (39–41). FN-α5β1 integrin binding depends on both the RGD cell-binding domain in module III10 and the synergy site in III9 (Fig. 4A) (42, 43). Friedland et al. (44) discovered that tensioned FN-integrin bonds require the synergy site, and the number of strong FN-α5β1 bonds correlates with the stiffness of the substrate. The strength of integrin binding to FN contributes to matrix assembly (17) because recombinant FN with a mutation at the synergy site is not assembled into matrix unless α5β1 is stimulated by treatment with Mn2+ (45). Based on this evidence, we tested how modulating integrin-FN binding affects matrix assembly by activating integrins with Mn2+ treatment. Addition of Mn2+ significantly increased the amount of FN matrix on all substrates as detected by immunostaining (Fig. 4B). Mn2+ treatment led to a noticeable increase in the apparent number and length of fibrils.
FIGURE 4.
Mn2+ treatment increases FN matrix assembly on soft substrates. A, the domain structure of an FN subunit is shown. Cell-binding modules containing synergy (in III9) and RGD (in III10) sites and the N-terminal 70 kDa fragment used to identify matrix assembly sites are labeled and underlined. FN-binding sites are indicated by overbars. B and C, NIH 3T3 cells were allowed to spread on gels for 2 h at which time 10 μg/ml rat FN and 1 mm MnCl2 were added. Cells were fixed or lysed after 6 h. B, cells were fixed and stained for rat FN. Scale bar, 25 μm. C, cells were lysed, and DOC-insoluble FN was analyzed by immunoblotting with IC3 antibody. Molecular mass markers of 250 kDa for FN blots and 37 kDa for GAPDH blots are indicated by dashes. D, band intensities from immunoblots of DOC-insoluble FN were quantified with and without Mn2+ or GdnHCl treatment. The band intensities were normalized to the signal for the untreated rigid sample. Values are the mean ± S.E. (error bars) for at least two experiments. interm, intermediate.
Treatment with Mn2+ also significantly increased the amounts of DOC-insoluble FN. No DOC-insoluble FN was detected on untreated soft or intermediate substrates, but Mn2+ treatment resulted in obvious bands similar in intensity to the signal on an untreated rigid substrate (Fig. 4C). In addition, the amount of DOC-insoluble FN on the rigid substrate became significantly higher with Mn2+ treatment. Quantification of band intensities shows that with Mn2+ treatment DOC-insoluble FN increased on soft and intermediate substrates from undetectable levels to levels similar to those of untreated cells on the rigid substrate (0.7- and 0.9-fold, respectively) (Fig. 4D). Mn2+ treatment also led to a greater than 2-fold increase in DOC-insoluble FN on rigid substrates (Fig. 4D). Therefore, integrin stimulation increases FN matrix assembly on all substrates, including softer ones, suggesting that in a soft environment integrin activity and the strength of FN-integrin binding are affected such that less matrix is assembled.
Extension of Substrate FN Stimulates Matrix Assembly on the Soft Substrate
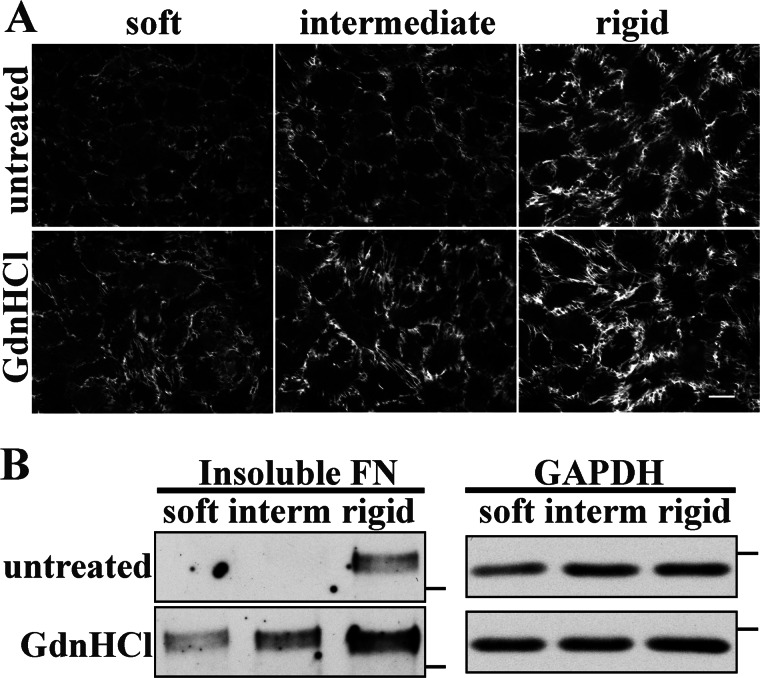
