Background: Specific interactions of peripheral membrane proteins with phosphatidylcholine (PC) are poorly characterized.
Results: Staphylococcus aureus phosphatidylinositol-specific phospholipase C has poor affinity for PC. Introduction of two tyrosines generates a specific PC binding site.
Conclusion: The PC choline cation interaction with amino acid π systems forms the PC-specific site.
Significance: Well defined choline cation-aromatic π interactions may be a general motif to anchor proteins to PC-rich bilayers.
Keywords: Membrane Enzymes, Nuclear Magnetic Resonance, Phosphatidylcholine, Phospholipase C, X-ray Crystallography
Abstract
Peripheral membrane proteins can be targeted to specific organelles or the plasma membrane by differential recognition of phospholipid headgroups. Although molecular determinants of specificity for several headgroups, including phosphatidylserine and phosphoinositides are well defined, specific recognition of the headgroup of the zwitterionic phosphatidylcholine (PC) is less well understood. In cytosolic proteins the cation-π box provides a suitable receptor for choline recognition and binding through the trimethylammonium moiety. In PC, this moiety might provide a sufficient handle to bind to peripheral proteins via a cation-π cage, where the π systems of two or more aromatic residues are within 4–5 Å of the quaternary amine. We prove this hypothesis by engineering the cation-π box into secreted phosphatidylinositol-specific phospholipase C from Staphylococcus aureus, which lacks specific PC recognition. The N254Y/H258Y variant selectively binds PC-enriched vesicles, and x-ray crystallography reveals N254Y/H258Y binds choline and dibutyroylphosphatidylcholine within the cation-π motif. Such simple PC recognition motifs could be engineered into a wide variety of secondary structures providing a generally applicable method for specific recognition of PC.
Introduction
Cells are dynamic systems where spatially and temporally localized signaling facilitates responses to the local environment. Variations in lipid composition between different organelles and between organelles and the plasma membrane (1) provide spatial localization of peripheral membrane proteins that recognize specific lipids. For example, a number of proteins specifically recognize rare, anionic phosphoinositides with sterospecific recognition of the phosphoinositide headgroups (2, 3). For pleckstrin homology, PROPPIN β propellers, and other domains, stereospecificity often depends on a network of hydrogen bonds between the phosphoinositide headgroup and conserved basic residues (2–4). Similarly, proteins specific to the anionic phospholipid phosphatidylserine (PS)2 may coordinate Ca2+ ions for PS binding using conserved loop motifs as observed in annexin V (5) or directly bind PS via C2 domains as in coagulation factor V (6) and lactadherin (7) where polar side chains allow specific recognition of PS. The affinity of the proteins for the membrane may be further modulated by insertion of hydrophobic and aromatic amino acids into the bilayer (5, 6, 8) and/or multivalent interactions (3) via domain repeats (9) or specificity for more than one type of lipid (9, 10).
Although there are numerous examples of specific binding to anionic lipids, less is known about specificity for zwitterionic lipids such as phosphatidylethanolamine (PE) and phosphatidylcholine (PC). Specific binding via a trimethylammonium moiety, such as that found in PC headgroups, has been observed in proteins that bind proline betaine and glycine betaine (11, 12) or choline (13, 14) as well as proteins that bind methylated lysine in histones (15–17) or methylated arginine (18). In these structures, the methylammonium is the center of a cation-π box with the faces of 2 to 4 aromatic residues located within 4–5 Å of the methylated amine allowing cation-π interactions with the aromatic residues. In search of the trimethylammonium binding motif, we have reviewed the Protein Data Bank database, and the only known structures containing PC with the choline held in a cation-π box are those for phosphatidylcholine transfer protein (supplemental Table S1 presents a list of crystal structures with choline and choline-related molecules bound). However, additional interactions with the acyl chains assist in PC binding by the transfer protein (19), and it is unclear whether the π-cation interaction by itself would provide sufficient binding energy to transiently bind a protein to the membrane.
To test the hypothesis that such a cation-π box might allow specific, but transient binding to a PC-rich membrane, we have engineered such a box into Staphylococcus aureus phosphatidylinositol-specific phospholipase C (PI-PLC). S. aureus PI-PLC and the related PI-PLC from Bacillus thuringiensis are secreted virulence factors for extracellular bacteria that target glycosylphosphatidylinositol-anchored proteins in the PC-rich outer membrane of eukaryotic cells (20, 21). Although B. thuringiensis PI-PLC has a high affinity for PC vesicles (8), the similar PI-PLC from S. aureus has virtually no affinity for PC vesicles. High resolution field-cycling NMR experiments on B. thuringiensis identified a discrete binding site for PC that is consistent with Tyr residues forming a cation-π box or sandwich (22). However, this motif is not present in the S. aureus enzyme, which displays much weaker binding to PC-rich vesicles and virtually no binding to pure PC vesicles (23). Interestingly, unlike most other cation-π boxes, which are often made up of aromatic residues located on β strands and/or on loops with the separation required to sandwich a methyl ammonium between at least two π systems, the putative B. thuringiensis PI-PLC cation-π box is proposed to be located on adjacent α helices on the outside of a β-barrel. The addition of two tyrosine residues to the S. aureus PI-PLC introduces a specific binding site for PC that we characterize with several biophysical techniques including fluorescence correlation spectroscopy, high resolution field cycling NMR, as well x-ray crystallography. The molecular details of the engineered PC site in S. aureus PI-PLC suggest that it should be feasible to engineer similar PC headgroup-specific membrane binding modules into other proteins.
EXPERIMENTAL PROCEDURES
S. aureus PI-PLC Expression, Purification, Modification, and Enzymatic Activity
Recombinant S. aureus PI-PLC was produced and purified as described previously (24). The gene for the N254Y/H258Y mutant was constructed from the wild-type recombinant PI-PLC gene using QuikChange site-directed mutagenesis methodology (Stratagene) and primers purchased from Operon. The sequence of the mutated gene was confirmed by Genewiz. The purity of the PI-PLC enzymes was above 95% as monitored by SDS-PAGE; concentrations were measured by the absorption at 280 using extinction coefficients of ϵ280 = 60280 m−1 cm−1 for WT and ϵ280 = 63260 m−1 cm−1 for N254Y/H258Y calculated by the web-based ProtParam software. Protein was stored at 4 °C until used. Other S. aureus PI-PLC variants constructed and verified in the same fashion include N254Y, D213C, D213C/N254Y/H258Y, and Y211A/N254Y/H258Y/Y290A; H258Y was characterized previously (24).
The fluorescent dye Alexa Fluor 488 carboxylic acid, succinimidyl ester from Invitrogen was used to modify the N terminus of PI-PLC proteins for FCS studies. For the field cycling NMR experiments, both wild-type and N254Y/H258Y proteins with D213C were modified with the spin labeling reagent 2,2,5,5-tetramethyl-l-oxyl-3-methyl methanethiosulfonate, obtained from Toronto Research Chemicals. Excess dye or spin labeling reagents were removed with three Bio-Spin 6 columns.
PI-PLC cleavage of PI in small unilamellar vesicles (SUV), prepared by sonication, with varying mole fractions of PC, XPC, was monitored by 31P NMR using a Varian VNMRS 600 spectrometer as described previously (23, 24). Phospholipids were obtained from Avanti Polar Lipids, Inc., and used without further purification. Most enzymatic assays were carried out in 50 mm MES with 1 mm EDTA and 0.1 mg/ml of BSA, pH 6.5, using 0.2 to 8 μg/ml of the PI-PLC enzymes. Buffers used in assays at other pH values have been described previously (23). Assays for each XPC were run in duplicate.
