Background: PACAP induces neuronal differentiation in PC12 cells via multiple pathways that are not fully delineated.
Results: PACAP elicits c-Rel nuclear translocation via ERK1/2 stimulation and Ca2+ mobilization to induce cell survival and neuritogenesis.
Conclusion: PACAP promotes PC12 cell survival and neuritogenesis by activating NF-κB signaling.
Significance: A novel molecular target of PACAP signaling during PC12 cell differentiation has been characterized.
Keywords: Cell Differentiation, Neurite Outgrowth, Neuropeptide, Neurotrophic Factor, NF-κB, ERK, PACAP, PC12 Cells, Calcium, Cell Survival
Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP) is a trophic factor that promotes neuronal survival and neurite outgrowth. However, the signaling pathways and the transcriptional mechanisms involved are not completely elucidated. Our previous studies aimed at characterizing the transcriptome of PACAP-differentiated PC12 cells revealed an increase in the expression of nuclear factor κB2 (NF-κB2) gene coding for p100/p52 subunit of NF-κB transcription factor. Here, we examined the role of the NF-κB pathway in neuronal differentiation promoted by PACAP. We first showed that PACAP-driven survival and neuritic extension in PC12 cells are inhibited following NF-κB pathway blockade. PACAP stimulated both c-Rel and p52 NF-κB subunit gene expression and nuclear translocation, whereas c-Rel down-regulation inhibited cell survival and neuritogenesis elicited by the neuropeptide. PACAP-induced c-Rel nuclear translocation was inhibited by ERK1/2 and Ca2+ blockers. Furthermore, the neuropeptide stimulated NF-κB p100 subunit processing into p52, indicative of activation of the NF-κB alternative pathway. Taken together, our data show that PACAP promotes both survival and neuritogenesis in PC12 cells by activating NF-κB pathway, most likely via classical and alternative signaling cascades involving ERK1/2 kinases, Ca2+, and c-Rel/p52 dimers.
Introduction
Differentiation is a key step of cell growth that allows the transition from proliferating progenitors to specialized, functionally oriented cells. Growth factors are major effectors of cell specification during development, acting through various signaling pathways that are translated into a cell decision to proliferate or differentiate. In the nervous system, several trophic cues are produced and secreted to support the development, function, maintenance, and plasticity of nerve cells (1). Besides the neurotrophin family of growth factors represented by nerve growth factor (NGF)2 or brain-derived neurotrophic factor, which act on different sensory, sympathetic, and central nervous system (CNS) neurons, it has been shown that the neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) also exerts multiple neurotrophic functions. PACAP affects neuronal cell cycle exit during CNS formation (2), promotes neuronal differentiation in cultured rat sympathetic neuroblasts (3), and stimulates neuritogenesis (4). PACAP prevents apoptotic cell death and protects cultured rat cerebellar and cortical neurons against cytotoxic insults (5–7). Whereas neurotrophins exert their effects through receptor tyrosine kinases (8), PACAP acts via two types of G protein-coupled receptors: a PACAP-selective receptor, PAC1-R, and two PACAP/vasoactive intestinal polypeptide mutual receptors, VPAC1-R and VPAC2-R (9). These receptors have been shown to activate different signal transduction pathways via cyclic AMP (cAMP) elevation and calcium (Ca2+) mobilization, leading to stimulation of several protein kinases, such as protein kinase A (PKA) and the MAP kinases ERK1/2, which in turn induce or repress transcription of genes associated with cell growth and differentiation (10, 11). PACAP has been shown to induce growth arrest and to promote survival and neuritic extension in PC12 cells (12), thus offering a paradigm to study the signaling cascades leading to neuronal differentiation induced by a ligand of G protein-coupled receptors.
In previous studies aimed at characterizing the phenotype of PACAP-differentiated PC12 cells, we identified genes and gene families potentially involved in the pleiotropic effects of the neuropeptide PACAP in neuronal development, neuroprotection and neurotransmission (13). These microarray studies revealed that among the genes activated during PACAP-induced neuronal differentiation, the expression of nuclear factor κB2 (NF-κB2) gene coding for the p100/p52 subunit of NF-κB transcription factor was significantly increased. Whereas the NF-κB pathway has been implicated in the neurotrophic effects induced by receptor tyrosine kinase activation (14–16), our finding was intriguing because no report had previously described the direct implication of NF-κB pathway in G protein-coupled receptor signaling or in the neurotrophic effects of neuropeptides such as PACAP.
The transcription factor NF-κB was best studied for its involvement in inflammation and immune responses (17), but it is also widely expressed in the CNS where it can promote neuronal survival and protection from toxic insults (18, 19). It has also been implicated in adaptive responses to neuroinflammatory conditions and neurodegenerative disorders (20). Many effects mediated by NF-κB in the nervous system are reminiscent of those of PACAP, suggesting that the neuropeptide may stimulate specific but yet uncharacterized signal transduction cascades ultimately involving activation of this transcription factor, which would lead to induction of different aspects of neuronal differentiation and protection. To test this hypothesis and to decipher the molecular mechanisms involving NF-κB, we studied the activation and role of the five members of the NF-κB family, i.e. RelA (p65), RelB, c-Rel, p50, and p52 subunits during PACAP-induced PC12 cell differentiation. We used a multilevel approach to characterize the role of NF-κB subunits in cell survival and neurite outgrowth, two prominent features occurring during cell differentiation induced by PACAP. Given the known signaling pathways implicated in the trophic effects of the neuropeptide (10, 11), we targeted specific transduction cascades that could be linked to NF-κB in neuronal cells. Our data presented below unveil a novel aspect of PACAP signaling through activation of ERK1/2 MAP kinases and Ca2+ to recruit c-Rel and p52 subunits of NF-κB to promote neuronal cell survival and neuritogenesis.
EXPERIMENTAL PROCEDURES
Drugs
PACAP38 was synthesized by the solid phase methodology as described previously (21). PS-1145, PD98059, NiCl2, EGTA, chelerythrine, and wortmannin were obtained from Sigma-Aldrich, and U0126 was purchased from Promega.
Cell Culture
Rat pheochromocytoma PC12 cells were obtained from the European Collection of Cell Culture (ECACC, Salisbury, Wiltshire, UK) and maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich), supplemented with 10% horse serum (Invitrogen), 5% fetal bovine serum (Sigma-Aldrich), 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) at 37 °C in 5% CO2 humidified atmosphere. The medium was renewed every 2–3 days. Twenty-four h after plating, differentiation of PC12 cells was initiated by adding 100 nm PACAP38.
Cell Viability Assay
Viable cell number was assessed using the MTT assay. Briefly, PC12 cells cultured in 100 μl of medium in 96-well culture plates were incubated with 10 μl of phosphate-buffered saline (PBS) containing 5 mg/ml MTT for 4 h at 37 °C in 5% CO2 atmosphere. Then, 100 μl of a solubilization solution of 50% N,N-dimethylformamide and 20% sodium dodecyl sulfate (SDS), pH 4.7, was added to the culture medium, and the plates were incubated at 37 °C overnight. The absorbance was then recorded using a microplate reader Bio-tek FL 600 (Bio-tek, Illkirsh, France). Specific optical density (ΔOD570–750) for the MTT product was obtained by subtracting the OD value at 750 nm from that at 570 nm, reflecting the number of viable cells.
Assessment of Caspase-3-like Activity
Caspase-3-like activity in cell culture was measured using an Apo-ONE Homogeneous Caspase-3/7 Assay Kit (Promega) according to the manufacturer's instructions. Briefly, cells (4 × 104 cells/well) in poly-l-lysine-coated 96-well plates (Corning) were rinsed twice with PBS and incubated in the presence or absence of the different effectors in DMEM (50 μl) at 37 °C in 5% CO2 atmosphere. Cells were then homogenized in 50 μl of Homogeneous Caspase-3/7 Buffer containing the caspase-3 substrate Z-DEVD-rhodamine 110, and the fluorescence intensity (excitation at 498 nm and emission at 521 nm) was measured in cell lysates during 3 h, using a Flexstation II spectrofluorophotometer.
Neurite Outgrowth Measurement
PC12 cells cultured in 6-well culture plates were observed under a macroconfocal Leica TCS LSI microscope. Neurite analysis was performed using ImageJ software (National Institutes of Health). Neurite outgrowth was evaluated by calculating the number of processes per cell and measuring neurite length. Neurite was defined as a cell process >10 μm.