FN in solution is in a compact conformation that is maintained by intramolecular interactions (46–48). Integrin binding to this FN promotes conformational changes that extend FN dimers and expose new FN-binding sites that participate in fibril formation (17). In fact, FN molecules within fibrils have been shown to exhibit varying degrees of extension (49, 50). Additionally, FRET studies suggest that FN is more extended in fibrils assembled on a rigid substrate than on a soft substrate (51). We tested the hypothesis that substrate rigidity affects the ability of cells to induce FN conformational changes that support fibril assembly. To do this, we compared FN matrix assembly on polyacrylamide gels coated either with untreated FN (in the compact conformation) or with FN that had been partially extended by treatment with GdnHCl. It is well established that 1 m GdnHCl opens the FN dimer by disrupting intramolecular interactions that maintain its compact conformation (50, 52, 53). In the presence of GdnHCl, partially unfolded FN dimers should have more sites available for contact with the polyacrylamide gel, and cross-linking will then maintain an extended conformation in the surface-bound FN. Conformational differences in FN with and without treatment either cross-linked to soft gels or absorbed onto a glass coverslip where FN is known to assume an extended conformation (54) were confirmed by an ELISA with MAB1890 (data not shown); no differences in binding of a control antibody (5E6) were detected.
Untreated FN and GdnHCl-treated FN were cross-linked to soft, intermediate, and rigid polyacrylamide gels, and NIH 3T3 cells on these substrates were then compared for the ability to assemble an FN matrix. FN fibrils were noticeably increased and appeared longer and denser on all substrates with GdnHCl-treated FN compared with untreated FN substrates (Fig. 5A). Providing cells with an extended form of FN appears to enhance their ability to assemble FN into fibrils even on the soft substrate.
FIGURE 5.
Guanidine HCl treatment of substrate FN rescues FN matrix assembly. A, NIH 3T3 cells were plated on untreated or GdnHCl-treated FN gel substrates and allowed to spread for 2 h before the addition of 10 μg/ml rat FN. Cells were fixed and stained for rat FN after 6 h. Scale bar, 25 μm. B, DOC-insoluble rat FN was detected by immunoblotting of cell lysates treated as in A. Molecular mass markers of 250 kDa for FN blots and 37 kDa for GAPDH blots are indicated by dashes. interm, intermediate.
DOC-insoluble matrix was also significantly increased on GdnHCl-FN substrates (Fig. 5B). As in previous experiments, no DOC-insoluble FN was detected on soft and intermediate substrates. However, on soft and intermediate gels with GdnHCl-treated FN, DOC-insoluble FN was detected, and DOC-insoluble FN was visibly increased on the rigid substrate (Fig. 5B). Quantification of band intensities showed that DOC-insoluble FN on the soft and intermediate substrates with GdnHCl-FN was similar to DOC-insoluble FN on the rigid substrate with untreated FN (0.8- and 1.3-fold, respectively) (Fig. 4D). Matrix on the rigid substrate with GdnHCl-FN was also significantly higher than that from cells on the untreated rigid substrate (3.2-fold). These results show that FN matrix assembly is affected by the conformation of the FN to which cells attach regardless of substrate stiffness. Increases observed with chemical extension of FN are similar to those obtained by treating cells with Mn2+, suggesting that the effects of integrin stimulation are due to an increased ability of cells to extend substrate FN.