Vesicle Binding Measured by Fluorescence Correlation Spectroscopy (FCS)
The partitioning of both wild-type recombinant S. aureus PI-PLC and the N254Y/H258Y mutant was measured by FCS as described previously (22, 25). The fluorescence was monitored at 22 °C with samples placed in chambered coverglass wells (Lab-Tek, Nunc), containing 10 nm labeled S. aureus PI-PLC protein and 1 mg/ml of BSA in 300 μl of 50 mm MES, pH 6.5 (the same buffer as used for enzymatic assays). SUVs of the anionic phospholipid dioleoylphosphatidylglycerol (used as the substrate analog) with different mole fractions of 1-palmitoyl-2-oleoyl-PC were prepared by sonication. FCS data were analyzed as previously described (23, 26). The fitted diffusion coefficient of free, Alexa Fluor 488-labeled S. aureus PI-PLC (Dfree) was 50 ± 2 μm2 s−1; Dbound, determined using vesicles containing fluorescent labeled lipids, was in the range of 12–15 μm2 s−1. Kd values for each XPC were determined in duplicate using different protein and SUV preparations. Once the binding profiles were established for WT and N254Y/H258Y proteins, a quicker centrifugation assay (27) to separate vesicle bound and free protein, with quantification of free protein with the Bio-Rad DC protein assay, was used to compare the fraction of other related protein variants bound to 1 mm PG/PC (1:4) in 50 mm MES, pH 6.5, with 140 mm salt added.
PI-PLC Line Broadening of DiC7PC 31P Resonance
DiC7PC (Avanti Polar Lipids, Inc.) was titrated into a solution of 3 mg/ml (0.085 mm) of PI-PLC in the same MES buffer with 1 mm EDTA. Protein-induced line broadening at 242.76 MHz was measured on a Varian VNMRS 600 spectrometer. For other bacterial PI-PLCs with significant PC affinity, the lipid linewidth increases dramatically around the critical micelle concentration (1.5 mm (10)), as protein-lipid micelle complexes form, then decreases as more diC7PC is added to reach a limiting line width. Proteins with weakened affinity for PC have very little effect on the diC7PC 31P line width (28). Line widths at a given diC7PC concentration were measured in duplicate samples.
Intrinsic Fluorescence of PI-PLC
Intrinsic fluorescence measurements of PI-PLC (0.2 μm) were carried out on a Fluorolog spectrometer (Horiba Jobin Yvon FL3–22). Samples were excited at 282 nm, and changes in the fluorescence intensity at the emission maximum, 337 nm, upon the addition of diC7PC were expressed as (I − I0)/I0, where I0 is the emission intensity of protein alone and I is the intensity in the presence of diC7PC. A small amount of background signal from pure buffer solution or buffer with different concentrations of diC7PC was subtracted from the control and sample intensities. The dependence of (I − I0)/I0 on diC7PC concentrations reflects protein binding affinity for that short chain lipid (10, 28).
High Resolution 31P Field Cycling NMR Spectroscopy
High resolution 31P field cycling NMR spin lattice (R1) relaxation measurements, using a custom-built high resolution field cycling system on a Varian Unityplus 500 spectrometer (29), were carried out with S. aureus PI-PLC spin-labeled at D213C. This position is comparable with D205C in B. thuringiensis PI-PLC, where a spin label had the largest effect on 31P relaxation (22). The much larger dipole of the unpaired electron can relax 31P fast-exchanging into and out of a discrete binding site and back into the bilayer. The field dependence of the increase in R1 caused by the spin label for each lipid (ΔR1) in a vesicle compared with the control, unlabeled protein, can be fit with the following expression: ΔR1 = RP-e(0)/(1 + ω2τP-e2) + c. Here RP-e(0) is the maximum relaxation enhancement for that fraction of ligand bound to the spin-labeled protein, and τP-e is the correlation time for the bound phospholipid/spin-labeled PI-PLC interaction. A constant residual R1 at higher fields, c, is likely to reflect a limiting chemical shift anisotropy contribution due to the paramagnetic interaction.
The parameters RP-e(0) and τP-e along with the total spin-labeled PI-PLC concentration, [PI-PLC-SL], and phospholipid concentration, Lo, are related to rP-e, the distance between the phospholipid 31P and the nitroxide when the ligand is bound (22). To a first approximation we assume that if there is a discrete site on the protein for an individual phosphorylated molecule, it is saturated with 5 mm of the ligand. We also assume that we are looking at a single PC or phosphatidylmethanol (PMe) binding in a given site for a time approaching 1–2 μs, long enough to suggest a specific complex as opposed to nonspecific lateral diffusion of the lipids around the protein, and that only phospholipid in the outer leaflet of the bilayer is in contact with the protein. For these small vesicles on average about 2/3 of the total of a specific phospholipid is in the outer monolayer. The average distance of the bound phospholipid on the protein at a specific site is calculated from the following expression.
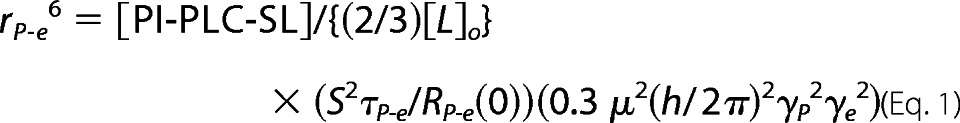 |
S2, the order parameter of the electron spin-31P dipolar interaction, is approximated as 1 because of the long rP-e compared with the size of local picosecond motions; μ, γP, and γe are well defined physical constants.
Crystallization and Structure Determination
S. aureus PI-PLC N254Y/H258Y was crystallized in three different conditions in 150 mm ammonium acetate, 100 mm sodium acetate, pH 4.6, and 100 mm magnesium nitrate with the following additives and treatments. (i) Crystals in the absence of choline compounds were grown with 26% PEG 4000; prior to crystallization, the protein was incubated with 100 mm myo-inositol for 2 h. (ii) Crystals with choline bound to the protein were grown with 26% PEG 4000; prior to crystallization, N254Y/H258Y was incubated with 30 mm glycerophosphocholine and 25 mm choline chloride. Before data collection, the crystals were soaked for 2 h in the crystallization buffer with the addition of 33% PEG 400 and 500 mm choline chloride. (iii) Crystals with diC4PC bound to the protein were grown with 27% PEG 4000; protein was pretreated with glycerophosphocholine and choline chloride. Before data collection, the crystals were soaked in the crystallization buffer with 90 instead of 100 mm magnesium nitrate, 30% PEG 4000 and 100 mm diC4PC (below its CMC ∼250 mm (30)).
For all crystallizations, purified PI-PLC proteins were diluted to a concentration of 10 mg/ml and crystallized at 20 °C by vapor diffusion, using hanging drops of 3 μl. Single large crystals (0.5–0.7 mm) appeared overnight. Suitable crystals were mounted in nylon loops and frozen in liquid nitrogen. Data were collected at 100 K using an in-house Rigaku MicroMax-07 HF high-intensity microfocus rotating Cu anode x-ray generator, coupled with Osmic VariMax Optics and a R-Axis IV++ image plate area detector. Data were indexed and reduced using HKL2000 (31). All structures were solved by molecular replacement in PHENIX (32) using PHASER (33), with the previous H258Y structure (24) as a model. All models were refined in PHENIX with manual model building in COOT (34). Ligands and ligand restraints were generated using sketcher in CCP4 (35). Structural comparisons were made using SSM superposition (36) in COOT and alignment in PyMOL (Schrodinger). PROCHECK was used for structure validation (37).