Real-time Polymerase Chain Reaction
Total RNA was isolated from PC12 cells using the Tri-Reagent (Sigma-Aldrich). Approximately 1 μg of total RNA was reverse-transcribed using the ImProm-IITM Reverse Transcription System (Promega). One tenth of the reaction mixture was used to quantify RelA, c-Rel, p52, and p50 expression by real-time PCR. Gene-specific forward and reverse primers were designed using the Primer Express software (Applied Biosystems) as follows: 5′-CTTCTGGGCCATATGTGGAGA-3′ and 5′-TCGCACTTGTAACGGAAACG-3′ for the RelA gene, 5′-CTTCCTGATGAACATGGTAACTTCA-3′ and 5′-GGGCACGGTTGTCGTAAATC-3′ for the c-Rel gene, 5′-AGCACCAAGACCGAAGCAA-3′ and 5′-TTATCCTGAAACCCCACATCCT-3′ for the p50 gene, 5′-CCAAGGACATGACTGCTCAATTTA-3′ and 5′-TGAATCATAATCTCCATCATGTTCTTC-3′ for the p52 gene, and 5′-AACTCCCTCAAGATTGTCAGCAA-3′ and 5′-TGGTCATGAGCCCTTCCA-3′ for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a reference gene. Real-time PCR was carried out in SYBR Green® PCR Master Mix in the presence of 300 nm specific primers, using an ABI Prism 7000 apparatus (Applied Biosystems). The relative efficiency of the amplification of each gene was assessed, and the amounts of each mRNA were determined by normalizing against GAPDH mRNA levels using the comparative Ct method as described by the apparatus manufacturer (Applied Biosystems).
Immunocytochemistry
PC12 cells cultured on coverslips in 12-well culture plates were washed twice with ice-cold PBS, fixed in a paraformaldehyde solution (4% w/v) for 15 min, then permeabilized with a Triton X-100 solution (0.1% Triton X-100 in PBS) for 20 min. After a saturation step of 1 h in BSA at 1 mg/ml in PBS, cells were incubated overnight at 4 °C with primary antibodies directed against RelA, c-Rel, RelB, p50, or p52 subunits of NF-κB (1:200 dilution, Santa Cruz Biotechnology). Immunodetection was revealed with FITC-conjugated goat anti-rabbit immunoglobulin G (1:300 dilution), and nuclei were visualized by DAPI staining (1 pg/ml; Sigma). After mounting, fluorescence was analyzed using a Leica TCS SP2 confocal microscope. For each staining analysis, three fields were selected. Before acquisition, we used a z stacking to select a z section containing the plain nucleus as visualized by DAPI staining. This method permits to avoid measurement of fluorescence of z sections located above or below the nucleus during analysis. For all images of the same experiment, the acquisition settings were unchanged except the z parameters. For each field, images of DAPI and NF-κB subunit stainings were acquired with the confocal microscope. The two images obtained were used to create layer masks of nuclear and cytoplasmic NF-κB staining thanks to the image treatment software GNU Image Manipulation Program (GIMP). The DAPI staining layer mask was used to define the nuclear region of interest by eliminating all regions except nuclear staining in the NF-κB staining layer mask. The DAPI layer mask was subtracted from the NF-κB layer mask to create a staining mask defining the cytoplasmic region of interest. This technique adapted from a procedure described by Noursadeghi et al. (22) was used to separate nuclear and cytoplasmic stainings within each image allowing thus to avoid measurement of fluorescence cross-contamination. The fluorescence of each region of interest was measured using the ImageJ software.
Western Blot Analysis
Antibodies against phospho-ERK1/2 and Lamin-A were obtained, respectively, from Cell Signaling Technology and Sigma-Aldrich. For cell fractionation, cytoplasmic and nuclear fractions were prepared by using a NE-PER® nuclear and cytoplasmic extraction kit (Thermo Scientific) according to the manufacturer's protocol. For whole protein analysis, PC12 cells cultured in 6-well plates (105 cells/well) were lysed in a buffer containing 10 mm Tris-HCl, pH 7.4, 0.05% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride. After centrifugation (12,000 × g, 15 min), the supernatant was precipitated with 10% trichloroacetic acid, and the whole protein precipitate was washed by ethanol/ether (v/v) and dissolved in electrophoresis-denaturing buffer. In all extracts, proteins were quantified by the Bradford assay (Bio-Rad) to ensure loading of equivalent amounts from all samples. Extracts were separated by SDS-polyacrylamide gel electrophoresis followed by electrotransfer onto nitrocellulose membranes (Amersham Biosciences). The different proteins were detected using the appropriated polyclonal antibody (1:1000 dilution) followed by horseradish peroxidase-conjugated secondary antibodies (1:5000). The antigen-antibody complexes were visualized by the chemiluminescence ECL Western blotting analysis system (Amersham Biosciences) and exposure to Kodak X-OMAT films (Sigma-Aldrich). Signals were quantified using ImageJ software.
siRNA Transfection
PC12 cells were transfected with siRNA (Qiagen) by using the Amaxa Nucleofector system and the Cell Line Nucleofector kit V electroporation method (Lonza, Switzerland) according to the manufacturer's protocol (program U-029). To assess the efficiency of siRNA-induced knockdown, mRNA levels of the target genes were determined by real-time quantitative PCR.
Statistical Analysis
Results were expressed as means ± S.E. Statistical significance was assessed through the nonparametric Mann-Whitney test and Student's t test. A probability level of p values < 0.05 was considered statistically significant.
RESULTS
NF-κB Is Required for PACAP-induced PC12 Cell Differentiation
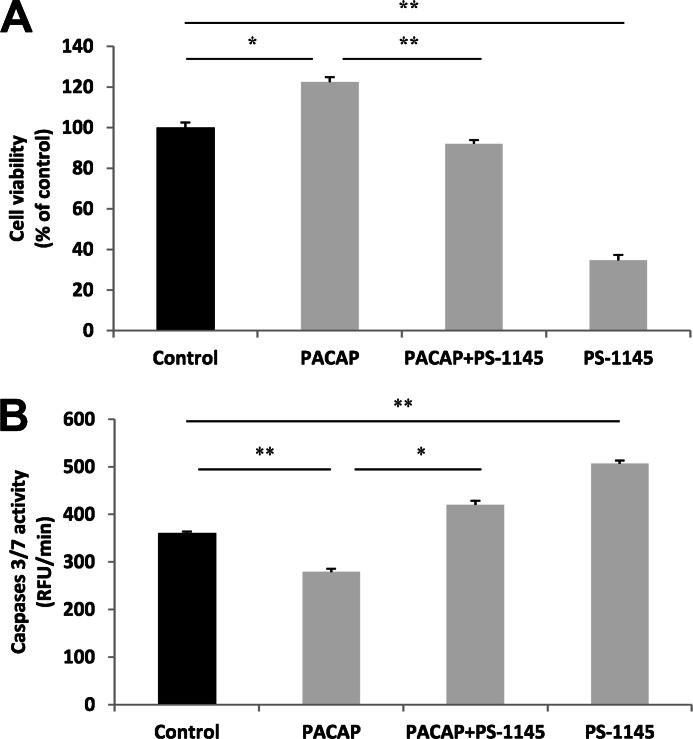
To determine the role of NF-κB in PACAP-induced PC12 cell neuronal differentiation, we evaluated the effect of PS-1145, a specific blocker of the kinase inhibitor of κB (IκB) kinase (IKK)-1 and IKK-2 which are responsible for activation of NF-κB signaling, on two major events occurring during this process, i.e. cell number increase and neurite outgrowth stimulation (21, 23). The MTT assay revealed that the PACAP-elicited increase in the number of living cells is prevented in the presence of PS-1145 (10 μm) (Fig. 1A). It is noteworthy that PS-1145 exerted a strong inhibitory effect on PC12 cell survival in basal condition (Fig. 1A), in accordance with previous reports showing the importance of NF-κB activity for neuronal survival (18, 24). Analysis of caspase-3/7 activity showed that PS-1145 (10 μm) reverted the inhibitory action of PACAP (100 nm) on these pro-apoptotic enzymes (Fig. 1B). In line with the PS-1145-induced decrease in cell number in basal condition, the NF-κB blocker stimulated caspase-3/7 activity in PC12 cells (Fig. 1B). These data indicate that the NF-κB pathway plays an important role in basal as well as PACAP-stimulated neuronal survival by inhibiting caspase activity and thereby apoptosis.
FIGURE 1.
Effect of PS-1145 on PACAP-regulated PC12 cell viability and caspase activity. A, PC12 cells were exposed to PACAP (100 nm) in the presence or absence of PS-1145 (10 μm) for 48 h. Cell viability was assessed using the MTT assay. B, PC12 cells were exposed to PACAP (100 nm) in the absence or presence of PS-1145 (10 μm) for 6 h. Caspase-3/7 activity was evaluated in PC12 cells by the Apo-ONE Kit and presented as relative fluorescence units (RFU) per min. Data are expressed as mean ± S.E. (error bars) relative to control values (n = 6). *, p < 0.05; **, p < 0.01 (Mann-Whitney U test). The experiments were repeated three times with similar results.
