Background: Kindlin 3 is mutated in LAD-III causing widespread infection, but its role in leukocyte function is incompletely understood.
Results: Deletion of the PH domain removes kindlin 3 from lymphocyte cell membrane and eliminates adhesion and migration.
Conclusion: The PH domain is intrinsic to kindlin 3 regulation of integrin LFA-1-mediated lymphocyte function.
Significance: A successful immune response depends on kindlin 3, and its PH subdomain is crucial.
Keywords: Adhesion, Cell Migration, Cell Signaling, Integrin, Lymphocyte, Receptors, PH Domain, Kindlin
Abstract
The protein kindlin 3 is mutated in the leukocyte adhesion deficiency III (LAD-III) disorder, leading to widespread infection due to the failure of leukocytes to migrate into infected tissue sites. To gain understanding of how kindlin 3 controls leukocyte function, we have focused on its pleckstrin homology (PH) domain and find that deletion of this domain eliminates the ability of kindlin 3 to participate in adhesion and migration of B cells mediated by the leukocyte integrin lymphocyte function-associated antigen 1 (LFA-1). PH domains are often involved in membrane localization of proteins through binding to phosphoinositides. We show that the kindlin 3 PH domain has binding affinity for phosphoinositide PI(3,4,5)P3 over PI(4,5)P2. It has a major role in membrane association of kindlin 3 that is enhanced by the binding of LFA-1 to intercellular adhesion molecule 1 (ICAM-1). A splice variant, kindlin 3-IPRR, has a four-residue insert in the PH domain at a critical site that influences phosphoinositide binding by enhancing binding to PI(4,5)P2 as well as by binding to PI(3,4,5)P3. However kindlin 3-IPRR is unable to restore the ability of LAD-III B cells to adhere to and migrate on LFA-1 ligand ICAM-1, potentially by altering the dynamics or PI specificity of binding to the membrane. Thus, the correct functioning of the kindlin 3 PH domain is central to the role that kindlin 3 performs in guiding lymphocyte adhesion and motility behavior, which in turn is required for a successful immune response.
Introduction
When infection or injury occurs, leukocytes leave the circulation and migrate into tissues using integrins such as LFA-12 (CD11a/CD18, αLβ2) (1–3). The integrins on circulating leukocytes bind only minimally to their ligands but become active following signaling through other membrane receptors and exposure to the shear force of blood flow. An essential component of this signaling pathway on leukocytes and platelets is kindlin 3, recently identified as the lesion in leukocyte adhesion deficiency III (LAD-III) patients (4, 5). To date, 10 different mutations have been identified in the KINDLIN3 gene (also known as FERMT3, URP2) in patients of diverse ethnicity but with consanguineous backgrounds (5–10). Deficient integrin function in the LAD-III disorder causes bleeding and widespread infection, both life-threatening conditions that are replicated in kindlin3−/− mice (11, 12).
The kindlin family comprises three members, kindlin 1, 2, and 3, each consisting of a FERM (four-point-one, ezrin, radixin, moesin) domain with four subdomains F0, F1, F2, and F3 that are highly homologous to the FERM head (H) domain of the cytoskeletal protein talin (13–16). Both the kindlins and the talin H domain bind the β subunit of integrin through their F3 subdomains, with kindlins showing specificity for the membrane distil NXXY site and talin, the membrane proximal NPXY site (11, 17). The talin H domain binding causes separation of α and β integrin subunits and a critical conformational shift associated with high affinity integrin (18–20). Kindlins optimize this interaction in a manner not yet fully understood (11, 14, 17).
There is increasing evidence that the other FERM subdomains of both talin and kindlin have importance in integrin activation. The talin H domain is positioned at the cell membrane via polybasic residues in the F1 loop and the F2 and F3 subdomains (21–23). These positively charged “patches” are aligned along one surface of the H domain, allowing interaction with membrane phosphoinositides (PI). Such extended contact optimally angles the talin H F3 subdomain for interaction with the β subunit favoring integrin αβ subunit separation. Disruption of these sites diminishes integrin activation and clustering, cell spreading, and assembly into focal adhesions (21–24). In terms of the kindlins, PI binding sites have been defined so far in F0 for kindlin 2 (25) and in the F1 loop for kindlins 1 and 2 (26). Deletion or mutation of these sites prevents cooperation with talin, integrin activation, and entry into focal adhesions (25–27). Thus the kindlins, like talin, must be membrane-associated to function.
The presence of the pleckstrin homology (PH) domain in the F2 subdomain of the kindlin family represents a major difference with the talin FERM domain. PH domains often act as membrane anchors through recognition of PI sites (28, 29). Crystal and NMR structures of kindlin 2 and kindlin 3 PH domains show them to be typical PH domain folds with an extra α helix (Protein Data Bank ID code 2YS3) (30, 31). The kindlin 2 PH domain binds several PIs but optimally binds phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) (30, 33).
In this study we have examined the role of kindlin 3 PH domain in the activity of the leukocyte integrin LFA-1 on B cells. We find that it binds PI(3,4,5)P3 and contributes to the association of kindlin 3 with the B cell membrane. An intact PH domain is required for LFA-1-mediated adhesion and migration on its ligand ICAM-1.
EXPERIMENTAL PROCEDURES
Antibodies and Other Reagents
Horseradish peroxidase-coupled anti-GFP mAb was purchased from Miltenyi Biotech. Full-length ICAM-1Fc protein was prepared as described previously (10). Pan-LFA-1 mAb 38 was prepared at CRUK LRI (34).
Generation of Human KINDLIN3 cDNAs
All human KINDLIN3 cDNAs were subcloned into vector pEGFP-N1 (Clontech) as reported previously (6). The following KINDLIN3 cDNA constructs were used in this study: wild type (NM_178443) (6); splice variant with IPRR insertion in the PH domain at residue 360 (NM_031471); PH domain-deleted (deletion of residues 344–455); isolated FERM domain 2 containing the PH domain (incorporating residues 265–550). The mutant cDNAs were made from wild type KINDLIN3-GFP using QuikChange Site-directed Mutagenesis Kit (Stratagene) as described previously (6). All cDNA constructs were verified by sequencing.