Formation of FN Matrix Assembly Sites and Integrin Signaling Are Affected by Stiffness
The correspondence in effects of Mn2+ and GdnHCl-FN treatments suggests that assembly steps that rely on integrin-dependent conformational changes in FN occur inefficiently on the soft substrate. FN fibril formation is initiated through intermolecular binding mediated by the N-terminal domain of FN. These early fibrils are formed at sites of cell contact with substrate FN termed FN matrix assembly sites (27, 55). If extending the conformation of substrate FN rescues FN matrix assembly on softer substrates by exposing FN-binding sites, then its effects should be detected in the earliest stages of assembly when FN fibrils are just beginning to form. The presence of FN matrix assembly sites can be detected by their association with the 70-kDa N-terminal fragment of FN (Fig. 4A) (56, 57). NIH 3T3 cells were plated on soft substrates cross-linked with either untreated FN or GdnHCl-treated FN, and they were allowed to spread for 2 h before the addition of 20 μg/ml biotinylated 70-kDa and 10 μg/ml exogenous rat FN. Cells were fixed 30 min later, and biotinylated 70-kDa was detected with fluorescently labeled streptavidin. Images of cell-bound biotinylated 70-kDa revealed punctate staining primarily near the peripheries of cells on untreated FN, whereas assembly sites that formed on GdnHCl-treated FN were larger and were found under the cell bodies as well as at cell edges (Fig. 6A). Assembly sites were quantified from the images of cells on soft substrates with GdnHCl-treated or untreated FN. The average intensity per cell area was measured for each cell, revealing that the overall intensity of matrix assembly sites formed by cells on GdnHCl-treated FN substrates was almost 2-fold that of assembly sites on cells plated on untreated FN substrates (Fig. 6B). These results demonstrate that the initial steps of FN matrix assembly on a soft substrate are influenced by the conformation of the substrate FN and that FN matrix assembly can be up-regulated on the soft substrate by manipulating the conformation of the FN cross-linked to it.
FIGURE 6.
Enhanced formation of FN matrix assembly sites and FAK activation with GdnHCl-FN. A, NIH 3T3 cells were plated on soft substrates coupled with untreated FN or GdnHCl-treated FN and allowed to spread for 2 h before the addition of 20 μg/ml biotinylated 70-kDa and 10 μg/ml rat FN. Cells were fixed and probed with fluorescently labeled streptavidin 30 min later. Representative images are shown. Scale bars, 10 μm. B, fluorescence signal intensity of FN matrix assembly sites was measured for 17 cells in each condition, and the average intensity per pixel was calculated. Error bars represent S.E. C, NIH 3T3 cells were plated on gels with untreated FN or GdnHCl-FN and lysed in modified radioimmunoprecipitation assay buffer after 2 h. Total FAK and FAK phosphorylated on Tyr-397 were measured by immunoblotting. Blots are representative of two independent experiments.
FN binding to α5β1 integrin initiates intracellular signaling through activation of FAK (58). FAK is a critical regulator of FN matrix assembly as FAK-null cells do not assemble an FN matrix (59). We found that phosphorylation on FAK Tyr-397 was significantly higher in cells on a rigid substrate after 2 h compared with those on a soft substrate. Importantly, FAK phosphorylation was also stimulated on GdnHCl-treated FN soft substrates to a similar degree as on the rigid substrate (Fig. 6C). This finding shows that GdnHCl-induced unfolding of FN promotes integrin signaling independently of substrate stiffness and indicates that exposure of FN-binding sites is the key step that is affected in stiffness-dependent FN matrix assembly.
DISCUSSION
Mechanical properties of the pericellular environment affect many important cell processes, including cell signaling, cytoskeletal function, and gene expression (60). Here we show using polyacrylamide substrates of different stiffnesses that the rigidity of the cellular environment regulates ECM assembly. Cells assemble a greater amount of stable FN matrix on a rigid substrate than on softer substrates where assembly is delayed considerably. Increasing cell contractility was not sufficient to rescue FN matrix assembly on a soft substrate. However, stimulation of integrin activity or unfolding of substrate FN significantly increased FN matrix assembly on softer substrates. Because FN conformational changes are downstream of integrin binding, our results indicate that limitations in integrin-FN binding strength are bypassed by extension of substrate-bound FN dimers. Furthermore, both FAK phosphorylation and formation of matrix assembly sites were enhanced on the soft substrate by an extended FN conformation, demonstrating that substrate stiffness exerts its effects during the initial steps of FN assembly.
Changing the conformation of substrate FN resulted in an increase in FN matrix assembly on softer substrates with levels equivalent to those on rigid gels with untreated FN. Soluble FN is kept in a compact conformation through electrostatic interactions between the III2–3 and III12–14 domains and possibly through other intramolecular interactions (46–48). GdnHCl disrupts these interactions, extending the FN dimer and exposing cryptic FN-binding sites. In particular, FN-binding sites in the III1–2 domain are exposed by denaturation or stretch (35, 53, 61). Conformational changes provide sites for association with other FN molecules via the N-terminal matrix assembly domain of FN and in this way initiate fibril formation. Our results indicate that substrate stiffness affects the ability of cells to induce unfolding of FN dimers, thereby limiting the exposure of FN-binding sites and reducing FN matrix assembly.