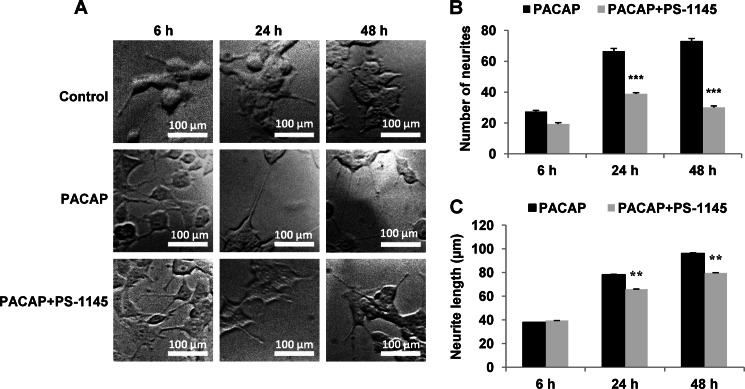
We next investigated whether the NF-κB pathway could be involved in PACAP-induced neurite outgrowth in PC12 cells. Our earlier studies showed that PACAP, as NGF, elicits a rapid stimulation of neurite outgrowth in PC12 cells which becomes visible at 6 h and prominent at 24 and 72 h after the application of the neuropeptide (21). Therefore, we determined the number of neurites and their length following incubation with PACAP (100 nm) for 6, 24, and 48 h, with or without PS-1145 (10 μm). Although the NF-κB inhibitor did not appreciably impair PACAP-induced neurite outgrowth at 6 h (Fig. 2A), the drug significantly reduced this process after 24 and 48 h of treatment (Fig. 2, A and B), indicating that NF-κB is involved in neurite formation in response to PACAP stimulation. Similarly, treatment with PS-1145 for 24 and 48 h altered neurite length (Fig. 2C), implying that NF-κB also contributes to neuritic extension induced by PACAP.
FIGURE 2.
Effect of PS-1145 on PACAP-induced neurite outgrowth. A, cells were left untreated or treated with PACAP (100 nm) in presence or absence of PS-1145 (10 μm) for 6, 24, or 48 h. B and C, the number of neurites for 100 cells (B) and neurite length (C) were determined using confocal macroscopy and ImageJ software. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). Scale bars, 100 μm. **, p < 0.01; ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results.
PACAP and Tumor Necrosis Factor-α (TNF-α) Differentially Regulate NF-κB Subunit Expression and Nuclear Translocation
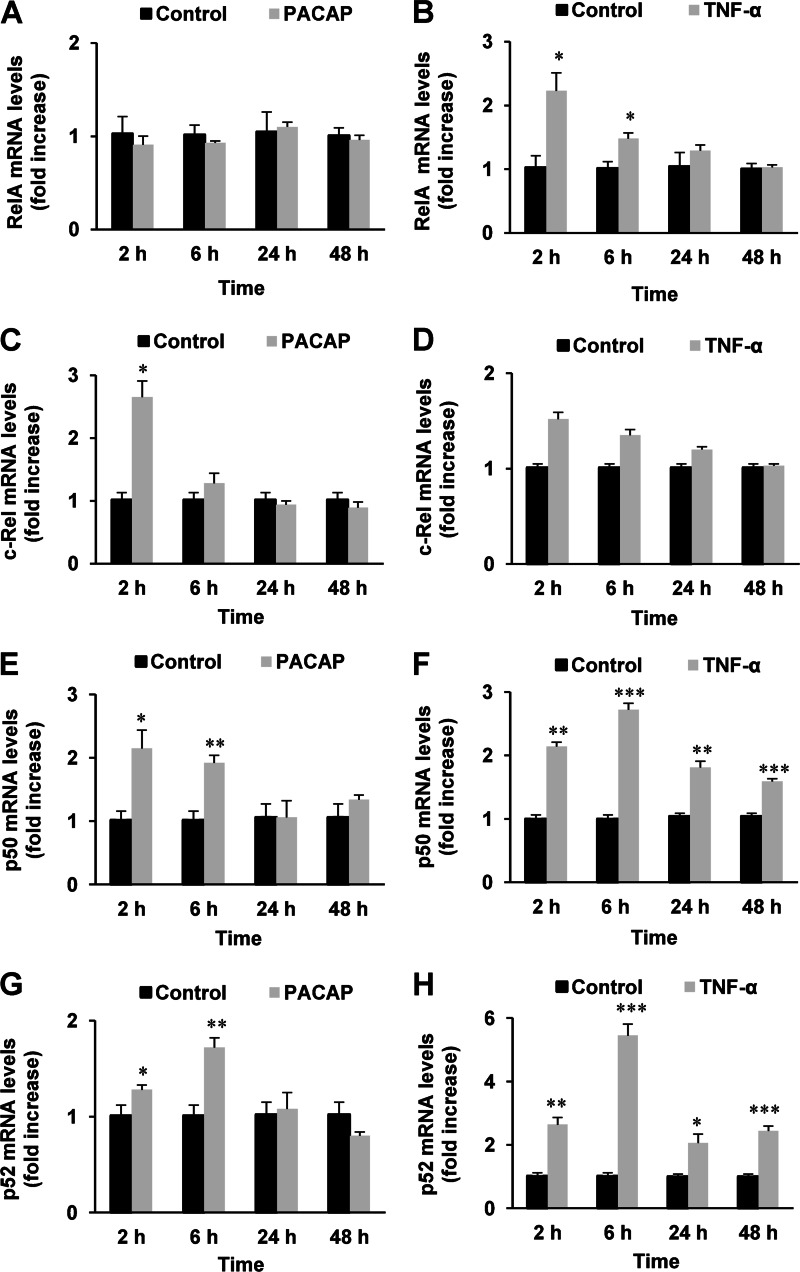
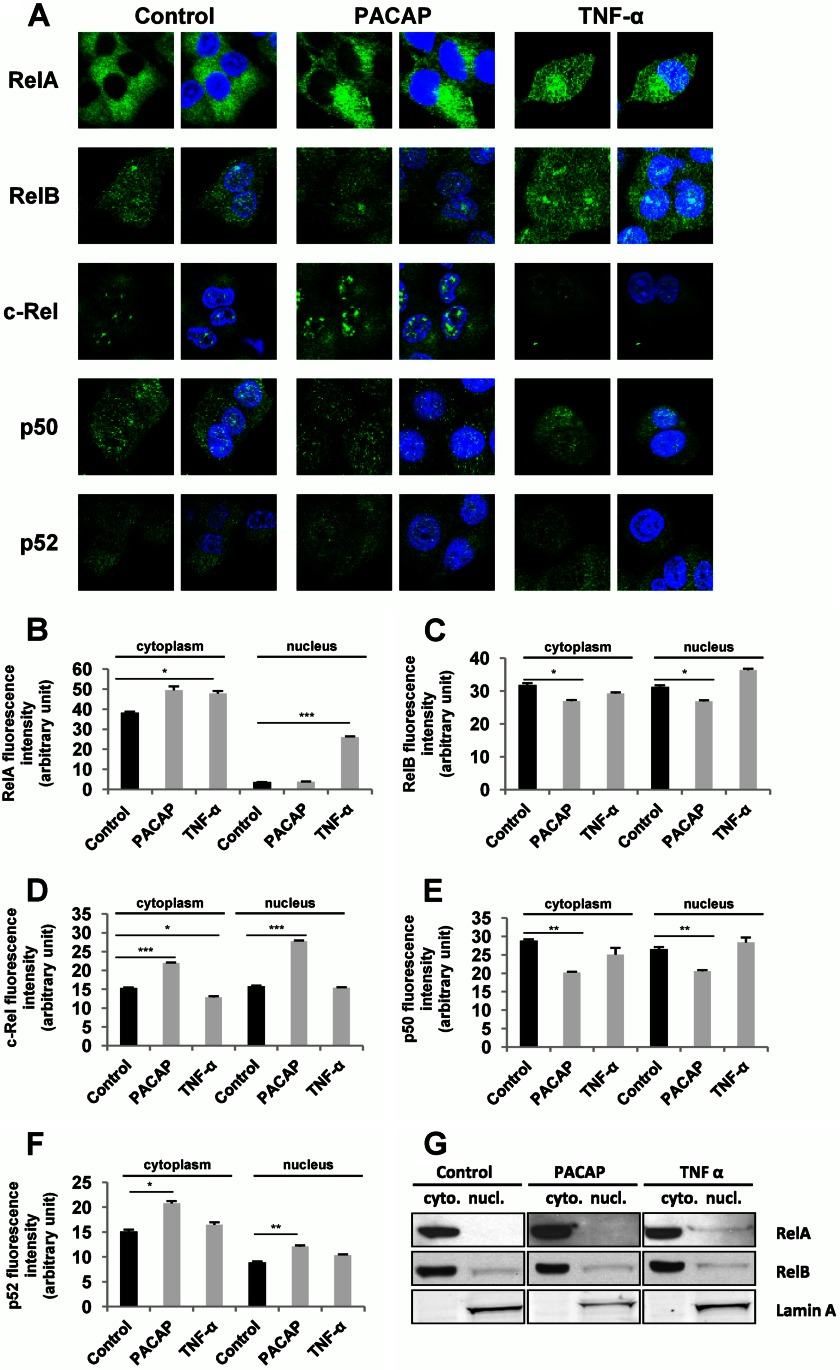
As a first approach to determine which NF-κB subunits are involved in PACAP trophic actions in PC12 cells, we evaluated the effect of the neuropeptide (100 nm) on RelA, c-Rel, p50, and p52 gene expression compared with the effect of TNF-α (10 nm), a cytokine known to activate NF-κB signaling but unable to induce PC12 cell differentiation. Quantitative PCR analysis revealed that PACAP, like TNF-α, is able to stimulate NF-κB subunit gene expression and that the two molecules acted either similarly or differently depending on the subunit (Fig. 3). PACAP induced a transient increase in c-Rel gene expression observed at 2 h of treatment, but had no effect on RelA mRNA levels (Fig. 3, A and C). Inversely, TNF-α was ineffective on c-Rel but stimulated RelA gene expression (Fig. 3B and D). Both PACAP and TNF-α increased the mRNA levels of p50 and p52 subunits (Fig. 3, E–H), albeit with different kinetics of stimulation. Thus, TNF-α elicited a persistent increase in p50 and p52 gene expression from 2 to 48 h which reached a peak value after 6 h of treatment (Fig. 3, F and H), whereas PACAP exerted a transient stimulation of gene expression observed at 2 and 6 h of treatment (Fig. 3, E and G). To determine whether the protein products of NF-κB subunit genes were also regulated by PACAP and whether this neuropeptide could affect their subcellular localization, we analyzed by immunofluorescence cytochemistry the cytoplasmic and nuclear occurrence of RelA, RelB, c-Rel, p50, and p52 subunits in PC12 cells. The effects of PACAP were compared with those of TNF-α (Fig. 4) to gain insight in the specific subunit complexes that may underlie the trophic activities of the neuropeptide. Consistent with the pro-survival effect of NF-κB in neuronal cells, several subunits including RelB, c-Rel, p50, and p52 were constitutively expressed in the nucleus of PC12 cells (Fig. 4, A and C–F). However, PACAP significantly increased c-Rel and p52 subunit levels in the cytoplasm and nucleus (Fig. 4, A, D, and F), but slightly decreased those of RelB and p50 in both compartments after 2 h of treatment (Fig. 4, A, C, and E). Finally, both PACAP and TNF-α induced a small increase in cytoplasmic RelA, whereas only TNF-α increased RelA levels in the nucleus (Fig. 4, A and B). Because Rel subunits hold the transactivation domain that ultimately