B Cell Transfection
Epstein-Barr virus (EBV)-transformed B lymphoblastoid cells (B cells) were derived from the LAD-III patients as reported previously (6) and were maintained in RPMI 1640 medium with 10% FCS. The B cells were washed in OptiMEM + GlutaMAX (Invitrogen), and electroporation was performed using 2 × 107 cells with 10 μg/reaction of KINDLIN3-GFP cDNA constructs or 2 μg of pEGFP-N1 (BD Biosciences). A Gene Pulser with Capacitance Extender (Bio-Rad) set at 960 microfarads and 260 mV was used for the electroporation. Transfected cells were maintained in RPMI 1640 medium/10% FCS overnight. The efficiency of transfection was evaluated the following day by flow cytometry, and the GFP-positive cells were sorted using a MoFlo Cell Sorter (Beckman Coulter). Sorted cells were left to recover in RPMI 1640 medium/10% FCS overnight. Cells expressing equivalent levels of GFP were compared in adhesion, migration, and total internal reflection fluorescence (TIRF) assays.
Phosphoinositide Binding Assay
IP3- and IP4-coated agarose beads and control beads (PIP beadsTM; Echelon) were blocked with 5% fatty-acid free BSA in PBS overnight at 4 °C. HEK293 cells were transfected with the GFP cDNA constructs using calcium phosphate. The cells were lysed on ice in 750 mm aminocaproic acid, 50 mm Bis-Tris, and 0.5 mm EDTA (pH 7.0) 48 h later, and soluble cellular fractions were generated by three cycles of freezing on dry ice and thawing at 4 °C. The supernatant was collected following centrifugation at 20,000 × g for 20 min at 4 °C, diluted in binding buffer (10 mm HEPES, 150 mm NaCl, and 0.25% Nonidet P-40) and allowed to bind to the preblocked beads for 2 h at 4 °C (input protein). The beads were washed four times with binding buffer, and bound protein was eluted with 2× Laemmli sample buffer at 95 °C for 5 min. These eluted samples (output), together with a sample of the input protein, were analyzed by Western blotting.
Migration Assay
μ-Slides VI (ibidi; Thistle Scientific) were coated overnight at 4 °C with 50 μl of 3 μg/ml ICAM-1Fc then blocked with 2% BSA. FACS-sorted EBV-transformed B cells (1 × 106/ml in Hanks' balanced salt solution/20 mm HEPES buffer) were allowed to settle on immobilized ICAM-1 for 10 min at 37 °C. Images were taken at 15-s intervals over 20 min with a Diaphot 300 microscope (Nikon Instruments), using a 20× lens and AQM2001 Kinetic Acquisition Manager software (Kinetic Imaging, Andor Technology PLC, Belfast, Northern Ireland). Cells were tracked using Motion Analysis software (Kinetic Imaging) and the data analyzed using a Mathematica notebook (Wolfram Research Inc., Champaign, IL) developed by Daniel Zicha (Cancer Research UK).
Interference Reflection Microscopy (IRM)
FACS-sorted EBV-transformed B cells were plated on ibiTreat μ-Slides coated with ICAM-1 as described above. Images of close substrate contact of the migrating cells were acquired between 10 and 30 min of ICAM-1 attachment at 37 °C in HBSS/20 mm HEPES using a Zeiss LSM510 Axioplan2 Inverted confocal microscope with a 63× NA1.4 Plan-Apochromat oil immersion objective lens. For evaluation of adhesion status, the area of contact was measured using ImageJ software.
TIRF
Glass-bottomed MatTek dishes (MatTek Corp, Ashland, MA) were precoated with 200 μl of ICAM-1Fc as above or 0.01% poly-l-lysine and blocked with 2% BSA. FACS-sorted B cells (2 × 105 cells/sample in HBSS/20 mm HEPES buffer plus 5 mm MgCl2/1 mm EGTA) were allowed to settle on the ICAM-1-coated dishes for 20 min at 37 °C. In some experiments the cells were pretreated with phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (Merck-Millipore) (5 μm) for 1 h. Adherent cells were fixed with fresh 3% paraformaldehyde in PBS for 10 min at room temperature and then blocked in 5% FCS + 1% BSA in PBS for 1 h. The cells were then incubated with anti-LFA-1 mAb 38 overnight at 4 °C, followed by Alexa Fluor 546-goat anti-mouse IgG (Invitrogen) for 1 h at room temperature.
TIRF images were acquired using a TIRF microscope system (Cell R; Olympus) based on an inverted microscope (IX 81, Olympus), TIRF illuminator with 488-nm and 561-nm lasers (Olympus), an objective (UAPO 150× TIRFM, NA 1.45; Olympus), and a sensitive EMCCD camera (iXon3 897; Andor) using Xcellence software (Cascadell; Photometrics). The mean fluorescence intensity/cell for GFP and the area of LFA-1 attachment to ICAM-1 were determined using ImageJ software.
Statistical Analysis
The phosphoinositide binding data were analyzed by two-way ANOVA with Sidak's multiple comparisons test or for broader comparisons, Tukey's multiple comparisons test; the IRM and migration data were analyzed by one-way ANOVA with Dunnett's multiple comparisons test; the TIRF data were analyzed using the Kruskal-Wallis test with Dunn's multiple comparison test or two-way ANOVA with Sidak's multiple comparison test. All analyses were performed using GraphPad Prism software. Significant differences are as indicated: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
RESULTS
The Kindlin 3 PH Domain Binds PI(3,4,5)P3
The solved structure of kindlin 3 PH domain shows it to be a typical 7-stranded anti-parallel β barrel that differs from the kindlin 2 PH domain by the presence of “split” β1 strand (β1.1 and β1.2) (Fig. 1A) (Protein Data Bank ID code 2YS3) (30, 31). This leads into the canonical positively charged PI binding site located on the β1 and β2 loop with secondary involvement of β3-β4 as is typical for PH domains (Fig. 1, A, inset, and B) (10, 30, 35). A naturally occurring longer splice variant of kindlin 3 exists which differs from the standard form by the addition of four residues Ile-Pro-Arg-Arg (IPRR) in the PH domain (kindlin 3-IPRR) (Fig. 1, A and B). The IPRR sequence is inserted close to the junction between the β1 strand and the β1-β2 loop with the implication that it might affect PI binding.
FIGURE 1.
Diagrammatic representation of kindlin 3 PH domain and kindlin 3-GFP constructs showing the FERM subdomains (residues 1–663). A, model of the kindlin 3 PH domain showing details of the β1-β2 loop and location of the IPRR insert at the junction of the β1 strand and β1-β2 loop. Inset, expansion of the β1-β2 loop indicating the amino acids that comprise the loop and illustrating the predominance of positively charged residues with IP4 interactive residues as in kindlin 2 highlighted in red (30). B, amino acid sequence covering the β1 (β1.1 and β1.2) and β2 strands and major phosphoinositide binding β1-β2 loop (IP4 binding residues, red). C, details of the human kindlin 3-GFP chimeric proteins that are used in the study. The wild type kindlin 3 protein contains an F0 domain, F1 domain, F2 domain intersected by a PH domain, and an F3 domain that binds to the β subunit of integrin (kindlin 3-GFP). Also illustrated are a second kindlin 3 splice variant with residues IPRR inserted into the PH domain (yellow line) (kindlin 3-IPRR-GFP), kindlin 3 with the PH domain deleted (kindlin 3-ΔPH-GFP), and the F2 subdomain that contains the PH domain (kindlin 3-PH-GFP).