RESULTS
Comparing B. thuringiensis and S. aureus PI-PLC
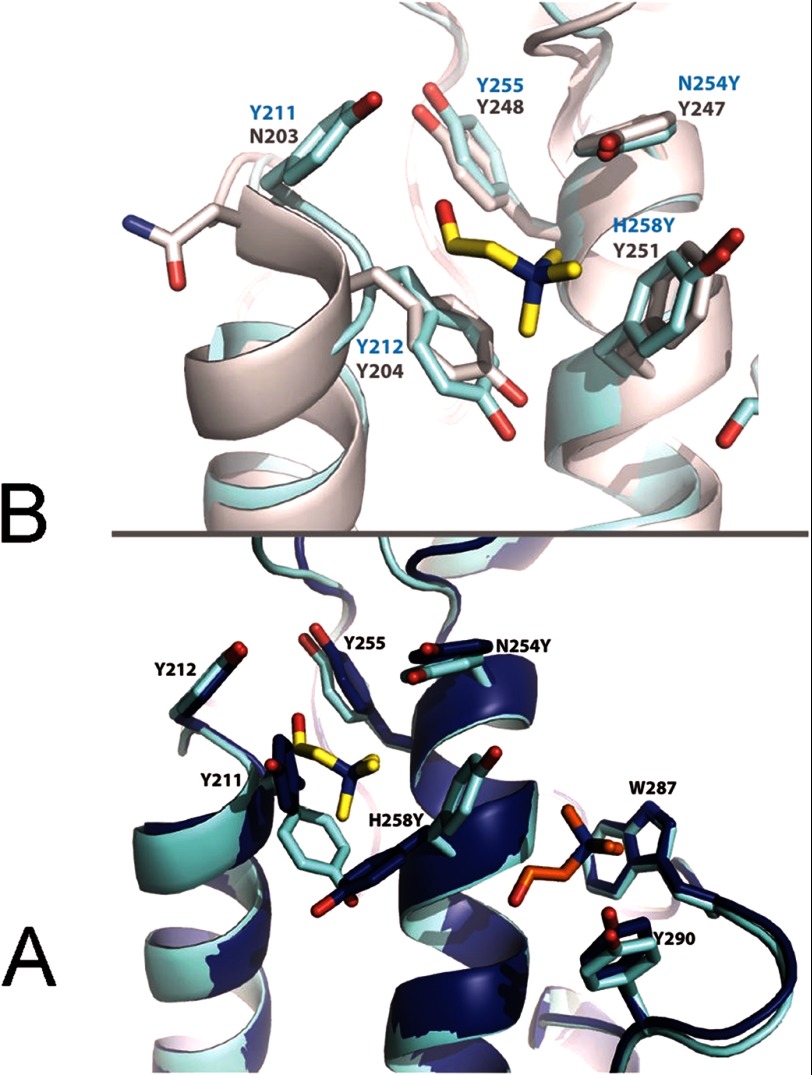
Bacterial PI-PLCs are single domain enzymes with active sites located at the center of a TIM-like barrel folding motif (24, 38, 39). PI-PLC from B. thuringiensis binds to PC-rich vesicles; residues located on the loop connecting helices F and G (notably Trp-242), as well several tyrosines located on helix G (Tyr-246, Tyr-247, Tyr-248, Tyr-251) were implicated in binding to PC interfaces (22, 28). Sequence and structural alignments of the B. thuringiensis and S. aureus PI-PLC enzymes show that whereas Tyr-246 and Tyr-248 in the Bacillus enzyme correspond to Tyr-253 and Tyr-255 in S. aureus, the other two tyrosine residues (247 and 251) in the Bacillus enzyme have been replaced with Asn-254 and His-258 in the S. aureus enzyme (Fig. 1A). S. aureus PI-PLC binds to PC-rich membranes with much lower affinity than does the Bacillus enzyme and has virtually no affinity for pure PC vesicles (23). This fact suggests that the higher affinity of the Bacillus enzyme for PC is associated with the presence of an aromatic box that creates a binding site for the choline. We hypothesized that mutating Asn-254 and His-258 to tyrosines (N254Y/H258Y) could introduce a specific PC binding site in S. aureus PI-PLC. This expectation was based on the observation that the site could utilize cation-π interactions to bind the PC headgroup.
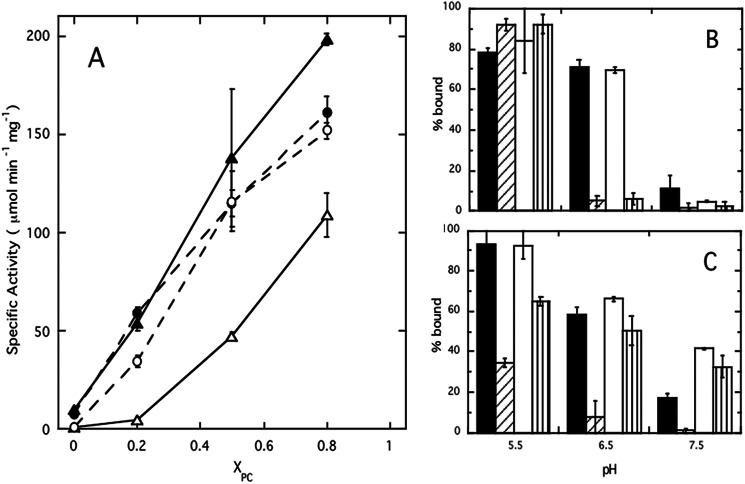
FIGURE 1.
Inserting the two missing Tyr residues generates PC specificity. A, alignment of the F/G helix region of B. thuringiensis (PDB 1T6M) and S. aureus (PDB 3V18) PI-PLC. The secondary structure is shown above the alignment. Tyr residues are shaded pink and S. aureus Asn-254 and His-258 are shaded yellow. B. thuringiensis Trp-242 and S. aureus Phe-249 are shaded blue, these residues are important for membrane binding. B, apparent binding constants at pH 6.5 for S. aureus wild-type (▴), Y253S/Y255S (○), and N254Y/H258Y (●) partitioning to SUVs as a function of mole fraction PC (XPC). C, 31P linewidth of diC7PC in the absence and presence (dashed lines) of 3 mg/ml of S. aureus PI-PLC variants: WT (▴) and N254Y/H258Y (●). The linewidth of diC7PC without protein shows a small increase as micelles form (solid line). For comparison, the diC7PC linewidth is shown in the presence of the same amount of B. thuringiensis PI-PLC (○). The inset shows the relative change in intrinsic fluorescence of S. aureus N254Y/H258Y (●) as a function of the amount of micellar diC7PC added (estimated as [diC7PC] ∼1.5 mm, where 1.5 mm is the critical micelle concentration for pure diC7PC at 25 °C). WT protein, which displays no change in fluorescence, is shown for comparison (▴). The line is a hyperbolic fit to the data.
Vesicle Binding and Enzymatic Activity of N254Y/H258Y S. aureus PI-PLC
FCS was used to measure binding of the WT and mutated enzymes to SUVs. We used low (∼10 nm) enzyme concentrations similar to those used for enzymatic assays. WT, Y253S/Y255S, and N254Y/H258Y proteins were fluorescently labeled at the N terminus with a succinimidyl ester of Alexa Fluor 488, and apparent Kd values for PG/PC SUVs were measured as a function of the mole fraction PC, XPC (Fig. 1B). All three proteins have similar affinities for XPC ≤ 0.2 vesicles, but both WT and Y253S/Y255S have low affinities once XPC ≥ 0.5, whereas N254Y/H258Y still has a millimolar affinity for these PC-rich vesicles. As the PC content increases the difference in binding between the double mutant protein and WT is quite pronounced. N254Y/H258Y has a 50-fold lower apparent Kd than WT for XPC = 0.8 vesicles. Although WT shows virtually no binding (<8% is bound with 55 mm PC) to pure PC SUVs, N254Y/H258Y binds with an apparent Kd of 3.3 ± 0.4 mm.
Moderate salt concentrations dramatically reduce S. aureus PI-PLC binding to vesicles (23), providing a simple assay to demonstrate whether PC is binding via one or both of the tyrosines that were introduced into the PI-PLC variant. Wild-type PI-PLC, N254Y, H258Y, and N254Y/H258Y (0.2 mg/ml) were each incubated with 1 mm PG/PC (0.2 mm/0.8 mm) SUVs in 50 mm MES, pH 7.5, with 140 mm salt, followed by centrifugation to separate free protein from vesicle bound protein. The total phospholipid concentration of 1 mm was chosen to be close to the Kd for N254Y/H258Y measured by FCS. Under these conditions, no WT or N254Y protein was bound to the vesicles; however, 12% of H258Y and 59% of N254Y/H258Y were bound to the vesicles. This suggests that a tyrosine at residue 258 is required, and that a tyrosine at 254 significantly increases PC binding in the H258Y background. This same binding assay carried out with 1 mm PG/PE (0.2 mm/0.8 mm) was used to examine if N254Y/H258Y exhibited a preference for PC compared with PE. When N254Y/H258Y was incubated with either the PC or PE containing SUVs, 68% of the protein was bound to the PG/PC SUVs, whereas only 36% of the protein was bound to the PG/PE SUVs. The interactions of the protein with PC appear significantly stronger than with PE.