regulates target genes, we performed a Western blot analysis of these subunits in nuclear and cytoplasmic preparations obtained by fractionation of treated and untreated PC12 cells to confirm the previous data obtained by immunofluorescence cytochemistry. This Western blot analysis confirmed the stimulatory effect of PACAP and TNF-α on RelA subunit in the cytoplasm and the nucleus, respectively, but little or no appreciable effect of PACAP and TNF-α on RelB subunit could be observed (Fig. 4G). Unfortunately, using four antibodies obtained from different suppliers, we were unable to detect a common c-Rel-specific band (inhibited by siRNA) in Western blot analysis (data not shown), suggesting that c-Rel antibodies are suitable for immunocytochemistry but not Western blot analysis of PC12 cell fractions. Overall, the data obtained by quantitative PCR and immunofluorescence cytochemistry analyses suggest that the neurotrophic effects of PACAP could be mediated by c-Rel and p52 whose gene expression and protein translocation to the nucleus were stimulated by the peptide after 2 h of treatment.
FIGURE 3.
Kinetic analysis of the effect of PACAP (100 nm) and TNF-α (10 nm) on the mRNA levels of NF-κB subunits. PC12 cells were treated with PACAP (A, C, E, and G) or TNF-α (B, D, F, and H) for the indicated times and RelA (A and B), c-Rel (C and D), p50 (E and F), and p52 (G and H) gene expression was quantified by real-time PCR. Data are expressed as mean ± S.E. (error bars) relative to control values (n = 4). *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Mann-Whitney U test). The experiment was repeated two times with similar results.
FIGURE 4.
Effect of PACAP and TNF-α on the subcellular localization of NF-κB subunits. A, PC12 cells were incubated with PACAP (100 nm) or TNF-α (10 nm) for 2 h, and immunocytochemistry was performed using antibodies directed against the different NF-κB subunits followed by a fluorochrome-conjugated secondary antibody. Nuclei were labeled with DAPI. B–F, nuclear and cytoplasmic fluorescence of RelA (B), RelB (C), c-Rel (D), p50 (E), and p52 (F) was measured using ImageJ software. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). **, p < 0.01; ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results. G, PC12 cells were treated with PACAP (100 nm) or TNF-α (10 nm) for 2 h, and cytoplasmic and nuclear fractions were prepared. Western blotting was performed by using anti-RelA and anti-RelB antibodies to assess nuclear translocation of the subunits. Lamin A was used as a marker of the nuclear fraction. Cytoplasmic and nuclear fractions are respectively referred to as cyto. and nucl.
PACAP-induced Pro-survival and Pro-differentiating Effects Rely on c-Rel Expression
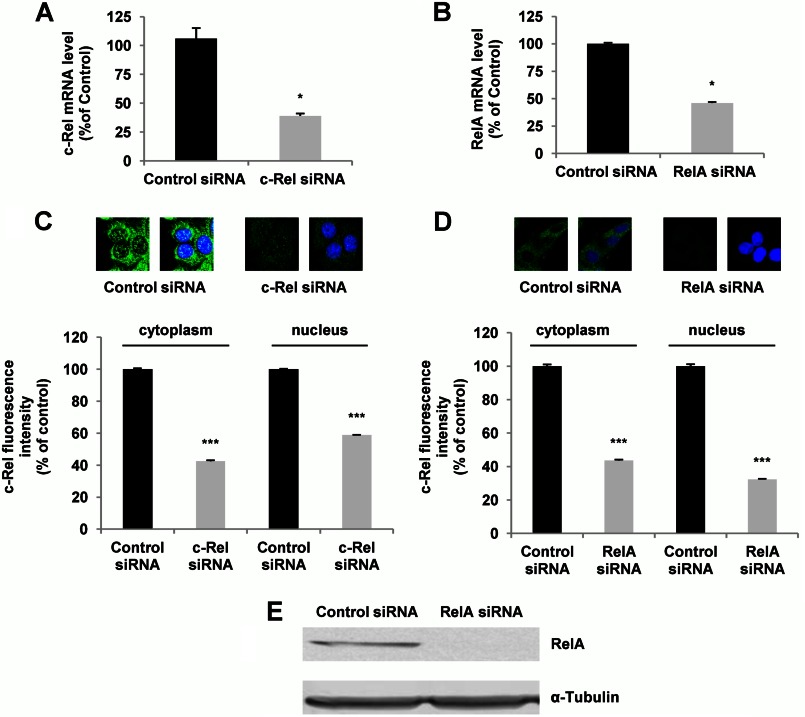
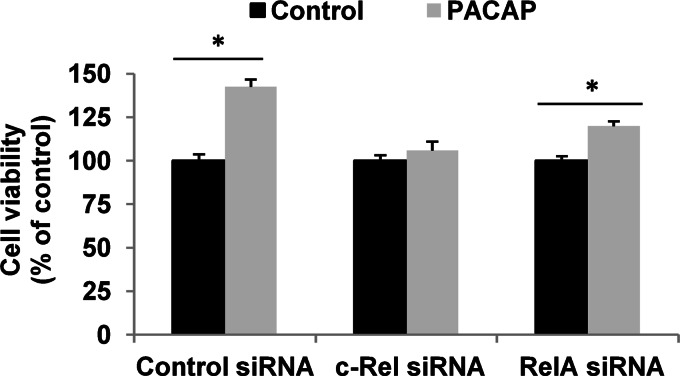
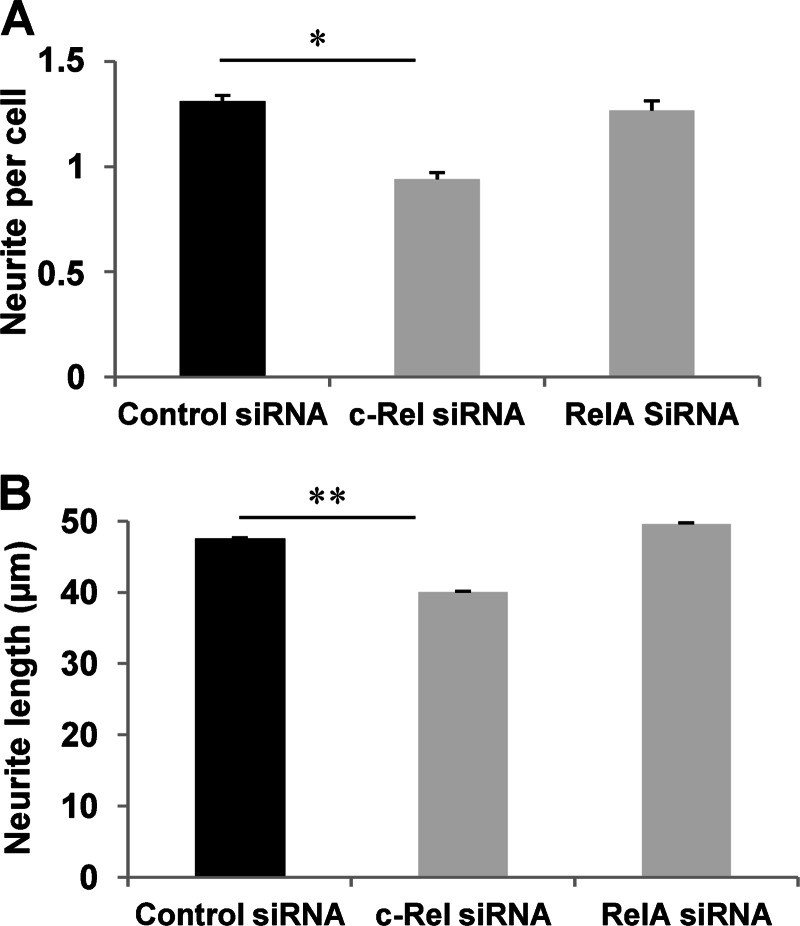
Having determined that PACAP exerts specific effects on NF-κB subunits, we proceeded with experiments aimed at examining the involvement of these subunits in its trophic activities. Because the c-Rel subunit was selectively and appreciably stimulated by PACAP and not by TNF-α and displays a transactivation domain allowing it to stimulate target gene expression, we sought to determine c-Rel implication in PACAP-induced neuronal survival and differentiation. We compared the role of c-Rel with that of RelA whose expression and translocation were not significantly altered by PACAP. We first determined that siRNA inhibition leads to 61 and 54% decrease in c-Rel and RelA mRNA levels, respectively, in PC12 cells (Fig. 5, A and B). The efficiency of siRNA-induced knockdown was confirmed by analysis of the target protein by immunofluorescence cytochemistry (Fig. 5, C and D) and by Western blot analysis for RelA siRNA (Fig. 5E). We found that PACAP-promoted PC12 cell viability is abolished when c-Rel, but not RelA, expression was inhibited by siRNA (Fig. 6). In contrast, control siRNA exerted no effect on PACAP-elicited increase in cell number (Fig. 6). To determine whether c-Rel was also required for PACAP-induced neuritogenesis, we counted the number of neurites and measured their length after treatment with c-Rel or RelA siRNA. Fig. 7 shows that c-Rel, but not RelA, silencing significantly reduced the formation of neuritic processes, indicating that the NF-κB subunit also plays a role in the neuritogenic effect triggered by PACAP in PC12 cells.