To test the PI binding properties of the kindlin 3 PH domain, we generated several kindlin 3 cDNA constructs (Fig. 1C). As well as the intact kindlin 3, we made kindlin 3 minus the PH domain, the isolated PH domain embedded in the kindlin 3 F2 subdomain, and finally the longer kindlin 3-IPRR with insert in the PH domain.
To investigate whether the function of the kindlin 3 PH domain was similar to the kindlin 2 PH domain (33), we first assessed the ability of wild type kindlin 3 protein to bind beads coated with the head group of PI(3,4,5)P3, inositol 1,3,4,5-tetraphosphate (IP4), compared with beads coated with inositol 3,4,5-trisphosphate (IP3), the head group for phosphatidylinositol 4,5-bisphosphate PI(4,5)P2, as well as control beads. The kindlin 3 protein displayed enhanced binding to IP4 compared with IP3 that resembled binding to the control beads (Fig. 2A).
FIGURE 2.
Phosphoinositide binding properties of the kindlin 3-GFP proteins. A, binding of wild type kindlin 3-GFP to control-, IP3-, and IP4-coupled beads revealed by blotting and anti-GFP mAb. The blot is representative of three independent experiments. B, comparison of control and IP4 bead binding properties of wild type kindlin 3-GFP and isolated FERM subdomain 2 incorporating the PH domain (kindlin 3 PH-GFP). Input, amount of GFP protein added to beads; output, amount of GFP protein bound to beads following incubation and washing. C, quantification of kindlin 3-GFP and kindlin 3 PH-GFP binding to control and IP4 beads from three independent experiments. Density was normalized to that of the relevant input band and binding to control beads. Data are shown as mean ± S.E. (error bars); NS, not significant. D, comparison of IP3 and IP4 bead binding of wild type kindlin 3-GFP, kindlin 3-ΔPH-GFP, and kindlin 3-IPRR-GFP. Input and output are as in B. Blot is representative of three independent experiments. E, quantification of kindlin 3-GFP, kindlin 3-ΔPH-GFP, and kindlin 3-IPRR-GFP binding to IP3 and IP4 beads from three independent experiments. Density was normalized to that of relevant input band and kindlin 3 binding to IP3 beads. Data are shown as mean ± S.E.; *, p < 0.05; NS, not significant.
We next tested the isolated PH domain (kindlin 3 PH) protein and found that it resembled wild type kindlin 3 in its enhanced binding of IP4-coated beads over control beads, providing evidence that that the PI binding activity was inherent in the PH domain itself (Fig. 2, B and C). This was further confirmed by testing kindlin 3 lacking the PH domain (the kindlin 3-ΔPH protein) that failed to bind either IP3- or IP4-coated beads (Fig. 2, D and E). Therefore, the kindlin 3 PH domain has binding specificity for PI(3,4,5)P3 with little evidence that it binds PI(4,5)P2 in a stable or easily detectable manner.
The longer splice variant of kindlin 3, the kindlin 3-IPRR protein, also bound IP4 and bound it comparably to the shorter protein (Fig. 2, D and E). However, in contrast, the splice variant also bound IP3 at an enhanced level such that both IP3 and IP4 were equivalently bound. Thus, alteration of the major PH domain by insertion of IPRR at the beginning of the β1-β2 loop caused an enhancement of binding to PI(4,5)P2 as well as binding to PI(3,4,5)P3.
The Kindlin 3 PH Domain Is Essential for Adhesion on ICAM-1
The absence of kindlin 3 in LAD-III patients results in a lack of integrin-mediated leukocyte adhesion (6, 7). As the kindlin 3 PH domain has so far not been linked to any leukocyte function, it was of interest to test whether its deletion had any affect on a critical leukocyte function such as adhesion. We therefore tested the ability of EBV-transformed B cells to attach and spread on LFA-1 ligand, ICAM-1, using IRM as an assay for adhesion. As expected, GFP-expressing B cells from the parent of the LAD-III patient (control B cells) displayed an adhesion pattern detected as an area of dark contrast (Fig. 3, A and B). As expected, the GFP-expressing LAD-III B cells failed to adhere. However, they resembled the parent cells when they were transfected with a wild type KINDLIN3 cDNA construct (Fig. 3, A and B).
FIGURE 3.
Adhesion characteristics of LAD-III B cells expressing human wild type or mutant kindlin 3-GFP. A, phase and IRM images of a LAD-III patient's EBV-transformed B cells transfected with eGFP cDNA compared with cells transfected with wild type KINDLIN3, KINDLIN3-IPRR, and KINDLIN3-ΔPH cDNAs. These cells were compared with parent B cells that expressed kindlin 3 endogenously and were transfected with eGFP cDNA (control). Images are representative of 20 fields/sample; n = 3; scale bar, 5 μm. B, quantification of the area of close contact from three independent experiments (>60 cells/group). Data are shown as mean ± S.E. (error bars); ***, p < 0.001; NS, not significant. C, expression levels of kindlin 3-GFP and GFP proteins in LAD-III EBV-transformed B cells. Gating shows that the KINDLIN3-GFP cDNAs (10 μg/2 × 107 cells) and eGFP cDNA (2 μg/2 × 107 cells) are translated in EBV-transformed B cells with a comparable efficiency at 24 h. D, typical experiment showing equal expression levels of GFP, kindlin 3-GFP, kindlin 3-ΔPH-GFP, and kindlin 3-IPRR-GFP proteins in transfected B cells compared with mock-transfected cells as determined by flow cytometry.
In contrast, the LAD-III B cells transfected with cDNA constructs of either KINDLIN3-ΔPH or KINDLIN3-IPRR failed to adhere and resembled the control LAD-III B cells (Fig. 3, A and B). This finding was not due to differences in expression of kindlin 3, kindlin 3-ΔPH, and kindlin 3-IPRR because the three proteins were tested at similar levels of expression as determined by flow cytometry and cell sorting (Fig. 3, C and D). Unfortunately, the isolated PH domain construct (KINDLIN3-PH) was not expressed in EBV-transformed B cells and therefore could not be tested (data not shown). Thus, an intact PH domain is required for LFA-1-mediated adhesion. Moreover, alteration of the PH domain by insertion of four amino acids IPRR at the beginning of the β1-β2 loop site interferes with kindlin 3 function despite the ability of this splice variant to bind both PI(4,5)P2 and PI(3,4,5)P3.