Additionally, binding of S. aureus PI-PLC enzymes to PC micelles was explored by monitoring the 31P line width of diC7PC in the presence of the protein (Fig. 1C). B. thuringiensis PI-PLC induces formation of large protein-micelle complexes right around the critical micelle concentration, and mutations that weaken association with PC reduce this change in 31P line width (28). S. aureus PI-PLC and the Y253S/Y255S variant had little or no effect on the diC7PC line width, consistent with poor binding to a PC interface. However, S. aureus N254Y/H258Y mimicked the behavior of the B. thuringiensis PI-PLC with a large increase in linewidth at the critical micelle concentration. The intrinsic fluorescence associated with the presence of aromatic residues in N254Y/H258Y also increased with the addition of micellar diC7PC; this effect was not observed with the S. aureus WT protein (Fig. 1C, inset).
Effect of the Added PC Site on Enzymatic Activity
The addition of two tyrosines to helix G in S. aureus PI-PLC has apparently created a site on S. aureus PI-PLC that can bind one or more PC molecules. The question arises whether this enhanced PC binding influences enzyme activity. S. aureus PI-PLC enzymatic activity toward PI in vesicles is sensitive to both pH and salt concentration (23, 24), and PC in the interface both alters the optimal pH for activity and ameliorates the salt sensitivity of the WT enzyme. These kinetic effects are not due to specific PC binding but rather result from competition between anion binding to a specific pocket in S. aureus PI-PLC and formation of a more active homodimer that occludes the anion binding site. Like WT, S. aureus PI-PLC N254Y/H258Y specific activity increases with increasing enzyme concentration indicating the active form is still a dimer. At pH 6.5 in the absence of salt, specific activity shows a large increase with PC content for both N254Y/H258Y and WT (Fig. 2A). If salt is present neither WT nor N254Y/H258Y exhibits much activity toward pure PI SUVs, and salt reduces the activity of WT S. aureus PI-PLC even toward SUVs containing PC. However, once PC is present in the SUVs, the salt sensitivity of N254Y/H258Y enzyme is virtually abolished and the engineered enzyme exhibits activities in the presence of salt that are close to the values obtained without salt, whereas the activities of the WT are much lower in the presence of salt.
FIGURE 2.
In the presence of PC, N254Y/H258Y S. aureus PI-PLC is less salt-sensitive than wild-type.
A, specific activity of S. aureus WT (triangles) and N254Y/H258Y (circles) PI-PLC at pH 6.5 toward different vesicle compositions (XPC = mole fraction PC) in the absence (filled symbols) and presence (open symbols) of 140 mm salt. The concentration of PI was kept at 4 mm with increasing amounts of PC. B and C, the apparent fraction of protein bound to (B) pure PG (6.2 mm) or (C) PG/PC (8.2:8.2 mm) SUVs (extracted from FCS data) is shown as a function of pH:WT in the absence (■) or presence (▨) of 140 mm salt; N254Y/H258Y in the absence (□) or presence ( ) of salt. Error bars represent the variation in parameters from experiments using different protein and SUV preparations.
) of salt. Error bars represent the variation in parameters from experiments using different protein and SUV preparations.
Specific activity toward PI in vesicles partially reflects how efficiently the protein partitions onto vesicles (24). For WT, the tightest binding is observed at pH 5.5, and added salt dramatically weakens binding to both pure PG and PG/PC (1:1) SUVs (Fig. 2, B and C). At pH > 5.5 in the absence of PC (Fig. 2B), the binding of both WT and N254Y/H258Y proteins are similarly inhibited by salt. However, once PC is present in the SUVs, N254Y/H258Y binding is not significantly affected by salt (Fig. 2C). The apparent Kd values for binding to XPC = 0.5 SUVs, at pH 6.5 in the presence of 140 mm salt, were ∼70 mm for WT and 2.6 ± 0.6 mm for N254Y/H258Y. Similarly, WT binding to pure PC SUVs was too low to measure, whereas N254Y/H258Y exhibited an apparent Kd of 5.4 ± 0.9 mm in the presence of salt (only about 50% higher than the apparent Kd in the absence of salt). Salt screens electrostatic interactions preventing WT S. aureus PI-PLC binding to these vesicles, but the engineered PC site in N254Y/H258Y allows binding to PC-containing interfaces even in the presence of salt.
Defining the PC Binding Site on a Molecular Level
Two experimental approaches were used to confirm the expectation that PC binds in direct proximity to the two introduced tyrosines (N254Y/H258Y): (i) high-resolution field cycling NMR analysis of the effect of spin-labeled protein on a mixed PC/PMe bilayer and (ii) x-ray crystallography structure determination for the N254Y/H258Y and H258Y S. aureus PI-PLC variants. Comparisons with the WT structure that has been described previously (24) offer insight not only into the conformational adaptability of the protein but also demonstrate that increased PC affinity results from PC binding mediated by the introduced tyrosine residues.
NMR Field Cycling Experiments with SUVs
Because the chemical shifts of different phospholipids are distinct, 31P field cycling NMR is useful for identifying specific phospholipid interactions with a spin-labeled protein in multicomponent vesicles (22). In these experiments, differences in phospholipid 31P relaxation rates provide a direct measure of the proximity of different phospholipid species to the spin label site. In particular, we have shown that for PC/PMe SUVs, B. thuringiensis PI-PLC spin labeled at D205C has a large effect on the 31P relaxation rate of PC with a much smaller effect on PMe consistent with a PC site near the spin label and a bound PC lifetime between 1 μs and 1 ms. PMe, an anionic lipid that also competes with PI substrate, was used in these experiments, because over the course of 24 h there is some hydrolysis of PG by the enzyme and generation of diacylglycerol that causes vesicle fusion. B. thuringiensis Asp-205 aligns with S. aureus Asp-213, and spin labeling S. aureus PI-PLC at D213C has little effect on the PMe or PC resonances (Fig. 3A) under conditions (140 mm salt and 0.5 mg/ml of protein and 5 mm each phospholipid) where ∼15–20% of this labeled WT protein is associated with the SUVs in the presence of salt. The field dependence data for D213C in the WT S. aureus PI-PLC background appears equivalent to what is seen for the SUVs in the absence of protein (22).
FIGURE 3.
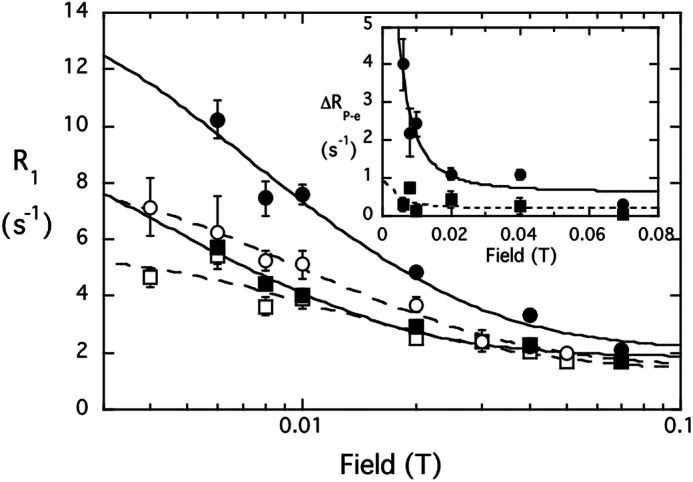
The spin label at D213C perturbs lipid signals for the N254Y/H258Y mutant but not WT. Effect of spin-labeled S. aureus PI-PLC (0.5 mg/ml) on PMe/PC (5:5 mm) SUVs:control PMe (□) and PC (○) mixed with spin-labeled D213C; PMe (■) and PC (●) with the spin-labeled D213C/N254Y/H258Y. The inset shows the difference in R1 for each phospholipid 31P specifically attributed to the spin label on D213C/N254Y/H258Y; the data are fit with τP-e = 2 μs.