FIGURE 5.
Effects of c-Rel and RelA silencing on NF-κB subunit mRNA and protein levels. Forty-eight h after transfection of PC12 cells with control, c-Rel, or RelA siRNA, c-Rel and RelA expression was analyzed. A and B, c-Rel (A) and RelA (B) gene expression was quantified by real-time PCR. Data are expressed as mean ± S.E. (error bars) relative to control values (n = 4). *, p < 0.05 (Mann-Whitney U test). The experiment was repeated three times with similar results. C and D, immunocytochemistry was performed using antibodies directed against c-Rel (C) and Rel-A (D) subunits followed by a fluorochrome-conjugated secondary antibody. Nuclei were labeled with DAPI. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results. E, cytoplasmic and nuclear fluorescence of c-Rel and RelA was measured using ImageJ software. Western blotting of RelA after siRNA treatment. α-Tubulin was used as a loading control.
FIGURE 6.
Effect of c-Rel and RelA silencing on PACAP-stimulated PC12 cell viability. Twenty-four h after transfection with control, c-Rel, or RelA siRNA, PC12 cells were treated by PACAP (100 nm) for 48 h. Cell viability was assessed using MTT assay. Data are expressed as mean ± S.E. (error bars) relative to control values (n = 6). *, p < 0.05 (Mann-Whitney U test). The experiment was repeated two times with similar results.
FIGURE 7.
Effect of c-Rel and RelA silencing on PACAP-induced neurite outgrowth. Twenty-four h after transfection with control, c-Rel, or RelA siRNA, PC12 cells were treated by PACAP (100 nm) for 48 h, and the number of neurites per cell (A) and neurite length (B) were determined by confocal macroscopy and ImageJ analysis. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). *, p < 0.05; **, p < 0.01 (Student's t test). The experiment was repeated three times with similar results.
PACAP Stimulates c-Rel Activity via ERK1/2 Kinases and Ca2+ Mobilization
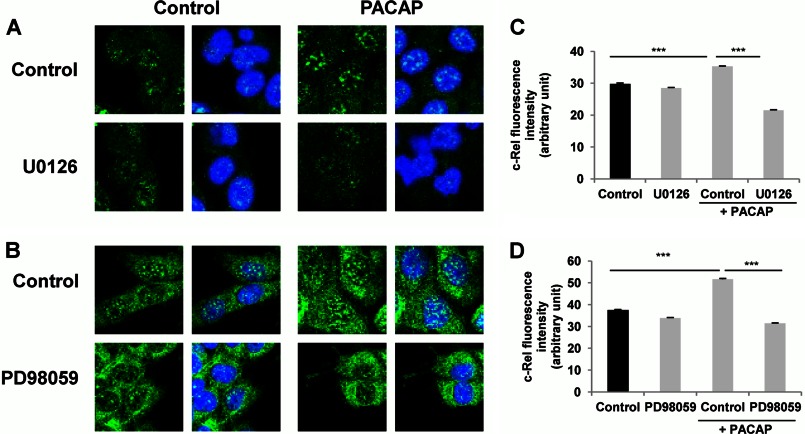
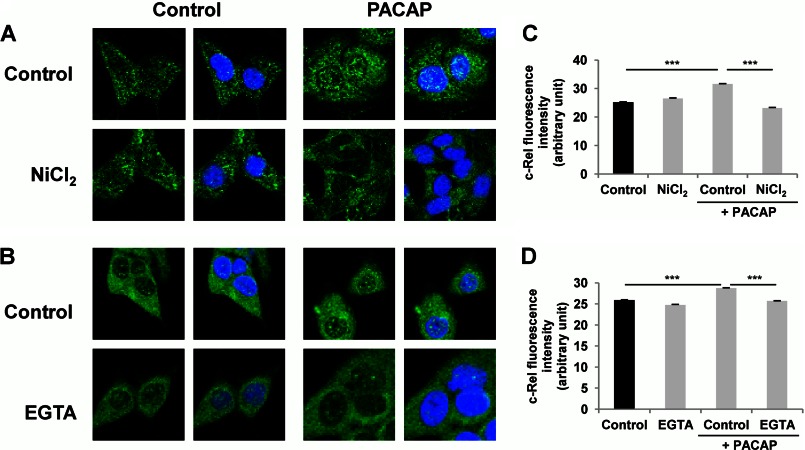
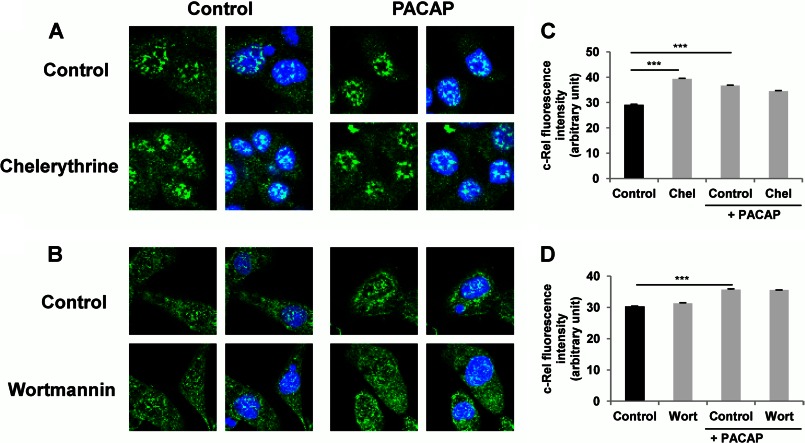
In sympathetic neurons including the sympathetic-like PC12 cells, PACAP exerts its actions through its PAC1 receptor coupled to Gs and Gq signaling proteins, which in turn activate multiple pathways including stimulation of several protein kinases and mobilization of Ca2+ (10, 25). In particular, MEK-dependent activation of the ERK1/2 MAP kinases and phosphatidylinositol 3-kinase (PI3K) play prominent roles in the neurotrophic effects exerted by PACAP in PC12 cells (26, 27). Likewise, PKA and protein kinase C (PKC) have also been shown to play important roles in mediating PACAP actions in different neuronal cells (10, 28, 29). In an attempt to link these major signaling cascades engaged in the neurotrophic effects of PACAP to the activation of the NF-κB subunit c-Rel, we first used the MEK1/2 inhibitor U0126 and the MEK1 inhibitor PD98059 to investigate the involvement of ERK1/2 in this process. Exposure of PC12 cells to the MEK inhibitors U0126 (10 μm) and PD98059 (50 μm) abrogated the PACAP-stimulated c-Rel nuclear translocation (Fig. 8). Likewise, the nonselective Ca2+ channel blocker NiCl2 (3 mm) and the extracellular Ca2+ chelator EGTA (300 μm) also impaired the PACAP-stimulated c-Rel nuclear translocation (Fig. 9). In contrast, the PKC inhibitor chelerythrine and the PI3K inhibitor wortmannin did not interfere with the PACAP effect (Fig. 10). These data suggest that PACAP stimulates NF-κB c-Rel subunit activity by inducing ERK1/2 activation and Ca2+ entry into the cell but not via PKC or PI3K activation.