The Kindlin 3 PH Domain Is Also Essential for Migration on ICAM-1
LFA-1 is a migration receptor guiding leukocytes such as T and B lymphocytes along the vasculature and into tissue (36, 37). As the absence of the kindlin 3 PH domain dramatically affected the adhesion of B cells, it was of interest to test whether it also had a role in their migration that would require additional properties of LFA-1 such as interaction with cytoskeletal proteins and the ability to be dynamic in terms of turnover of active forms of the integrin.
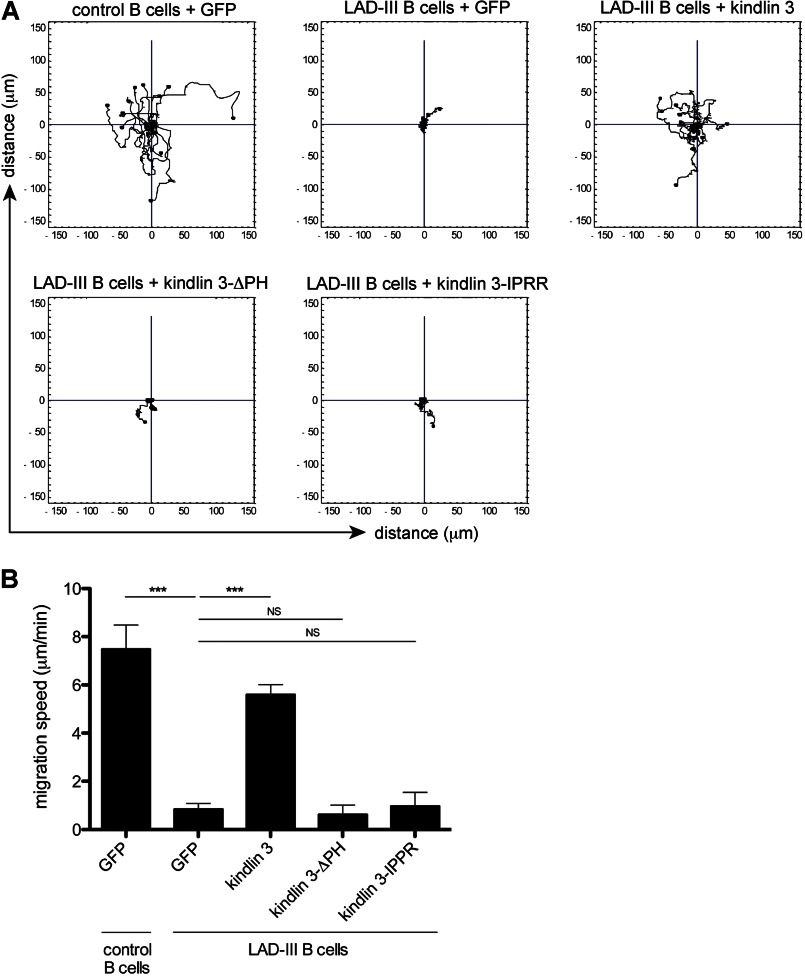
GFP-expressing B cells from the parents of the LAD-III patients (control B cells) migrated in a random fashion with an average speed of 7.48 ± 1.00 μm/min similar to normal B lymphocytes (38). Control GFP-expressing LAD-III B cells did not migrate, but, following transfection with the wild type KINDLIN3 cDNA construct, B cells were able to migrate randomly and with a similar speed to the parent B cells as determined previously (Fig. 4, A and B) (6). In contrast, LAD-III B cells transfected with either KINDLIN3-ΔPH or KINDLIN3-IPRR were unable to migrate and resembled the control LAD-III B cells (Fig. 4, A and B). Thus, the PH domain is required for LFA-1-mediated migration as well as normal levels of adhesion, and its role in migration is compromised by insertion of four amino acids IPRR.
FIGURE 4.
Migration characteristics of LAD-III B cells expressing human wild type or mutant kindlin 3-GFP. A, representative single cell tracking images showing migration characteristics of control B cells and LAD-III B cells transfected with eGFP cDNA compared with cells transfected with wild type KINDLIN3, KINDLIN3-IPRR, and KINDLIN3-ΔPH cDNAs; n = 3 for each cDNA construct. B, quantification of average speed of migration for n = 15 cells/group from a representative experiment. Data are shown as mean ± S.E. (error bars); ***, p < 0.001; NS, not significant.
Localization of Kindlin 3 at the Membrane
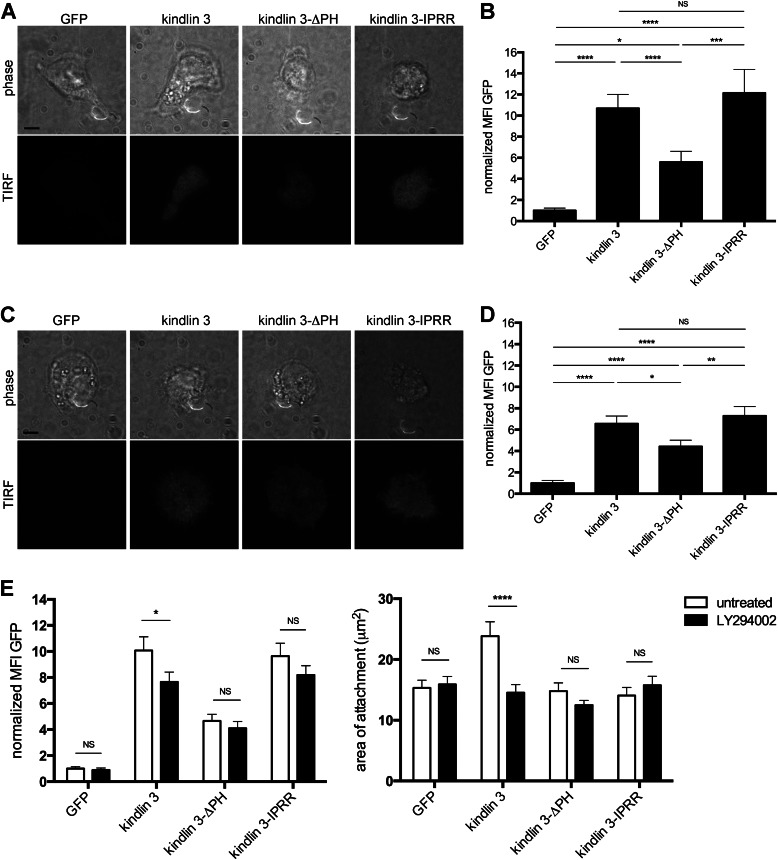
Kindlin 3 is located in microridges in monocytic U937 cells, thus is closely membrane-associated (39). To determine whether kindlin 3 is also membrane-localized in transfected LAD-III B cells and whether its distribution in the cell is influenced by the PH domain, we induced LFA-1 binding to ICAM-1 using Mg2+/EGTA to activate LFA-1 directly (40) and used TIRF microscopy to focus on kindlin 3-GFP at membrane level. We observed wild type kindlin 3 to be well expressed at the membrane level, whereas control GFP was not, indicating a positive association of kindlin 3 with the B cell membrane (Fig. 5, A and B). The form of kindlin 3 with inserted IPRR was also located at membrane level where its presence was equivalent to the shorter kindlin 3. Of interest was the substantially reduced membrane localization of kindlin 3 lacking the PH domain, indicating that kindlin 3 association with the membrane was influenced by its PH domain (Fig. 5, A and B).