However, a very different profile is observed for spin-labeled D213C/N254Y/H258Y under the same conditions, and the results for this variant resemble those for B. thuringiensis PI-PLC (Fig. 3). A potential complication to the average distance determination arises from the fact that S. aureus PI-PLC forms transient dimers on vesicle surfaces via helix B side chains (23). However, based on the dimer x-ray crystal structure, a spin label at residue 213 should be >35 Å from the active site of the opposing monomer, and 34–35 Å from H258Y on the opposing monomer. Because the distance dependence of the spin label falls off as 1/r6, the field cycling NMR should only report on an intramonomer PC site. The stronger effect exerted by the spin-labeled protein on the PC NMR relaxation rate compared with PMe therefore indicates that in the engineered protein (i) PC has a discrete binding site and (ii) it is near the region identified in the B. thuringiensis enzyme (22).
If we assume a single PC binds to the protein for the observed correlation time of the low field dispersion, then we can further use the ratio τP-e/ΔRP-e(0) to estimate a distance for each of these phospholipids to the spin label at residue 213. These parameters are obtained from fitting the relaxation as a function of field that is specifically due to the introduction of the spin label on N254Y/H258Y at D213C (Fig. 3, inset). The correlation time for the 31P-electron dipolar interaction is 2 ± 1 μs and the extrapolated ΔRP-e(0) values for the two phospholipids are 8.52 ± 1.28 s−1 (PC) and 0.71 ± 0.61 s−1 (PMe). Although τP-e is not known precisely, what is relevant in determining rP-e is the ratio τP-e/ΔRP-e(0), and this is very similar when fitting the data at 1 to 3 μs. For PC with τP-e = 2 μs, this yields rP-e = 15.2 ± 0.4 Å. If the plot of ΔR1 versus field is fit at 1- or 3-μs correlation times, there is at most a 0.5-Å variation in the estimated rP-e. Interestingly, this value is a little longer than the rP-e extrapolated for the B. thuringiensis PI-PLC with a spin label attached at the same site (13.5 ± 0.2 Å (22)). The difference in rP-e for PC binding to the two PI-PLC proteins may look small, however, it is real because τP-e/ΔRP-e(0) (which differs by a factor of two for the two proteins) is proportional to rP-e6. There is a small effect on PMe, which should only occupy the active site with this amount of salt in the buffer (23). If one uses the fit with 2 μs rP-e, bound PMe must be ∼22–24 Å away, roughly about where the active would be from the spin label on residue 213. The key result is that the PC binding site we introduced in S. aureus N254Y/H258Y is located in the same area of the protein as it is in the B. thuringiensis PI-PLC.
Crystal Structures of N254Y/H258Y with Choline and DiC4PC
To further explore the location of the introduced PC site, we obtained crystal structures of the N254Y/H258Y double mutant and compared it with those previously obtained for WT. The overall structure of the double mutant follows closely that of the native structure in basic conditions (24), with only slight deviations in the positioning of the mobile loop. The refined model of S. aureus PI-PLC N254Y/H258Y closely resembles that of the Bacillus enzyme especially in the region spanning the top halves of helices F and G. In this region, the structure of the N254Y/H258Y (PDB entry 4I8Y) differs only slightly from the basic form of the S. aureus native structure, aside from the mutations and the rotation of Tyr-212 and Tyr-255 by 83° and 22°, respectively. These rotamer differences are necessary to accommodate the larger side chains of the mutated residues. The rest of the structure shows only small variations associated with the inherent mobility of the enzyme and different amino acid content.
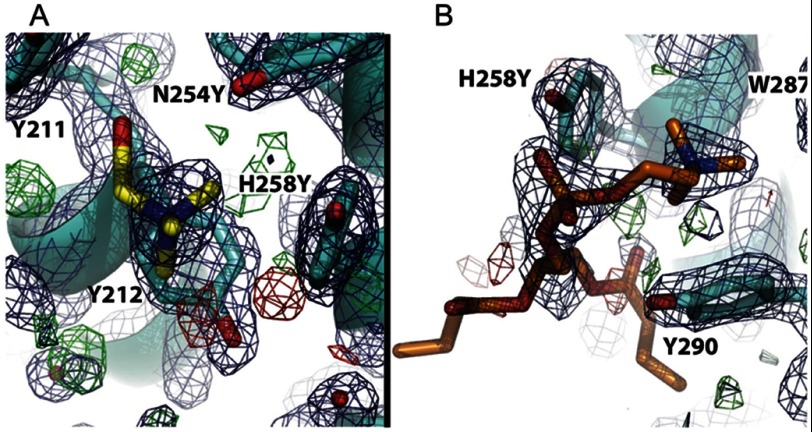
Initial crystallization trials to produce a crystal with a PC molecule bound to the N254Y/H258Y protein used solutions with 100 mm HEPES. Serendipitously, we found that rather than the PC, two HEPES molecules could be refined in the vicinity of the introduced tyrosines (supplemental Fig. S1 and Table S2). Because HEPES is cationic, the thought was that we might be able to get choline-containing molecules to bind if we soaked crystals with high enough concentrations of the potential ligands. Further evidence for the existence and the location of the binding site for a choline moiety was provided by crystal structures of N254Y/H258Y with choline and diC4PC. These crystals were initially grown in 30 mm glycerol phosphocholine and 25 mm choline chloride. Under these conditions, no density was observed for either ligand in the vicinity of the new tyrosine residues. However, when these crystals were soaked for 2 h in a solution containing 500 mm choline or 100 mm diC4PC, distinct density for these choline ligands was observed (representative density with the models superimposed is shown in Fig. 4). Statistics for the different crystal structures are presented in Table 1.
FIGURE 4.
Representative electron density for the choline binding site. Electron density, in dark blue and contoured at 1σ, is shown with the model superimposed: A, binding site 1 with a choline molecule refined (PDB 4I9O); B, binding site 2 showing a molecule of diC4PC fit to the electron density (PDB 4I9J). Residues that make up the binding pocket are indicated.
TABLE 1.
Crystallographic data for critical S. aureus PI-PLC crystals examined
| Crystal | N254Y/H258Y | N254Y/H258Y + choline | N254Y/H258Y +diC4PC |
|---|---|---|---|
| PDB ID | 4I8Y | 4I90 | 4I9J |
| Diffraction data | |||
| Resolution range (Å) | 2.10–30.69Å | 1.65–30.08 | 1.85–37.76 |
| No. of reflections | 18,210 | 36,554 | 24,758 |
| Reflections in free set | 933 | 1840 | 1279 |
| Space group | P212121 | P212121 | P212121 |
| Unit cell | |||
| a (Å) | 85.55 | 85.98 | 85.65 |
| b (Å) | 57.78 | 57.58 | 57.47 |
| c (Å) | 61.38 | 61.69 | 61.75 |
| Completeness | 99.2% | 97.2% | 92.5 |
| Rmerge | 9.9 | 5.8 | 8.4 |
| Protein molecules in A.U. | 1 | 1 | 1 |
| Refinement | |||
| Rcrysta | 0.1781 | 0.1772 | 0.1627 |
| Rfreeb | 0.2312 | 0.2136 | 0.2076 |
| No. residues | 303 | 301 | 301 |
| No. non-hydrogen protein atoms | 2441 | 2449 | 2465 |
| No. H2O molecules | 138 | 195 | 239 |
| No. acetate molecules | 1 | 2 | 1 |
| No. chloride ions | 1 | 1 | 0 |
| Root mean square bonds (Å) | 0.009 | 0.007 | 0.019 |
| Root mean square deviation angles (°) | 1.11 | 1.05 | 1.94 |
| Ramachandran plot (%) | |||
| Most favored | 98.02 | 98.69 | 98.70 |
| Additionally allowed | 1.98 | 1.31 | 1.30 |
| Generously allowed | 0 | 0 | 0 |
| Disallowed | 0 | 0 | 0 |
| Average B-factor (Å2) | 33.74 | 29.40 | 28.998 |
a Rcryst = {Σ (‖Fo| − |Fc‖)/|Fo|}, where |Fo| and |Fc| are the observed and calculated structure factor amplitudes, respectively.
b Brunger (1992).