FIGURE 8.
Effect of MEK inhibitors on PACAP-induced c-Rel nuclear translocation. A and B, PC12 cells were pretreated with U0126 (10 μm, A) or PD98059 (50 μm, B) for 1 h in the absence or presence of PACAP (100 nm) for 2 h. Immunocytochemistry was performed using an antibody directed against the c-Rel subunit of NF-κB followed by a fluorochrome-conjugated secondary antibody. Nuclei were labeled with DAPI. C and D, nuclear fluorescence of c-Rel was measured using ImageJ software. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results.
FIGURE 9.
Effect of Ca2+ mobilization inhibitors on PACAP-induced c-Rel nuclear translocation. A and B, PC12 cells were pretreated with NiCl2 (3 mm, A) or EGTA (0.3 mm, B) for 1 h in the absence or presence of PACAP (100 nm) for 2 h. Immunocytochemistry was performed using an antibody directed against the c-Rel subunit of NF-κB followed by a fluorochrome-conjugated secondary antibody. Nuclei were labeled with DAPI. C and D, nuclear fluorescence of c-Rel was measured using ImageJ software. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results.
FIGURE 10.
Effect of protein kinase inhibitors on PACAP-induced c-Rel nuclear translocation. A and B, PC12 cells were pretreated with chelerythrine (Chel, 5 μm, A) or wortmannin (Wort, 0.5 μm, B) for 1 h in the absence or presence of PACAP (100 nm) for 2 h. Immunocytochemistry was performed using an antibody directed against the c-Rel subunit of NF-κB followed by a fluorochrome-conjugated secondary antibody. Nuclei were labeled with DAPI. C and D, nuclear fluorescence of c-Rel was measured using ImageJ software. Data are expressed as mean ± S.E. (error bars) relative to control values (n >30). ***, p < 0.001 (Student's t test). The experiment was repeated three times with similar results.
We next tested the effect of the inhibitors of MEK activity and Ca2+ mobilization on ERK phosphorylation in response to PACAP to ascertain that the enzyme is activated prior to c-Rel nuclear translocation in PC12 cells and that Ca2+ is involved in this effect. As expected, PACAP treatment increased ERK phosphorylation that was prevented by the MEK1/2 inhibitor U0126 and the MEK1 inhibitor PD98059 (Fig. 11). In addition, treatment with NiCl2 induced a decrease in PACAP-stimulated ERK phosphorylation (Fig. 11). Taken together, these data indicate that PACAP stimulates c-Rel activity via MEK- and Ca2+-dependent ERK activation.
FIGURE 11.
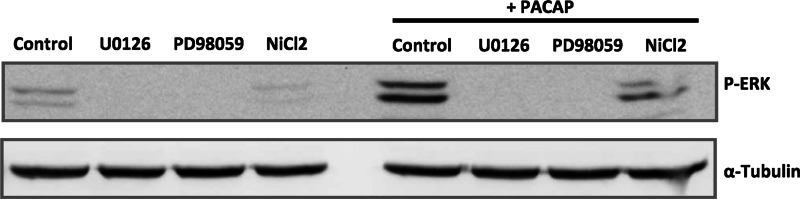
Effect of PACAP on ERK activation. PC12 cells were pretreated with U0126 (10 μm), PD98059 (50 μm), or NiCl2 (3 mm) for 1 h before incubation with PACAP (100 nm) for 5 min. Western blotting was performed by using antibodies against the phosphorylated form of ERK (P-ERK), and α-tubulin antibody was used as a loading control.
PACAP Induces p100 Processing
To activate PACAP target genes during PC12 cell differentiation, c-Rel can either homodimerize or heterodimerize with another NF-κB partner. These two possibilities are not mutually exclusive depending on the target gene and the DNA binding sequence in their promoters (30). Because PACAP stimulated p52 expression and nuclear translocation (see above, Fig. 3G and Fig. 4, A and F), it is possible that this NF-κB subunit could also be involved, in combination with c-Rel, in the trophic effects of the neuropeptide. The p52 protein is synthesized as a cytoplasmic precursor p100 which is proteolytically processed in a signal-dependent manner to yield the active p52 transcription regulator (31). To further support a role of p52 subunit in conjunction with c-Rel in PACAP-induced trophic activities, we analyzed by Western blotting the processing of the p100 protein into p52 after treatment of PC12 cells by the neuropeptide. PACAP strongly stimulated p100 processing into p52, as the ratio p52/p100 was increased by ∼8-fold in PACAP-treated cells (Fig. 12), suggesting that stimulation of NF-κB pathway by PACAP implicates p52 activation through p100 processing.
FIGURE 12.
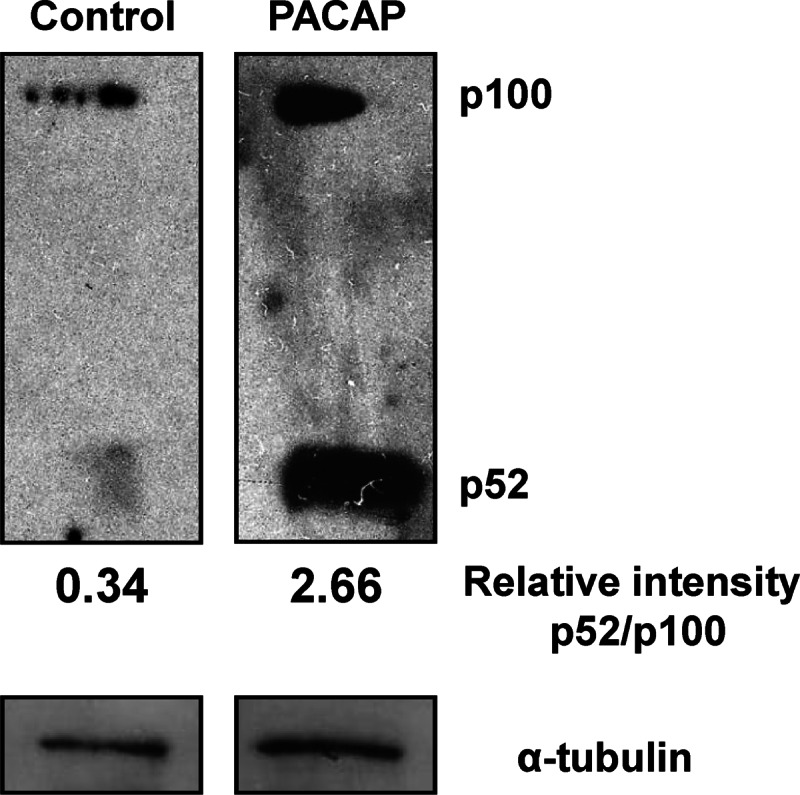
Effect of PACAP on p100 maturation. Protein extracts from cells treated or not with PACAP (100 nm) for 2 h were used to analyze the processing of p100 by Western blotting. Relative intensity was measured using ImageJ software.