FIGURE 5.
TIRF analysis of LAD-III B cells attached to ICAM-1 and poly-l-lysine. A, phase and TIRF images at the plasma membrane of LAD III B cells attached to ICAM-1. Cells transfected with eGFP cDNA were compared with cells transfected with wild type KINDLIN3, KINDLIN3-IPRR, and KINDLIN3-ΔPH cDNAs. Images are representative of 20 fields/sample; n = 4; scale bar, = 5 μm. B, quantification of the mean fluorescence intensity of GFP at the cell membrane level with ICAM-1, normalized to that of the LAD-III B cells transfected with eGFP cDNA. Data are shown as mean ± S.E. (error bars) from four independent experiments (>95 cells/group); *, p < 0.05; ***, p < 0.01; ****, p < 0.0001; NS, not significant. C, phase and TIRF images of the plasma membrane of LAD-III B cells at the interface with poly-l-lysine. Cells transfected with eGFP cDNA were compared with cells transfected with wild type KINDLIN3, KINDLIN3-IPRR, and KINDLIN3-ΔPH cDNAs taken at the level of the cell membrane with poly-l-lysine. Images are representative of 20 fields/sample; n = 4; scale bar, 5 μm. D, quantification of the mean fluorescence intensity of GFP at the cell membrane level with poly-l-lysine, normalized to that of the LAD-III B cells transfected with eGFP cDNA. Data are shown as mean ± S.E. from four independent experiments (>95 cells/group); *, p < 0.05; **, p < 0.01; ****, p < 0.0001; NS, not significant. E, left, quantification of the mean fluorescence intensity of GFP at the cell membrane level with ICAM-1, normalized to that of the LAD-III B cells transfected with eGFP cDNA. E, right, quantification of the area of cell membrane LFA-1 at the interface with ICAM-1. Cells were treated ± 5 μm LY294002 for 1 h. Data are shown as mean ± S.E. from three independent experiments (>60 cells/group); *, p < 0.05; ****, p < 0.0001; NS, not significant.
To test whether LFA-1 adhesion was responsible for PH domain membrane localization, nonspecific contact with substrate was generated through use of poly-l-lysine. The key result was that the presence of the kindlin 3 and kindlin 3-IPRR proteins at the membrane was significantly decreased compared with the ICAM-1 substrate, indicating enhanced PH domain-dependent localization upon LFA-1 binding. Moreover, a background level of LFA-1 activation in the EBV-transformed B cells was suggested by the increased membrane association of wild type kindlin 3 and kindlin 3-IPRR over the kindlin 3-ΔPH form (Fig. 5, C and D). However, kindlin 3 lacking the PH domain was also membrane-localized above background GFP levels. This is consistent with the PH domain-independence of a certain level of kindlin 3 at the membrane.
Active PI3K converts PI(4,5)P2 to PI(3,4,5)P3, and it was also of interest to test whether interfering with its activity would alter the membrane recruitment of kindlin 3. Following treatment with PI3K inhibitor LY294002, the membrane level of intact kindlin 3 was reduced, but kindlin 3-ΔPH was not influenced (Fig. 5E). Of interest was the lack of reduction of kindlin 3-IPRR which could be explained by its extra PI(4,5)P2 binding ability being strong enough to maintain membrane presence.
We next investigated whether PI3K blockade affected the extent of the spread area of B cells on ICAM-1 and whether this correlated with expression of the PH domain at the membrane. LY294002 reduced the area of intact kindlin 3-expressing cells to background level of GFP-expressing cells (Fig. 5E). However, the compound had no effect on the contact footprint of either kindlin 3-ΔPH- or kindlin 3-IPRR-expressing B cells which were already at background levels without LY294002 treatment. These findings were in agreement with the reduced adhesion and migration displayed by B cells expressing these proteins.
These findings indicate that the binding of LFA-1 to ICAM-1 has a downstream effect in the recruitment of kindlin 3 to the membrane that is PH-dependent. Additionally the data also indicate that a certain level of kindlin 3 is present at the membrane in a manner that is PH domain-independent. Thus, kindlin 3 is already associated with the B cell membrane but is then further recruited in a PH-dependent manner following an adhesion event.
DISCUSSION
In this study we have investigated the role of the kindlin 3 PH domain in the function of the leukocyte integrin LFA-1 and show that it is essential for the major LFA-1 functions of lymphocyte adhesion and migration. The focus in the literature has hitherto been on the homologous family member, kindlin 2, where the PH domain similarly controls podocyte adhesion and also deposition of fibronectin matrix (33).
The kindlin 2 PH domain has been demonstrated to have highest specificity for PI(3,4,5)P3 and its head group IP4 (30, 41). PI(3,4,5)P3 is generated from PI(4,5)P2 in the membrane by PI3K recognized as a component of the signaling pathway leading to integrin activation (42, 43). Using a bioinformatics approach we previously described the kindlin 3 PH domain to have a canonical PI binding site (10), and in this study we report that, like kindlin 2, it also has a preference for the PI(3,4,5)P3 head group IP4 over the PI(4,5)P2 headgroup IP3. Further evidence for the PI(3,4,5)P3 specificity comes from the negative effect on membrane recruitment of kindlin 3 following interference with PI3K activity. This correlated with a reduction in LFA-1-mediated spreading on ICAM-1 in keeping with other reports (44–47). Thus, interfering with PI3K diminishes kindlin 3 access to the membrane in an LFA-1-dependent manner linking PI3K, kindlin 3 binding to PI(3,4,5)P3, and LFA-1 function.