Stimulating integrins with Mn2+ induced assembly on soft and intermediate substrates to levels similar to that of matrix on the rigid untreated substrate or on a soft GdnHCl-FN substrate, indicating that integrin binding is impaired on soft substrate. Friedland et al. (44) demonstrated that the total number of FN-α5β1 bonds is independent of substrate stiffness, but the number of “tensioned” FN-α5β1 bonds increases with substrate stiffness. In addition, they determined that formation of tensioned bonds is dependent on the synergy site in FN (44). Mn2+ activation of integrins has been shown to compensate for weak integrin-FN binding in the absence of a synergy site (62) and to promote assembly of a recombinant FN that lacks the synergy site (40). These data explain why cells are able to assemble FN matrix on a rigid substrate independently of Mn2+ treatment and why they require integrin activation for assembly on soft substrate. Cells on rigid substrates have a sufficient number of strong FN-integrin bonds, which would allow them to apply enough force to unfold FN and expose FN-binding sites. However, cells on a soft substrate bind FN weakly, and Mn2+ treatment would strengthen these bonds and enable them to cause FN conformational changes.
Given the relationship between integrin binding and FN conformational changes during assembly, we propose that the two treatments that stimulate assembly in a stiffness-dependent manner are correcting the same deficiency. It has been shown that the integrin-FN binding strength is lower on soft substrates compared with rigid substrates (44). Mn2+ activation of integrins enhances FN-integrin binding (39, 40) and likely induces sufficient FN-integrin engagement to initiate FN unfolding. Cross-linking of GdnHCl-FN to the polyacrylamide gel surface would directly affect FN-FN interactions by providing a substrate with FN in a conformation that displays previously buried FN-binding sites. GdnHCl treatment unmasks FN-binding sites without the need to unfold the FN dimers using strong integrin bonds. FAK activation correlates with integrin bond strength with strong FN-integrin bonds resulting in an increase in downstream FAK phosphorylation (44). Our demonstration that FAK phosphorylation is stimulated when cells contact GdnHCl-FN on a soft gel similarly to cells on a rigid substrate suggests a mechanism whereby cells can sense and respond to the stiffness of their environment by regulating integrin binding and signaling activity. Thus, our results show that extension of FN with GdnHCl circumnavigates the need for integrins to forcefully unfold FN, allowing cells to form fibrils and activate intracellular signaling through a mechanism that is independent of substrate stiffness.
Stimulation of contractility has been shown to increase matrix assembly on stiff substrates (35, 38, 63) and in our experiments led to a considerable increase in FN matrix on the rigid substrate. However, neither activation of Rho nor inhibition of myosin light chain phosphatase was sufficient to promote assembly on a soft gel, indicating that decreased cell contractility is not the primary reason for down-regulation of FN matrix assembly. Therefore, stimulating intracellular pathways to cell contractility is not sufficient to rescue assembly in the absence of essential extracellular events.
Several intracellular proteins involved in mechanotransduction have been shown to become mechanically extended in response to force, revealing cryptic interaction sites that mediate their activity (64–66). Similar to these intracellular proteins, the correlation between substrate stiffness and FN conformational changes may allow cells to use FN itself to probe the stiffness of the environment. Cells sense the stiffness of their environment by pulling on their substrate and feeling the resistance (67). FN in a rigid environment might be more extensible than in a soft environment, providing an FN-dependent environmental signal to cells. Because FN conformation can differ with substrate stiffness, cells might be able to detect differences in stiffness through their varied ability to expose and interact with certain FN domains. In this way, FN conformation itself can act as a microenvironment mechanosensor.
Tissues become progressively more rigid during pathological progression. For example, stiff ECM has been shown to promote tumor progression by breast cancer cells (10, 68), and FN contributes to development of a tumorigenic phenotype by mammary epithelial cells (30). In addition, progression of liver fibrosis has been found to correlate with a progressive increase in liver stiffness (11, 69), and interestingly, an increase in liver stiffness has been shown to precede the pathological deposition of ECM proteins (70). Deposition of other ECM proteins such as collagen (20) depends on FN matrix. This could make FN a master regulator of stiffness-dependent ECM accumulation. This idea is supported by our data that show increased collagen with up-regulation of FN matrix. Perhaps stiffness-dependent FN matrix assembly creates a pathological feedback loop whereby FN matrix promotes collagen deposition, which then increases tissue stiffness and in turn up-regulates FN matrix assembly. Our finding that an FN conformational change is the terminal event in regulation of matrix assembly by stiffness implicates FN in a mechanosensing mechanism that may control disease progression.