DISCUSSION
Although the neurotrophic attributes of PACAP through its G protein-coupled PAC1 receptor and the associated complex downstream signaling pathways have been extensively studied, the unifying or diverging transcriptional mechanisms and factors required to ultimately elicit these neurotrophic responses are not well delineated. The PC12 cell line has been widely used to investigate the neurotrophic effects of PACAP and their underlying molecular events and thus represents a suitable model to decipher the role of genes regulated by this neuropeptide and potentially implicated in neuronal cell differentiation (23). The major finding of the present study is the implication of NF-κB signaling pathway in PACAP-induced neuritogenic and neuroprotective activities in PC12 cells, most likely by recruiting c-Rel/p52 dimers via activation of Ca2+ signaling and ERK1/2 MAP kinases.
The importance of NF-κB signaling pathways to central and peripheral neuron survival and neurite process formation has been well established using in vivo and in vitro models (18, 32). In the present study, we confirmed that constitutive activation of the NF-κB pathway plays an important role in PC12 cell survival and found that PACAP also required this pathway to exert its pro-survival action. The anti-apoptotic effect promoted by the NF-κB pathway in response to PACAP involved caspase-3/7 inhibition, presumably as a result of the stimulation of the expression of genes encoding survival factors like B cell lymphoma 2 (Bcl-2) which has been shown to be activated by the neuropeptide (33, 34). Likewise, NF-κB pathway activation was necessary for neurite outgrowth because PS-1145 reduced both the number of neurites per cell and the neurite length observed in the presence of PACAP. However, the gene targets that could be stimulated by the neuropeptide in a NF-κB-dependent manner to trigger neuritogenesis remain to be identified. Overall, these data add the neuropeptide PACAP to the list of neurotrophins involving the NF-κB pathway to induce neuronal differentiation.
The pro-inflammatory cytokine TNF-α is known to activate NF-κB signaling pathway (35), but unlike PACAP or NGF, it induces apoptotic cell death thanks to the death domain of TNF-α receptor 1 which is expressed in PC12 cells (36). In fact, NF-κB acts as an autoregulator of TNF-α-induced cell death because the transcription factor reduces apoptosis provoked by the cytokine (37). Similarly, TNF-α, unlike PACAP or NGF, does not induce neurite outgrowth in PC12 cells. Comparison of the effects of PACAP and TNF-α on NF-κB subunit mRNA expression showed that PACAP specifically stimulates c-Rel subunit gene expression whereas TNF-α specifically increases RelA subunit gene expression, suggesting that the trophic effects of PACAP could be mediated by c-Rel expression and that the opposing effects of PACAP and TNF-α could be explained by the differential activation of NF-κB subunits. Both PACAP and TNF-α increased p50 and p52 subunit gene expression, suggesting that these subunits are not responsible for PACAP-specific effects. This notion is consistent with the fact that p50 and p52 do not display a transactivation domain and therefore only act as transcription regulators when associated with RelA, RelB, or c-Rel (38, 39). Selective activation of NF-κB subunits by PACAP and TNF-α was confirmed by analysis of their nuclear translocation which revealed that (i) all NF-κB subunits are constitutively present in the nucleus of PC12 cells except RelA which exhibited a very low nuclear levels, (ii) PACAP increased c-Rel and p52 nuclear localization whereas TNF-α robustly augmented nuclear RelA amounts, and finally (iii) PACAP seems to diminish RelB and p50 nuclear translocation. Some discrepancies were observed for the effects of PACAP or TNF-α on NF-κB mRNA and protein levels (Figs. 3 and 4), which could be ascribed to different kinetics of stimulation at the posttranscriptional and translational levels and to posttranslational regulations that may affect protein levels. Nevertheless, the data clearly show that PACAP selectively and differentially modulates the nuclear translocation of some subunits to promote its pro-survival and neuritogenic activities. The fact that PACAP selectively increased c-Rel and p52 expression and nuclear translocation suggests that these two subunits could support as c-Rel/p52 dimers the transcriptional activity of NF-κB underlying the trophic effects of the neuropeptide. In fact, our experiments confirmed that c-Rel nuclear translocation mediates PACAP-induced NF-κB-specific activities during PC12 cell differentiation because c-Rel, but not RelA, silencing impaired the trophic effects of the neuropeptide. These observations are consistent with the data reported by Azotei et al. (40) showing that PC12 cells stably expressing c-Rel spontaneously extend neurites whereas RelA-expressing cells do not. Thus, under PACAP action, activation of c-Rel/p52 dimers could stimulate expression of anti-apoptotic and pro-differentiating genes, whereas inhibition of RelB/p50 activity may allow the repression of genes that could inhibit neurite outgrowth and cell survival. In accordance, expression of genes involved in cell survival, like the anti-apoptotic proteins Bcl-xL and Bcl-2, is known to be activated by both c-Rel (41–43) and PACAP (33, 34, 44), but targets of RelB/p50 in PC12 cells remain to be identified.
In the nervous system, PACAP exerts its trophic effects mainly through its capacity to stimulate cAMP production and Ca2+ mobilization (11), which recruit different signaling cascades to induce cell growth arrest, to promote neurite outgrowth, and to inhibit apoptotic cell death. Using the IKK blocker PS-1145, we showed that NF-κB pathway could be activated by PACAP to convey anti-apoptotic and neuritogenic effects, raising the possibility that cAMP and/or Ca2+-dependent signaling mechanisms activated by the neuropeptide elicit different components of neuronal differentiation in PC12 cells by recruiting IKK/NF-κB cascade. There is a wealth of literature available on cAMP-regulating agents that exert anti-inflammatory effects in the immune system by modulating NF-κB activity (45), but evidence is lacking for the involvement of this transcription factor in the neurotrophic effects exerted by cAMP-modulating cues such as PACAP. Although the link between cAMP elevation and NF-κB pathway remains to be fully delineated, the requirement for ERK1/2 activity to stimulate c-Rel translocation to the nucleus as observed in the present study suggests that these MAP kinases most likely represent the link between cAMP production and the activation of NF-κB in response to PACAP. Indeed, several reports have shown that in PC12 cells, PACAP-elicited cAMP increase triggers ERK1/2 phosphorylation, leading to neuronal differentiation (26, 46). Similarly, PACAP-induced mobilization of Ca2+ has also been linked to ERK1/2 activation (47), as also found in the present study, which again would link this second messenger to NF-κB stimulation in PC12 cells, in accordance with other studies performed in different cell types showing interactions between Ca2+ and ERK1/2 (48) or Ca2+ and NF-κB (49).
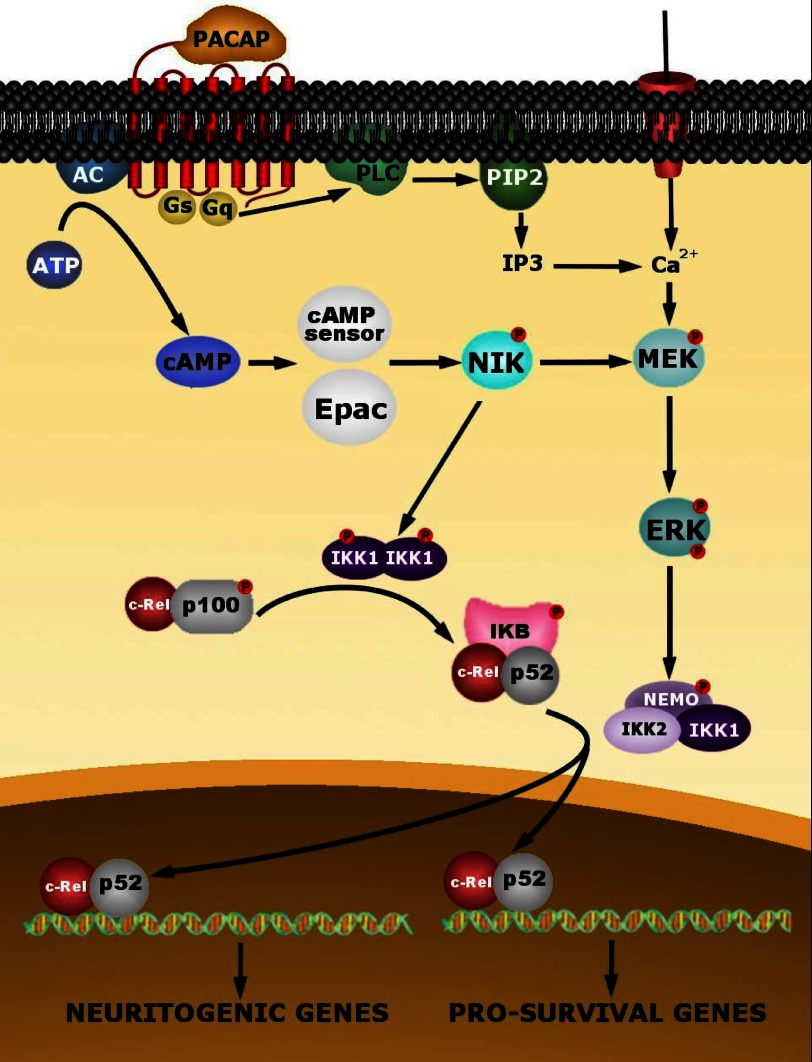
Analysis of the processing of p100 subunit gene product revealed that PACAP significantly increases p52/p100 protein ratio, implying that the neuropeptide controls p100 maturation into p52 in addition to the regulation of its gene expression and nuclear translocation. The processing of p52 has been associated with a particular NF-κB signaling pathway called the NF-κB alternative pathway (50). This pathway is initiated by p100 processing by the NF-κB-inducing kinase (NIK) in a manner dependent on IKK1 (Refs. 51–53 and Fig. 13). However, c-Rel/p52 activation is not only induced by NIK/IKK1 but occurs at the intersection between the alternative pathway and the classical IκB-involving pathway (Ref. 54 and Fig. 13). In fact, c-Rel/p52 dimers produced by NIK/IKK1 are maintained as a pool in the cytoplasm by the IκB protein and are liberated by IKK2, allowing nuclear translocation (54). It has been shown that constitutive activation of NIK is able to stimulate survival and neurite outgrowth in PC12 cells (55), but only IKK2 and not IKK1 is able to induce neuritogenesis (40). These observations suggest that PACAP could activate NIK and IKK1/2 as key kinases to ensure c-Rel/p52 translocation and neuronal differentiation. This effect of PACAP could be linked to ERK1/2 kinases which have been shown to be activated by NIK (55).