The kindlin 2 and kindlin 3 PH domains are highly structurally homologous, but a difference exists in the β1 strand leading into the major PI binding site in the β1-β2 loop that is split in kindlin 3 (Protein Data Bank ID code 2YS3) (30, 31). This change would be predicted to give added flexibility to the end of the β barrel leading into the PI binding site. In addition, comparison of the kindlin 2 PH domain structure with a second NMR structure with ligand IP4 of PI(3,4,5)P3 in place highlights a conformational shift in the β1-β2 loop (30, 31). When the kindlin 2 PI domain binds IP4, this loop undergoes a dramatic shift, moving inwardly toward the “pocket center.” Thus, an “induced-fit” change occurs upon ligand binding involving movement of the β1-β2 loop. Such movement that leads to firmer binding of kindlin 2 to IP4 appears not to occur with IP3 binding (30).
The splice variant of kindlin 3 is longer than the major form by the four amino acids Ile-Pro-Arg-Arg. This longer kindlin 3 is unable to support LFA-1 adhesion or migration, mirroring the behavior of kindlin 3 that lacks the PH domain. Unexpectedly, the splice variant bound both IP3 and IP4 and was present at the plasma membrane as determined by TIRF, raising the question as to why it failed to support LFA-1-mediated function. The four IPRR residues of the longer kindlin 3 isoform are inserted into the β1 loop close to the junction between the split β1 strand and the β1-β2 loop that is highly positively charged. The insert is therefore in position to influence the PI binding site. A speculation would be that the IPRR sequence with its two vicinal positively charged arginine residues might alter the inward movement of this flexible loop by enhancing binding in the pocket via further positive charge. This would be predicted to make PI binding to the site more rigid and make the inward shift less dynamic.
Stronger binding of PI(4,5)P2 might also be expected to affect the arrangement or orientation of kindlin 3 in the membrane potentially by misdirecting it away from PI(3,4,5)P3. Of note was the lack of effect of PI3K block on the minimal adhesion footprint of the kindlin 3-IPRR B cells. This provides some evidence that the malfunction of this form of kindlin 3 might have to do with mislocalization at the membrane due to its “extra” PI(4,5)P2 binding activity. Similarly, the GRP1 PH domain has a splice variant with one added Gly in the β1-β2 loop that causes increased affinity for IP3 without altering the affinity for IP4 (48, 49).
This altered binding ability of the IPRR-containing kindlin 3 is accompanied by a lack of support for LFA-1 function. Along with the shorter kindlin 3, the IPRR-containing form is also expressed by T and B cells,3 but future work is needed to determine how its presence affects lymphocyte function. It cannot account for the lack of integrin activity in LAD-III because there is an absence of total kindlin 3 protein in the patients' leukocytes (6–8, 10). A possibility is that it might influence responses requiring adhesion over time by interference at the membrane with the shorter form of kindlin 3. Additionally, it might interfere indirectly with kindlin 3 function away from the membrane. We observed kindlin 3 to be expressed throughout the B cell (data not shown) as has been reported for T cells (39).
It is relevant that an intact PH domain is essential for localization of wild type levels of kindlin 3 at the B cell membrane with its recruitment enhanced by LFA-1 binding to ICAM-1. Although this indicates the PH domain to be a major factor in bringing kindlin 3 to the cell membrane, some membrane association of kindlin 3 exists in its absence and in the absence of LFA-1 signaling. This is reminiscent of kindlin 2 which is located in focal adhesions in a partially PH domain-dependent manner (33). Phosphoinositide binding in F0 and F1 loop supports kindlin 1 and kindlin 2 binding at the cell membrane so it possible that PI binding by other FERM subdomains of kindlin 3 may have a similar role (25, 26).
The kindlins including kindlin 3 are well described to work together with talin. A relevant question is how these two proteins become co-localized when talin is activated by PI(4,5)P2 binding and the kindlins, at least kindlin 2 and kindlin 3, having highest binding specificity for PI(3,4,5)P3. A first point is that kindlin 2 and talin bind independently to the integrin αIIbβ3 (50, 51). Deletion of PIPKIγ that generates PI(4,5)P2 in focal adhesions abolishes talin entry, but has no particular effect on kindlin 2 (41). Together these data suggest that talin and the kindlins are recruited independently to the membrane.
How these events might be temporally regulated is currently a matter of speculation. There is evidence that the role of the kindlins follows on from that of talin. For example, talin becomes active in the absence of kindlin 3 when neutrophils are migrating under shear flow conditions (52). Kindlin then reinforces the role of talin (51, 52) potentially through the LFA-1 adhesions that are stabilized by outside-in signaling (53). An absence of kindlin 3 is also associated with a loss of microclusters that are a feature of integrin postligand binding (3, 32).
Thus, activation of PI3K and localization at the membrane of kindlin 3 via PI(3,4,5)P3 appears to occur later in the sequence of events. It is also possible that kindlin 3 is initially recruited via interactions outside the PH domain and forms unstable attachments that are strengthened or conformationally altered when PI(3,4,5)P3 is generated. The negative effect of PI3K blockade on membrane localization as detected by TIRF indicates the importance of PI(3,4,5)P3 for retaining kindlin 3 at the membrane. This was also observed for kindlin 2 membrane expression on podocytes (33). A final outcome applicable to all of the kindlins is that they would finally be in position to support the binding of talin to the β tail of integrin in a manner that would fully support stable adhesion.
In summary, the PH domain has a controlling effect on the function of kindlin 3 that in turn has an essential role in the LFA-1 signaling required for leukocyte adhesion and migration on ICAM-1. The importance of the correct functioning of kindlin 3 is fully demonstrated by the life-threatening infections experienced by LAD-III patients, indicating that such responses of leukocytes are essential for an immune response to be successful.
Acknowledgments
We thank our Cancer Research UK London Research Institute colleagues: Banafshe Larijani, Cell Biophysics, for discussion and advice concerning the phosphoinositide assays; Raphael Chaleil and Paul Bates, Biomolecular Modeling, for discussion about kindlin structures; Andreas Bruckbauer and Christoph Feest, Lymphocyte Interaction, for help with TIRF analysis; Stuart Horswell for help with statistical analysis.