Acknowledgments
We thank Adam Engler and Purva Singh for valuable technical assistance and Robert Prud'homme and Nathalie Pinkerton for help with rheometry.
This work was supported, in whole or in part, by National Institutes of Health Grants GM059383, CA160611, and 5T32GM007388, a predoctoral training grant from the NIGMS.
- ECM
- extracellular matrix
- FN
- fibronectin
- FAK
- focal adhesion kinase
- DOC
- deoxycholate
- GdnHCl
- guanidine hydrochloride
- LPA
- lysophosphatidic acid
- kPa
- kilopascals
- EPPS
- 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid.
REFERENCES
- 1. Hynes R. O., Yamada K. M. (eds) (2011) Extracellular Matrix Biology, pp. 1–16, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2. Wang H. B., Dembo M., Wang Y. L. (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 279, C1345–C1350 [DOI] [PubMed] [Google Scholar]
- 3. Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P. A. (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 [DOI] [PubMed] [Google Scholar]
- 4. Pelham R. J., Jr., Wang Y. (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U.S.A. 94, 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isenberg B. C., Dimilla P. A., Walker M., Kim S., Wong J. Y. (2009) Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 97, 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engler A. J., Sweeney H. L., Discher D. E., Schwarzbauer J. E. (2007) Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal Interact. 7, 335. [PubMed] [Google Scholar]
- 7. Engler A. J., Griffin M. A., Sen S., Bönnemann C. G., Sweeney H. L., Discher D. E. (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 [DOI] [PubMed] [Google Scholar]
- 9. Mammoto T., Ingber D. E. (2010) Mechanical control of tissue and organ development. Development 137, 1407–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paszek M. J., Zahir N., Johnson K. R., Lakins J. N., Rozenberg G. I., Gefen A., Reinhart-King C. A., Margulies S. S., Dembo M., Boettiger D., Hammer D. A., Weaver V. M. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 [DOI] [PubMed] [Google Scholar]
- 11. Yin M., Talwalkar J. A., Glaser K. J., Manduca A., Grimm R. C., Rossman P. J., Fidler J. L., Ehman R. L. (2007) Assessment of hepatic fibrosis with magnetic resonance elastography. Clin. Gastroenterol. Hepatol. 5, 1207–1213.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto T., Abe H., Ohashi T., Kato Y., Sato M. (2002) Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol. Meas. 23, 635–648 [DOI] [PubMed] [Google Scholar]
- 13. Samani A., Bishop J., Luginbuhl C., Plewes D. B. (2003) Measuring the elastic modulus of ex vivo small tissue samples. Phys. Med. Biol. 48, 2183–2198 [DOI] [PubMed] [Google Scholar]
- 14. Gefen A., Dilmoney B. (2007) Mechanics of the normal woman's breast. Technol. Health Care 15, 259–271 [PubMed] [Google Scholar]
- 15. Muro A. F., Moretti F. A., Moore B. B., Yan M., Atrasz R. G., Wilke C. A., Flaherty K. R., Martinez F. J., Tsui J. L., Sheppard D., Baralle F. E., Toews G. B., White E. S. (2008) An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177, 638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kischer C. W., Hendrix M. J. (1983) Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res. 231, 29–37 [DOI] [PubMed] [Google Scholar]
- 17. Singh P., Carraher C., Schwarzbauer J. E. (2010) Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26, 397–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halliday N. L., Tomasek J. J. (1995) Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 217, 109–117 [DOI] [PubMed] [Google Scholar]
- 19. Schwarzbauer J. E., DeSimone D. W. (2011) Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sottile J., Shi F., Rublyevska I., Chiang H. Y., Lust J., Chandler J. (2007) Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am. J. Physiol. Cell Physiol. 293, C1934–C1946 [DOI] [PubMed] [Google Scholar]
- 21. Wilson C. L., Schwarzbauer J. E. (1992) The alternatively spliced V region contributes to the differential incorporation of plasma and cellular fibronectins into fibrin clots. J. Cell Biol. 119, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguirre K. M., McCormick R. J., Schwarzbauer J. E. (1994) Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J. Biol. Chem. 269, 27863–27868 [PubMed] [Google Scholar]