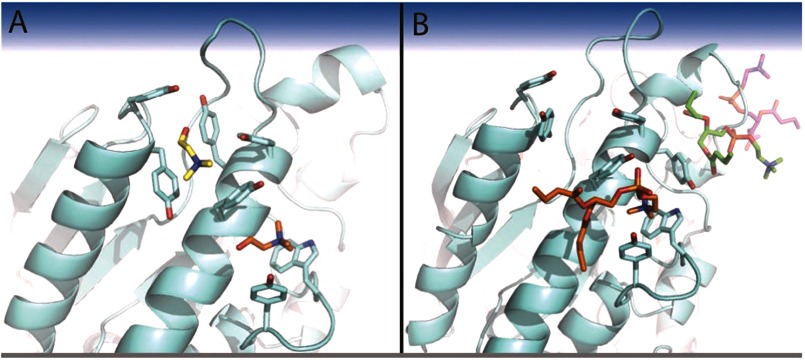
In the N254Y/H258Y structure with choline (PDB entry 4I9O), two molecules of that ligand could be identified (Fig. 5A). The quaternary amines of each choline were observed to bind on either side of H258Y, with the quaternary amine of choline 1 making cation-π interactions with the aromatic side chains of H258Y and Tyr-212, whereas choline 2 makes similar cation-π interactions with Trp-287 and Tyr-290. Comparison with an unliganded structure of N254Y/H258Y shows that the side chains of Tyr-211 and H258Y are rotated 77° and 100°, respectively (Fig. 6A). The rotation of Tyr-211 creates binding pocket 1, whereas the rotation of H258Y forms the right side of binding pocket 1, as well as the left side of binding pocket 2.
FIGURE 5.
Cationic ligand binding pockets on S. aureus PI-PLC N254Y/H258Y. View of the two choline binding pockets (A) occupied by choline shown in yellow in site 1 and orange in site 2 (PDB entry 4I9O) or (B) diC4PC (PDB entry 4I9J) with the lipid in choline site 2 in orange, diC4PC below helix B in green, and diC4PC associated with the anion binding pocket in magenta.
FIGURE 6.
A, overlay of the S. aureus PI-PLC N254Y/H258Y structure without choline (dark blue, PDB entry 4I8Y) and with choline bound (teal, PDB entry 4I9O) showing that choline site 2 is preformed, whereas site 1 requires rotamer changes in side chains. B, overlay of choline site 1 in S. aureus N254Y/H258Y (teal) with the (gray) B. thuringensis PI-PLC (PDB entry 1T6M). Residues interacting with the cholines are identified.
The structure for the mutant protein in the presence of the short-chain lipid diC4PC (PDB entry 4I9J) showed three molecules of diC4PC bound (Fig. 5B). One molecule of diC4PC (refined with 80% occupancy) was located in choline binding pocket 2. The quaternary amines of the choline and diC4PC ligands are completely superimposable. The phosphate moiety of diC4PC makes additional polar contacts to the side chain hydroxyls of Tyr-290 and Tyr-258. Density for this ligand (Fig. 4B) is quite strong for the phosphocholine portion of the molecule and the glycerol moiety. However, there is substantial disorder at the lipid chains. No diC4PC lipids were seen occupying choline binding site 1. However, the high concentration of lipid led to the observation of two additional diC4PC molecules in the structure at sites not occupied by choline. One diC4PC, found at 70% occupancy, was bound through polar contacts of the phosphate to the backbone carbonyls of Leu-37 and Lys-38 and the side chain hydroxyl of Ser-43 (supplemental Fig. S2A). An additional molecule of diC4PC (80% occupancy) was observed (supplemental Fig. S2B) with the phosphate occupying an anion binding pocket (23, 24). Under the conditions this crystal was formed, an acetate ion would be a typical occupant of the anion pocket. The high levels of diC4PC in the soaking liquor could easily outcompete the acetate ion. The observation of a diC4PC phosphate interacting at the anion binding pocket is consistent with the proposal that anionic phospholipids (including substrate PI) in a vesicle can bind in this region (23), and are likely to be displaced when the surface concentration of the anionic lipid decreases. These two additional lipids are held by polar contacts that are not specific to choline or phosphatidylcholine.
Do Both Choline Sites in N254Y/H258Y Bind PC?
A comparison of S. aureus N254Y/H258Y PI-PLC structures in the absence or presence of a trimethylammonium ligands provides insights into the nature of the specific choline and PC sites. To accommodate the cationic ligand there are clear rotations of side chains in choline binding site 1, relative to the unliganded enzyme (Fig. 6A). Interestingly, in the choline bound conformation, binding pocket 1 of the double mutant overlays well with the side chains of the B. thuringiensis PI-PLC structure (PDB entry 1T6M) (Fig. 6B), whereas choline site 2 appears unique to the S. aureus PI-PLC mutant but resembles other choline binding sites that can use a Trp residue to form part of the cation-π box (supplemental Table S1). Although the secondary structure of the two proteins is quite different, the spatial arrangement of tyrosine residues in choline site 1 is very similar to how choline is bound in OpuBC (14). However, we only see soluble diC4PC binding in choline site 2 and not in choline site 1. This could be the result of weak PC binding, at least in the absence of a bilayer (which could locally increase the choline headgroup concentration so that binding occurs more readily), or it could be attributed to the difficulty in moving side chains in site 1 to accommodate a choline moiety, which might be more difficult when the choline is part of a phospholipid molecule.
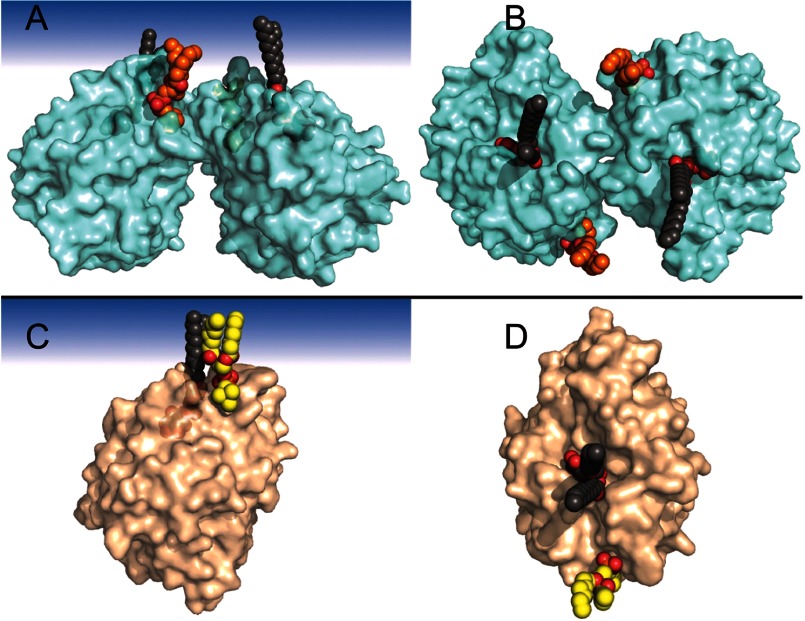
The field cycling 31P NMR experiments provide insight into where PC presented in a bilayer binds on the protein, for at least 2 μs (22). For the same amount of protein and phospholipids, τP-e/ΔRP-e(0), which is proportional to rP-e6, is 2.35 × 10−7 s2 for N254Y/H258Y, and 1.48 × 10−7 s2 for B. thuringiensis PI-PLC, consistent with PC binding closer to the spin label site in B. thuringiensis PI-PLC than in the engineered S. aureus protein. The Bacillus protein only has choline site 1 available for PC binding. Furthermore, if a PC molecule occupied both choline sites when N254Y/H258Y was bound to PC/PMe SUVs, there should be a considerably stronger relaxation of the 31P nucleus that is roughly twice as effective as when a single PC binds to the protein. Because τP-e/ΔRP-e(0) is larger for the S. aureus N254Y/H258Y, only one PC binds well with a ≥2 μs lifetime. In turn, the observation that the averaged distance of the spin label to the bound PC 31P is larger in N254Y/H258Y than in B. thuringiensis PI-PLC is consistent with PC binding to the S. aureus protein in choline site 2. Energy minimization of the crystal structure placed the isolated chains of diC4PC against the protein, but the same orientation of the choline moiety could easily be occupied with a longer chain phospholipid anchored in a bilayer (Fig. 7A).