R. Hart and I. Patzak, data not shown.
- LFA-1
- lymphocyte function-associated antigen 1
- ANOVA
- analysis of variance
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- FERM
- four-point-one, ezrin, radixin, moesin
- H
- head
- ICAM-1
- intercellular adhesion molecule 1
- IP3
- inositol 3,4,5-trisphosphate
- IP4
- inositol 1,3,4,5-tetraphosphate
- IRM
- interference reflection microscopy
- LAD-III
- leukocyte adhesion deficiency III
- PH
- pleckstrin homology
- PI
- phosphoinositide
- PI(3,4,5)P3
- phosphatidylinositol 3,4,5-trisphosphate
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- TIRF
- total internal reflection fluorescence.
REFERENCES
- 1. Kinashi T. (2005) Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5, 546–559 [DOI] [PubMed] [Google Scholar]
- 2. Alon R., Dustin M. L. (2007) Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27 [DOI] [PubMed] [Google Scholar]
- 3. Hogg N., Patzak I., Willenbrock F. (2011) The insider's guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 11, 416–426 [DOI] [PubMed] [Google Scholar]
- 4. Etzioni A., Alon R. (2004) Leukocyte adhesion deficiency III: a group of integrin activation defects in hematopoietic lineage cells. Curr. Opin. Allergy Clin. Immunol. 4, 485–490 [DOI] [PubMed] [Google Scholar]
- 5. van de Vijver E., Maddalena A., Sanal Ö., Holland S. M., Uzel G., Madkaikar M., de Boer M., van Leeuwen K., Köker M. Y., Parvaneh N., Fischer A., Law S. K., Klein N., Tezcan F. I., Unal E., Patiroglu T., Belohradsky B. H., Schwartz K., Somech R., Kuijpers T. W., Roos D. (2012) Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol. Dis. 48, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Svensson L., Howarth K., McDowall A., Patzak I., Evans R., Ussar S., Moser M., Metin A., Fried M., Tomlinson I., Hogg N. (2009) Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat. Med. 15, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malinin N. L., Zhang L., Choi J., Ciocea A., Razorenova O., Ma Y. Q., Podrez E. A., Tosi M., Lennon D. P., Caplan A. I., Shurin S. B., Plow E. F., Byzova T. V. (2009) A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat. Med. 15, 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuijpers T. W., van de Vijver E., Weterman M. A., de Boer M., Tool A. T., van den Berg T. K., Moser M., Jakobs M. E., Seeger K., Sanal O., Unal S., Cetin M., Roos D., Verhoeven A. J., Baas F. (2009) LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113, 4740–4746 [DOI] [PubMed] [Google Scholar]
- 9. Mory A., Feigelson S. W., Yarali N., Kilic S. S., Bayhan G. I., Gershoni-Baruch R., Etzioni A., Alon R. (2008) Kindlin 3: a new gene involved in the pathogenesis of LAD-III. Blood 112, 2591. [DOI] [PubMed] [Google Scholar]
- 10. McDowall A., Svensson L., Stanley P., Patzak I., Chakravarty P., Howarth K., Sabnis H., Briones M., Hogg N. (2010) Two mutations in the KINDLIN3 gene of a new leukocyte adhesion deficiency III patient reveal distinct effects on leukocyte function in vitro. Blood 115, 4834–4842 [DOI] [PubMed] [Google Scholar]
- 11. Moser M., Nieswandt B., Ussar S., Pozgajova M., Fässler R. (2008) Kindlin 3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 [DOI] [PubMed] [Google Scholar]
- 12. Moser M., Bauer M., Schmid S., Ruppert R., Schmidt S., Sixt M., Wang H. V., Sperandio M., Fässler R. (2009) Kindlin 3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat. Med. 15, 300–305 [DOI] [PubMed] [Google Scholar]
- 13. Larjava H., Plow E. F., Wu C. (2008) Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9, 1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moser M., Legate K. R., Zent R., Fässler R. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 15. Meves A., Stremmel C., Gottschalk K., Fässler R. (2009) The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19, 504–513 [DOI] [PubMed] [Google Scholar]
- 16. Malinin N. L., Plow E. F., Byzova T. V. (2010) Kindlins in FERM adhesion. Blood 115, 4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma Y. Q., Qin J., Wu C., Plow E. F. (2008) Kindlin 2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tadokoro S., Shattil S. J., Eto K., Tai V., Liddington R. C., de Pereda J. M., Ginsberg M. H., Calderwood D. A. (2003) Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 [DOI] [PubMed] [Google Scholar]
- 19. Kim M., Carman C. V., Springer T. A. (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 20. Ye F., Hu G., Taylor D., Ratnikov B., Bobkov A. A., McLean M. A., Sligar S. G., Taylor K. A., Ginsberg M. H. (2010) Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 188, 157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goult B. T., Bouaouina M., Elliott P. R., Bate N., Patel B., Gingras A. R., Grossmann J. G., Roberts G. C., Calderwood D. A., Critchley D. R., Barsukov I. L. (2010) Structure of a double ubiquitin-like domain in the talin head: a role in integrin activation. EMBO J. 29, 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elliott P. R., Goult B. T., Kopp P. M., Bate N., Grossmann J. G., Roberts G. C., Critchley D. R., Barsukov I. L. (2010) The structure of the talin head reveals a novel extended conformation of the FERM domain. Structure 18, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anthis N. J., Campbell I. D. (2011) The tail of integrin activation. Trends Biochem. Sci. 36, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saltel F., Mortier E., Hytönen V. P., Jacquier M. C., Zimmermann P., Vogel V., Liu W., Wehrle-Haller B. (2009) New PI(4,5)P2- and membrane proximal integrin-binding motifs in the talin head control β3-integrin clustering. J. Cell Biol. 187, 715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perera H. D., Ma Y. Q., Yang J., Hirbawi J., Plow E. F., Qin J. (2011) Membrane binding of the N-terminal ubiquitin-like domain of kindlin 2 is crucial for its regulation of integrin activation. Structure 19, 1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouaouina M., Goult B. T., Huet-Calderwood C., Bate N., Brahme N. N., Barsukov I. L., Critchley D. R., Calderwood D. A. (2012) A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation. J. Biol. Chem. 287, 6979–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goult B. T., Bouaouina M., Harburger D. S., Bate N., Patel B., Anthis N. J., Campbell I. D., Calderwood D. A., Barsukov I. L., Roberts G. C., Critchley D. R. (2009) The structure of the N-terminus of kindlin 1: a domain important for αIIbβ3 integrin activation. J. Mol. Biol. 394, 944–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blomberg N., Baraldi E., Nilges M., Saraste M. (1999) The PH superfold: a structural scaffold for multiple functions. Trends Biochem. Sci. 24, 441–445 [DOI] [PubMed] [Google Scholar]