- 23. Sechler J. L., Takada Y., Schwarzbauer J. E. (1996) Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J. Cell Biol. 134, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moshayedi P., Costa, Lda F., Christ A., Lacour S. P., Fawcett J., Guck J., Franze K. (2010) Mechanosensitivity of astrocytes on optimized polyacrylamide gels analyzed by quantitative morphometry. J. Phys. Condens. Matter 22, 194114. [DOI] [PubMed] [Google Scholar]
- 25. Guo H. B., Lee I., Kamar M., Pierce M. (2003) N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell-cell adhesion and intracellular signaling pathways. J. Biol. Chem. 278, 52412–52424 [DOI] [PubMed] [Google Scholar]
- 26. Boudou T., Ohayon J., Picart C., Tracqui P. (2006) An extended relationship for the characterization of Young's modulus and Poisson's ratio of tunable polyacrylamide gels. Biorheology 43, 721–728 [PubMed] [Google Scholar]
- 27. McKeown-Longo P. J., Mosher D. F. (1983) Binding of plasma fibronectin to cell layers of human skin fibroblasts. J. Cell Biol. 97, 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen H., Mosher D. F. (1996) Formation of sodium dodecyl sulfate-stable fibronectin multimers. Failure to detect products of thiol-disulfide exchange in cyanogen bromide or limited acid digests of stabilized matrix fibronectin. J. Biol. Chem. 271, 9084–9089 [DOI] [PubMed] [Google Scholar]
- 29. Ohashi T., Erickson H. P. (2009) Revisiting the mystery of fibronectin multimers: the fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol. 28, 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams C. M., Engler A. J., Slone R. D., Galante L. L., Schwarzbauer J. E. (2008) Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 68, 3185–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Velling T., Risteli J., Wennerberg K., Mosher D. F., Johansson S. (2002) Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins α11β1 and α2β1. J. Biol. Chem. 277, 37377–37381 [DOI] [PubMed] [Google Scholar]
- 32. Li S., Van Den Diepstraten C., D'Souza S. J., Chan B. M., Pickering J. G. (2003) Vascular smooth muscle cells orchestrate the assembly of type I collagen via α2β1 integrin, RhoA, and fibronectin polymerization. Am. J. Pathol. 163, 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kadler K. E., Hill A., Canty-Laird E. G. (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 20, 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu C., Keivens V. M., O'Toole T. E., McDonald J. A., Ginsberg M. H. (1995) Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 83, 715–724 [DOI] [PubMed] [Google Scholar]
- 35. Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A. M., Burridge K. (1998) Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hynes R. O. (1990) Fibronectins, Springer-Verlag, New York, pp. 231–248 [Google Scholar]
- 37. Hall A. (2005) Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33, 891–895 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Q., Checovich W. J., Peters D. M., Albrecht R. M., Mosher D. F. (1994) Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J. Cell Biol. 127, 1447–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gailit J., Ruoslahti E. (1988) Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 263, 12927–12932 [PubMed] [Google Scholar]
- 40. Mould A. P., Akiyama S. K., Humphries M. J. (1995) Regulation of integrin α5β1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+. J. Biol. Chem. 270, 26270–26277 [DOI] [PubMed] [Google Scholar]
- 41. Elices M. J., Urry L. A., Hemler M. E. (1991) Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J. Cell Biol. 112, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aota S., Nomizu M., Yamada K. M. (1994) The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269, 24756–24761 [PubMed] [Google Scholar]
- 43. García A. J., Schwarzbauer J. E., Boettiger D. (2002) Distinct activation states of α5β1 integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry 41, 9063–9069 [DOI] [PubMed] [Google Scholar]
- 44. Friedland J. C., Lee M. H., Boettiger D. (2009) Mechanically activated integrin switch controls α5β1 function. Science 323, 642–644 [DOI] [PubMed] [Google Scholar]
- 45. Sechler J. L., Corbett S. A., Schwarzbauer J. E. (1997) Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol. Biol. Cell 8, 2563–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson K. J., Sage H., Briscoe G., Erickson H. P. (1999) The compact conformation of fibronectin is determined by intramolecular ionic interactions. J. Biol. Chem. 274, 15473–15479 [DOI] [PubMed] [Google Scholar]