FIGURE 7.
In silico models of PC and PI binding to discrete sites on S. aureus PI-PLC N254Y/H258Y and B. thuringiensis PI-PLC. The S. aureus mutant structure is shown as a dimer (mediated through helix B) with one molecules of diC7PC in site 2 (orange) and one molecule of PI (dark gray) in the active site in views (A) with the membrane interface at the top, and (B) looking down from the membrane. The B. thuringiensis monomer is shown with diC7PC modeled in site 1 (yellow) along with one molecule of PI (dark gray) in the active site in views (C) with the membrane at the top or (D) looking down from the membrane.
As further evidence for occupation of a single PC site in N254Y/H258Y, we generated Y211A/N254Y/H258Y/Y290A. Mutation of Tyr-290 to alanine should abolish choline affinity for site 2. Tyr-211 is not conserved in B. thuringiensis PI-PLC and must rotate by 77° to accommodate the choline cation. Its removal might be expected to enhance PC binding, if site 1 is significantly populated by a PC molecule. Using the centrifugation assay for partitioning of Y211A/N254Y/H258Y/Y290A onto PG/PC (0.2 mm/0.8 mm) SUVs, we found little protein associated with the SUVs with 1 mm total phospholipid. With 8 mm total phospholipid 7.7 ± 3.8% of the protein was bound. In essence, abolishing choline site 2 prevents S. aureus N254Y/H258Y from binding to PC-containing SUVs. This confirms that although two choline sites were introduced into S. aureus N254Y/H258Y, only preformed site 2 interacts significantly with a PC headgroup.
DISCUSSION
Sandwiches, cages, or boxes composed of aromatic amino acid π systems of aromatic amino acids are a facile way to bind trimethylammonium moieties via cation-π interactions. In so doing they provide a specific recognition motif for the headgroups of zwitterioinic phospholipids. Bacillus PI-PLC is activated by PC (10), in large part by enhancing vesicle binding (25). The G helix region of Bacillus PI-PLCs has several Tyr residues that could form a sandwich or cage around a choline group (22, 28), but how and whether PC binds to this box was unclear. Attempts to transplant this box into S. aureus PI-PLC by introducing two ”missing“ tyrosine side chains in helix G generated catalytically active protein that binds much more tightly to PC-rich interfaces. Field cycling NMR suggest there are similarities in the position of PC bound to B. thuringiensis PI-PLC and to S. aureus N254Y/H258Y protein when the protein is transiently anchored on a vesicle. Although x-ray crystallography reveals two spatially close choline binding sites on the engineered protein (site 1 between tyrosines on helix F and helix G and site 2 between helix G and a loop), only choline site 2 between helix G and a loop is occupied by a PC molecule in the S. aureus protein. B. thuringiensis PI-PLC does not have the key tryptophan residue needed for choline site 2. However, all the side chains are correctly oriented for binding a choline (or PC) in what is analogous to S. aureus N254Y/H258Y site 1.
Although the specific cation-π PC binding site in B. thuringiensis PI-PLC (equivalent of choline site 1) and that in S. aureus PI-PLC (choline site 2) are different, the energetic contribution of these aromatic π-choline cation interactions to PC binding should be similar and different from values for partitioning a tyrosine side chain into a bilayer (40). We can use the apparent Kd values as a way to assess this. The relative change in vesicle binding affinity when the cation-π site is abolished, Kd(no PC site)/Kd(intact PC site), should be similar for both enzymes. Data for XPC = 0.8 SUVs were chosen for the comparison, because no Kd could be obtained for S. aureus WT binding to pure PC SUVs (23). For the S. aureus PI-PLC, Kd(WT)/Kd(N254Y/H258Y) = 48. For B. thuringiensis PI-PLC, the Y251A mutant was selected as one where the site analogous to choline site 1 should be abolished (Tyr-251 is the equivalent of S. aureus H258Y). Binding data at the same mole fraction PC (41) yield Kd(Y251A)/Kd(WT) = 45. Thus, the absence of a PC site, whether it is analogous to choline site 1 or choline site 2 in the S. aureus mutant, has essentially equivalent effects on vesicle binding. This translates to a change in free energy (at 22 °C, the temperature of the FCS experiments) of 9.5 and 9.3 kJ/mol for losing this cation-π interaction. For comparison, partitioning of a tyrosine or a tryptophan side chain from the interior of a bilayer to water is estimated as 3.9 or 7.7 kJ/mol, respectively (40). Clearly, cation-π interactions between proteins and the PC headgroup can stabilize the transient membrane binding needed for peripheral proteins. Invoking these interactions provides an explanation for why S. aureus PI-PLC N254Y/H258Y binds to pure PC bilayers with significantly weaker affinity than B. thuringiensis PI-PLC. Tallying up the tyrosine residues around the rim of the αβ-barrel it is clear that the B. thuringiensis PI-PLC has far more aromatic residues that could either (i) insert into the bilayer or (ii) form transient cation-π complexes.
These results, combined with structural studies of proteins that bind methyl-Lys, methyl-Arg, or choline using similar cation-π cages/boxes suggest that this cation-π motif has evolved in a variety of secondary structural contexts ranging from β barrels to β propellers to α helices and loops, anywhere that aromatic side chains can be separated by 8–10 Å to allow space for binding a methylammonium moiety. In the case of membranes, such π system motifs could provide a general way to introduce specific yet transient interactions of peripheral membrane proteins with PC headgroups.
Supplementary Material
Acknowledgment
We thank Prof. Alfred G. Redfield for access to his high-resolution field cycling device and for sealing samples.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM60418 (to M. F. R.).

This article contains supplemental Tables S1 and S2 and Figs. S1 and S2.
The atomic coordinates and structure factors (codes 4I9J and 4I8Y) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- PS
- phosphatidylserine
- diC4PC
- 1,2-dibutyroyl-sn-glycero-3-phosphocholine
- diC7PC
- 1,2-diheptanoyl-sn-glycero-3-phosphocholine
- FCS
- fluorescence correlation spectroscopy
- PC
- 1-palmitoyl-2-oleoylphosphatidylcholine
- PE
- 1,2-dioleoylphosphatidylethanolamine
- PG
- 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol)
- PI
- l-α-phosphatidylinositol
- PI-PLC
- phosphatidylinositol-specific phospholipase C
- PMe
- 1,2-dioleoylphosphatidylmethanol
- SUV
- small unilamellar vesicle
- WT
- wild-type recombinant S. aureus PI-PLC
- XPC
- mole fraction PC
- PDB
- Protein Data Bank.