- 29. Lemmon M. A. (2008) Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9, 99–111 [DOI] [PubMed] [Google Scholar]
- 30. Liu J., Fukuda K., Xu Z., Ma Y. Q., Hirbawi J., Mao X., Wu C., Plow E. F., Qin J. (2011) Structural basis of phosphoinositide binding to kindlin 2 protein pleckstrin homology domain in regulating integrin activation. J. Biol. Chem. 286, 43334–43342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y., Zhu Y., Ye S., Zhang R. (2012) Crystal structure of kindlin 2 PH domain reveals a conformational transition for its membrane anchoring and regulation of integrin activation. Protein Cell 3, 434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng C., Li Y. F., Yau Y. H., Lee H. S., Tang X. Y., Xue Z. H., Zhou Y. C., Lim W. M., Cornvik T. C., Ruedl C., Shochat S. G., Tan S. M. (2012) Kindlin 3 mediates integrin αLβ2 outside-in signaling, and it interacts with scaffold protein receptor for activated-C kinase 1 (RACK1). J. Biol. Chem. 287, 10714–10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qu H., Tu Y., Shi X., Larjava H., Saleem M. A., Shattil S. J., Fukuda K., Qin J., Kretzler M., Wu C. (2011) Kindlin 2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J. Cell Sci. 124, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dransfield I., Hogg N. (1989) Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunits. EMBO J. 8, 3759–3765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Isakoff S. J., Cardozo T., Andreev J., Li Z., Ferguson K. M., Abagyan R., Lemmon M. A., Aronheim A., Skolnik E. Y. (1998) Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 17, 5374–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinashi T. (2007) Integrin regulation of lymphocyte trafficking: lessons from structural and signaling studies. Adv. Immunol. 93, 185–227 [DOI] [PubMed] [Google Scholar]
- 37. Evans R., Patzak I., Svensson L., De Filippo K., Jones K., McDowall A., Hogg N. (2009) Integrins in immunity. J. Cell Sci. 122, 215–225 [DOI] [PubMed] [Google Scholar]
- 38. Miller M. J., Wei S. H., Parker I., Cahalan M. D. (2002) Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 [DOI] [PubMed] [Google Scholar]
- 39. Hyduk S. J., Rullo J., Cano A. P., Xiao H., Chen M., Moser M., Cybulsky M. I. (2011) Talin-1 and kindlin 3 regulate α4β1 integrin-mediated adhesion stabilization, but not G protein-coupled receptor-induced affinity up-regulation. J. Immunol. 187, 4360–4368 [DOI] [PubMed] [Google Scholar]
- 40. Dransfield I., Cabañas C., Craig A., Hogg N. (1992) Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 116, 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legate K. R., Takahashi S., Bonakdar N., Fabry B., Boettiger D., Zent R., Fässler R. (2011) Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 30, 4539–4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ward S. G., Marelli-Berg F. M. (2009) Mechanisms of chemokine and antigen-dependent T-lymphocyte navigation. Biochem. J. 418, 13–27 [DOI] [PubMed] [Google Scholar]
- 43. Zhang T. T., Li H., Cheung S. M., Costantini J. L., Hou S., Al-Alwan M., Marshall A. J. (2009) Phosphoinositide 3-kinase-regulated adapters in lymphocyte activation. Immunol. Rev. 232, 255–272 [DOI] [PubMed] [Google Scholar]
- 44. Mueller K. L., Daniels M. A., Felthauser A., Kao C., Jameson S. C., Shimizu Y. (2004) Cutting edge: LFA-1 integrin-dependent T cell adhesion is regulated by both antigen specificity and sensitivity. J. Immunol. 173, 2222–2226 [DOI] [PubMed] [Google Scholar]
- 45. Sánchez-Martín L., Sánchez-Sánchez N., Gutiérrez-López M. D., Rojo A. I., Vicente-Manzanares M., Pérez-Alvarez M. J., Sánchez-Mateos P., Bustelo X. R., Cuadrado A., Sánchez-Madrid F., Rodríguez-Fernández J. L., Cabañas C. (2004) Signaling through the leukocyte integrin LFA-1 in T cells induces a transient activation of Rac-1 that is regulated by Vav and PI3K/Akt-1. J. Biol. Chem. 279, 16194–16205 [DOI] [PubMed] [Google Scholar]
- 46. Smith D. F., Deem T. L., Bruce A. C., Reutershan J., Wu D., Ley K. (2006) Leukocyte phosphoinositide-3 kinase γ is required for chemokine-induced, sustained adhesion under flow in vivo. J. Leukoc. Biol. 80, 1491–1499 [DOI] [PubMed] [Google Scholar]
- 47. Raab M., Smith X., Matthess Y., Strebhardt K., Rudd C. E. (2011) SKAP1 protein PH domain determines RapL membrane localization and Rap1 protein complex formation for T cell receptor (TCR) activation of LFA-1. J. Biol. Chem. 286, 29663–29670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cronin T. C., DiNitto J. P., Czech M. P., Lambright D. G. (2004) Structural determinants of phosphoinositide selectivity in splice variants of Grp1 family PH domains. EMBO J. 23, 3711–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klarlund J. K., Tsiaras W., Holik J. J., Chawla A., Czech M. P. (2000) Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. J. Biol. Chem. 275, 32816–32821 [DOI] [PubMed] [Google Scholar]
- 50. Kahner B. N., Kato H., Banno A., Ginsberg M. H., Shattil S. J., Ye F. (2012) Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3. PLoS One 7, e34056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bledzka K., Liu J., Xu Z., Perera H. D., Yadav S. P., Bialkowska K., Qin J., Ma Y. Q., Plow E. F. (2012) Spatial coordination of kindlin 2 with talin head domain in interaction with integrin β cytoplasmic tails. J. Biol. Chem. 287, 24585–24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lefort C. T., Rossaint J., Moser M., Petrich B. G., Zarbock A., Monkley S. J., Critchley D. R., Ginsberg M. H., Fässler R., Ley K. (2012) Distinct roles for talin-1 and kindlin 3 in LFA-1 extension and affinity regulation. Blood 119, 4275–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feigelson S. W., Grabovsky V., Manevich-Mendelson E., Pasvolsky R., Shulman Z., Shinder V., Klein E., Etzioni A., Aker M., Alon R. (2011) Kindlin 3 is required for the stabilization of TCR-stimulated LFA-1:ICAM-1 bonds critical for lymphocyte arrest and spreading on dendritic cells. Blood 117, 7042–7052 [DOI] [PubMed] [Google Scholar]