- 47. Vakonakis I., Staunton D., Ellis I. R., Sarkies P., Flanagan A., Schor A. M., Schor S. L., Campbell I. D. (2009) Motogenic sites in human fibronectin are masked by long range interactions. J. Biol. Chem. 284, 15668–15675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maurer L. M., Ma W., Eickstaedt N. L., Johnson I. A., Tomasini-Johansson B. R., Annis D. S., Mosher D. F. (2012) Ligation of the fibrin-binding domain by β-strand addition is sufficient for expansion of soluble fibronectin. J. Biol. Chem. 287, 13303–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baneyx G., Baugh L., Vogel V. (2001) Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U.S.A. 98, 14464–14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baneyx G., Baugh L., Vogel V. (2002) Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc. Natl. Acad. Sci. U.S.A. 99, 5139–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antia M., Baneyx G., Kubow K. E., Vogel V. (2008) Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 139, 229–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan M. Y., Medow M. S., Newman S. A. (1990) Unfolding transitions of fibronectin and its domains. Stabilization and structural alteration of the N-terminal domain by heparin. Biochem. J. 270, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karuri N. W., Lin Z., Rye H. S., Schwarzbauer J. E. (2009) Probing the conformation of the fibronectin III1–2 domain by fluorescence resonance energy transfer. J. Biol. Chem. 284, 3445–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baugh L., Vogel V. (2004) Structural changes of fibronectin adsorbed to model surfaces probed by fluorescence resonance energy transfer. J. Biomed. Mater. Res. A 69, 525–534 [DOI] [PubMed] [Google Scholar]
- 55. Christopher R. A., Kowalczyk A. P., McKeown-Longo P. J. (1997) Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J. Cell Sci. 110, 569–581 [DOI] [PubMed] [Google Scholar]
- 56. McKeown-Longo P. J., Mosher D. F. (1985) Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 100, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wierzbicka-Patynowski I., Schwarzbauer J. E. (2002) Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J. Biol. Chem. 277, 19703–19708 [DOI] [PubMed] [Google Scholar]
- 58. Schaller M. D. (2001) Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 1540, 1–21 [DOI] [PubMed] [Google Scholar]
- 59. Ilić D., Kovacic B., Johkura K., Schlaepfer D. D., Tomasević N., Han Q., Kim J. B., Howerton K., Baumbusch C., Ogiwara N., Streblow D. N., Nelson J. A., Dazin P., Shino Y., Sasaki K., Damsky C. H. (2004) FAK promotes organization of fibronectin matrix and fibrillar adhesions. J. Cell Sci. 117, 177–187 [DOI] [PubMed] [Google Scholar]
- 60. Hoffman B. D., Grashoff C., Schwartz M. A. (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hocking D. C., Sottile J., McKeown-Longo P. J. (1994) Fibronectins III-1 module contains a conformation-dependent binding-site for the amino-terminal region of fibronectin. J. Biol. Chem. 269, 19183–19187 [PubMed] [Google Scholar]
- 62. Danen E. H., Aota S., van Kraats A. A., Yamada K. M., Ruiter D. J., van Muijen G. N. (1995) Requirement for the synergy site for cell adhesion to fibronectin depends on the activation state of integrin α5β1. J. Biol. Chem. 270, 21612–21618 [DOI] [PubMed] [Google Scholar]
- 63. Zhang Q., Magnusson M. K., Mosher D. F. (1997) Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol. Biol. Cell 8, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. del Rio A., Perez-Jimenez R., Liu R., Roca-Cusachs P., Fernandez J. M., Sheetz M. P. (2009) Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006) Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Golji J., Mofrad M. R. (2010) A molecular dynamics investigation of vinculin activation. Biophys. J. 99, 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Discher D. E., Janmey P., Wang Y. L. (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 [DOI] [PubMed] [Google Scholar]
- 68. Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D. L., Weaver V. M. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yeh W. C., Li P. C., Jeng Y. M., Hsu H. C., Kuo P. L., Li M. L., Yang P. M., Lee P. H. (2002) Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 28, 467–474 [DOI] [PubMed] [Google Scholar]
- 70. Georges P. C., Hui J. J., Gombos Z., McCormick M. E., Wang A. Y., Uemura M., Mick R., Janmey P. A., Furth E. E., Wells R. G. (2007) Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1147–G1154 [DOI] [PubMed] [Google Scholar]