REFERENCES
- 1. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids. Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lemmon M. A. (2008) Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9, 99–111 [DOI] [PubMed] [Google Scholar]
- 3. Kutateladze T. G. (2010) Translation of the phosphoinositide code by PI effectors. Nat. Chem. Biol. 6, 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krick R., Busse R. A., Scacioc A., Stephan M., Janshoff A., Thumm M., Kühnel K. (2012) Structural and functional characterization of the two phosphoinositide binding sites of PROPPINs, a β-propeller protein family. Proc. Natl. Acad. Sci. U.S.A. 109, E2042–E2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Concha N. O., Head J. F., Kaetzel M. A., Dedman J. R., Seaton B. A. (1993) Rat annexin V crystal structure. Ca2+-induced conformational changes. Science 261, 1321–1324 [DOI] [PubMed] [Google Scholar]
- 6. Macedo-Ribeiro S., Bode W., Huber R., Quinn-Allen M. A., Kim S. W., Ortel T. L., Bourenkov G. P., Bartunik H. D., Stubbs M. T., Kane W. H., Fuentes-Prior P. (1999) Crystal structures of the membrane-binding C2 domain of human coagulation factor V. Nature 402, 434–439 [DOI] [PubMed] [Google Scholar]
- 7. Shao C., Novakovic V. A., Head J. F., Seaton B. A., Gilbert G. E. (2008) Crystal structure of lactadherin C2 domain at 1.7-Å resolution with mutational and computational analyses of its membrane binding motif. J. Biol. Chem. 283, 7230–7241 [DOI] [PubMed] [Google Scholar]
- 8. Feng J., Wehbi H., Roberts M. F. (2002) Role of tryptophan residues in interfacial binding of phosphatidylinositol-specific phospholipase C. J. Biol. Chem. 277, 19867–19875 [DOI] [PubMed] [Google Scholar]
- 9. Knight J. D., Lerner M. G., Marcano-Velázquez J. G., Pastor R. W., Falke J. J. (2010) Single molecule diffusion of membrane-bound proteins. Window into lipid contacts and bilayer dynamics. Biophys. J. 99, 2879–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou C., Qian X., Roberts M. F. (1997) Allosteric activation of phosphatidylinositol-specific phospholipase C. Specific phospholipid binding anchors the enzyme to the interface. Biochemistry 36, 10089–10097 [DOI] [PubMed] [Google Scholar]
- 11. Schiefner A., Breed J., Bösser L., Kneip S., Gade J., Holtmann G., Diederichs K., Welte W., Bremer E. (2004) Cation-π interactions as determinants for binding of the compatible solutes glycine betaine and proline betaine by the periplasmic ligand-binding protein ProX from Escherichia coli. J. Biol. Chem. 279, 5588–5596 [DOI] [PubMed] [Google Scholar]
- 12. Tschapek B., Pittelkow M., Sohn-Bösser L., Holtmann G., Smits S. H., Gohlke H., Bremer E., Schmitt L. (2011) Arg149 is involved in switching the low affinity, open state of the binding protein AfProX into its high affinity, closed state. J. Mol. Biol. 411, 36–52 [DOI] [PubMed] [Google Scholar]
- 13. Oswald C., Smits S. H., Höing M., Sohn-Bösser L., Dupont L., Le Rudulier D., Schmitt L., Bremer E. (2008) Crystal structures of the choline/acetylcholine substrate-binding protein ChoX from Sinorhizobium meliloti in the liganded and unliganded-closed states. J. Biol. Chem. 283, 32848–32859 [DOI] [PubMed] [Google Scholar]
- 14. Pittelkow M., Tschapek B., Smits S. H., Schmitt L., Bremer E. (2011) The crystal structure of the substrate-binding protein OpuBC from Bacillus subtilis in complex with choline. J. Mol. Biol. 411, 53–67 [DOI] [PubMed] [Google Scholar]
- 15. Nielsen P. R., Nietlispach D., Mott H. R., Callaghan J., Bannister A., Kouzarides T., Murzin A. G., Murzina N. V., Laue E. D. (2002) Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416, 103–107 [DOI] [PubMed] [Google Scholar]
- 16. Jacobs S. A., Khorasanizadeh S. (2002) Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295, 2080–2083 [DOI] [PubMed] [Google Scholar]
- 17. Collins R. E., Northrop J. P., Horton J. R., Lee D. Y., Zhang X., Stallcup M. R., Cheng X. (2008) The ankyrin repeats of G9a and GLP histone methyltransferases are mono- and dimethyllysine binding modules. Nat. Struct. Mol. Biol. 15, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tripsianes K., Madl T., Machyna M., Fessas D., Englbrecht C., Fischer U., Neugebauer K. M., Sattler M. (2011) Structural basis for dimethylarginine recognition by the Tudor domains of human SMN and SPF30 proteins. Nat. Struct. Mol. Biol. 18, 1414–1420 [DOI] [PubMed] [Google Scholar]
- 19. Roderick S. L., Chan W. W., Agate D. S., Olsen L. R., Vetting M. W., Rajashankar K. R., Cohen D. E. (2002) Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat. Struct. Biol. 9, 507–511 [DOI] [PubMed] [Google Scholar]
- 20. Lovgren A., Carlson C. R., Eskils K., Kolsto A. B. (1998) Localization of putative virulence genes on a physical map of the Bacillus thuringiensis subsp. gelechiae chromosome. Curr. Microbiol. 37, 245–250 [DOI] [PubMed] [Google Scholar]
- 21. Daugherty S., Low M. G. (1993) Cloning, expression, and mutagenesis of phosphatidylinositol-specific phospholipase C from Staphylococcus aureus. A potential staphylococcal virulence factor. Infect. Immun. 61, 5078–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pu M., Orr A., Redfield A. G., Roberts M. F. (2010) Defining specific lipid binding sites for a peripheral membrane protein in situ using subtesla field cycling NMR. J. Biol. Chem. 285, 26916–26922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng J., Goldstein R., Stec B., Gershenson A., Roberts M. F. (2012) Competition between anion binding and dimerization modulates S. aureus phosphatidylinositol-specific phospholipase C enzymatic activity. J. Biol. Chem. 287, 40317–40327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldstein R., Cheng J., Stec B., Roberts M. F. (2012) Structure of the S. aureus PI-specific phospholipase C reveals modulation of active site access by a titratable π-cation latched loop. Biochemistry 51, 2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pu M., Roberts M. F., Gershenson A. (2009) Fluorescence correlation spectroscopy of phosphatidylinositol-specific phospholipase C monitors the interplay of substrate and activator lipid binding. Biochemistry 48, 6835–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng J., Karri S., Grauffel C., Wang F., Reuter N., Roberts M. F., Wintrode P. L., Gershenson A. (2013) Does changing the predicted dynamics of a phospholipase C alter activity and membrane binding? Biophys. J. 104, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wehbi H., Feng J., Kolbeck J., Ananthanarayanan B., Cho W., Roberts M. F. (2003) Investigating the interfacial binding of bacterial phosphatidylinositol-specific phospholipase C. Biochemistry 42, 9374–9382 [DOI] [PubMed] [Google Scholar]
- 28. Shi X., Shao C., Zhang X., Zambonelli C., Redfield A. G., Head J. F., Seaton B. A., Roberts M. F. (2009) Modulation of Bacillus thuringiensis phosphatidylinositol-specific phospholipase C activity by mutations in the putative dimerization interface. J. Biol. Chem. 284, 15607–15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Redfield A. G. (2012) High-resolution NMR field cycling device for full range relaxation and structural studies of biopolymers on a shared commercial instrument. J. Biomol. NMR 52, 159–177 [DOI] [PubMed] [Google Scholar]
- 30. Bian J., Roberts M. F. (1992) Thermodynamic comparison of lyso- and diacylphosphatidylcholines. J. Colloid. Interface Sci. 153, 420–428 [Google Scholar]
- 31. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 32. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX. A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emsley P., Cowtan K. (2004) Coot. Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 35. Collaborative Computational Project, Number 4 (1994) The CCP4 Suite. Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 36. Krissinel E., Henrick K. (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 [DOI] [PubMed] [Google Scholar]
- 37. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) PROCHECK. A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 38. Heinz D. W., Ryan M., Bullock T. L., Griffith O. H. (1995) Crystal structure of the phosphatidylinositol-specific phospholipase C from Bacillus cereus in complex with myo-inositol. EMBO J. 14, 3855–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moser J., Gerstel B., Meyer J. E., Chakraborty T., Wehland J., Heinz D. W. (1997) Crystal structure of the phosphatidylinositolspecific phospholipase C from the human pathogen, Listeria monocytogenes. J. Mol. Biol. 273, 269–282 [DOI] [PubMed] [Google Scholar]
- 40. Wimley W. C., White S. H. (1996) Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat. Struct. Biol. 3, 842–848 [DOI] [PubMed] [Google Scholar]
- 41. Grauffel C., Yang B., He T., Roberts M. F., Gershenson A., Reuter N. (2013) Cation-π interactions as lipid-specific anchors for phosphatidylinositol-specific phospholipase-C. J. Am. Chem. Soc. 135, 5740–5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.