FIGURE 13.
Schematic representation summarizing the putative intracellular events involved in PACAP-induced NF-κB activation in PC12 cells. The effect of PACAP triggers a cAMP-dependent activation of NIK, probably through Epac or the new cAMP sensor (26). NIK, in turn, would activate IKK1 subunit of the IKK complex composed of a homodimer of the catalytic IKK1 permitting the production of c-Rel/p52 dimers which are maintained in the cytoplasm by the IκB protein. NIK also activates IKK2, a subunit of the heterodimeric IKK complex comprising the catalytic IKK1 and IKK2 subunits and the regulatory protein NEMO, through activation of the ERK1/2 kinases. The MAP kinase pathway could also be activated by Ca2+ mobilization. Activated IKK2 induces the release of c-Rel/p52 from IκB and its nuclear translocation to regulate the expression of genes implicated in PACAP-induced survival and neuritogenesis.
In summary, our results show that PACAP exerts its trophic effects in neuronal cells through classical and alternative NF-κB pathways involving ERK1/2 activation and presumably c-Rel/p52 NF-κB dimers. These data unveil the important role of NF-κB in PACAP signaling, which likely contributes to the neurodevelopmental effects of the neuropeptide and its beneficial actions in neuropathological conditions such as ischemia and neurodegenerative diseases.
This work was supported by INSERM (U982), the Conseil Régional de Haute-Normandie, and The Institute for Research and Innovation in Biomedicine.
- NGF
- nerve growth factor
- IKK
- inhibitor of NF-κB kinase
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIK
- NF-κB inducing kinase
- PACAP
- pituitary adenylate cyclase-activating polypeptide
- Z
- benzyloxycarbonyl.
REFERENCES
- 1. Alsina F. C., Ledda F., Paratcha G. (2012) New insights into the control of neurotrophic growth factor receptor signaling: implications for nervous system development and repair. J. Neurochem. 123, 652–661 [DOI] [PubMed] [Google Scholar]
- 2. Carey R. G., Li B., DiCicco-Bloom E. (2002) Pituitary adenylate cyclase activating polypeptide anti-mitogenic signaling in cerebral cortical progenitors is regulated by p57Kip2-dependent CDK2 activity. J. Neurosci. 22, 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiCicco-Bloom E., Deutsch P. J., Maltzman J., Zhang J., Pintar J. E., Zheng J., Friedman W. F., Zhou X., Zaremba T. (2000) Autocrine expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Dev. Biol. 219, 197–213 [DOI] [PubMed] [Google Scholar]
- 4. Falluel-Morel A., Vaudry D., Aubert N., Galas L., Benard M., Basille M., Fontaine M., Fournier A., Vaudry H., Gonzalez B. J. (2005) Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc. Natl. Acad. Sci. U.S.A. 102, 2637–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morio H., Tatsuno I., Hirai A., Tamura Y., Saito Y. (1996) Pituitary adenylate cyclase-activating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 741, 82–88 [DOI] [PubMed] [Google Scholar]
- 6. Takei N., Skoglösa Y., Lindholm D. (1998) Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J. Neurosci. Res. 54, 698–706 [DOI] [PubMed] [Google Scholar]
- 7. Vaudry D., Pamantung T. F., Basille M., Rousselle C., Fournier A., Vaudry H., Beauvillain J. C., Gonzalez B. J. (2002) PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur. J. Neurosci. 15, 1451–1460 [DOI] [PubMed] [Google Scholar]
- 8. Schecterson L. C., Bothwell M. (2010) Neurotrophin receptors: old friends with new partners. Dev. Neurobiol. 70, 332–338 [DOI] [PubMed] [Google Scholar]
- 9. Sherwood N. M., Krueckl S. L., McRory J. E. (2000) The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 21, 619–670 [DOI] [PubMed] [Google Scholar]
- 10. Ravni A., Bourgault S., Lebon A., Chan P., Galas L., Fournier A., Vaudry H., Gonzalez B., Eiden L. E., Vaudry D. (2006) The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J. Neurochem. 98, 321–329 [DOI] [PubMed] [Google Scholar]
- 11. Vaudry D., Falluel-Morel A., Bourgault S., Basille M., Burel D., Wurtz O., Fournier A., Chow B. K., Hashimoto H., Galas L., Vaudry H. (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 61, 283–357 [DOI] [PubMed] [Google Scholar]
- 12. Deutsch P. J., Sun Y. (1992) The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 267, 5108–5113 [PubMed] [Google Scholar]
- 13. Grumolato L., Elkahloun A. G., Ghzili H., Alexandre D., Coulouarn C., Yon L., Salier J. P., Eiden L. E., Fournier A., Vaudry H., Anouar Y. (2003) Microarray and suppression subtractive hybridization analyses of gene expression in pheochromocytoma cells reveal pleiotropic effects of pituitary adenylate cyclase-activating polypeptide on cell proliferation, survival, and adhesion. Endocrinology 144, 2368–2379 [DOI] [PubMed] [Google Scholar]
- 14. Foehr E. D., Lin X., O'Mahony A., Geleziunas R., Bradshaw R. A., Greene W. C. (2000) NF-κB signaling promotes both cell survival and neurite process formation in nerve growth factor-stimulated PC12 cells. J. Neurosci. 20, 7556–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bui N. T., Livolsi A., Peyron J. F., Prehn J. H. (2001) Activation of nuclear factor κB and Bcl-x survival gene expression by nerve growth factor requires tyrosine phosphorylation of IκBα. J. Cell Biol. 152, 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taglialatela G., Robinson R., Perez-Polo J. R. (1997) Inhibition of nuclear factor κB (NFκB) activity induces nerve growth factor-resistant apoptosis in PC12 cells. J. Neurosci. Res. 47, 155–162 [PubMed] [Google Scholar]
- 17. Hayden M. S., Ghosh S. (2011) NF-κB in immunobiology. Cell Res. 21, 223–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhakar A. L., Tannis L. L., Zeindler C., Russo M. P., Jobin C., Park D. S., MacPherson S., Barker P. A. (2002) Constitutive nuclear factor-κB activity is required for central neuron survival. J. Neurosci. 22, 8466–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaltschmidt B., Widera D., Kaltschmidt C. (2005) Signaling via NF-κB in the nervous system. Biochim. Biophys. Acta 1745, 287–299 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill L. A., Kaltschmidt C. (1997) NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258 [DOI] [PubMed] [Google Scholar]
- 21. Grumolato L., Louiset E., Alexandre D., Aït-Ali D., Turquier V., Fournier A., Fasolo A., Vaudry H., Anouar Y. (2003) PACAP and NGF regulate common and distinct traits of the sympathoadrenal lineage: effects on electrical properties, gene markers and transcription factors in differentiating PC12 cells. Eur. J. Neurosci. 17, 71–82 [DOI] [PubMed] [Google Scholar]
- 22. Noursadeghi M., Tsang J., Haustein T., Miller R. F., Chain B. M., Katz D. R. (2008) Quantitative imaging assay for NF-κB nuclear translocation in primary human macrophages. J. Immunol. Methods 329, 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaudry D., Chen Y., Hsu C. M., Eiden L. E. (2002) PC12 cells as a model to study the neurotrophic activities of PACAP. Ann. N.Y. Acad. Sci. 971, 491–496 [DOI] [PubMed] [Google Scholar]
- 24. Koulich E., Nguyen T., Johnson K., Giardina C., D'mello S. (2001) NF-κB is involved in the survival of cerebellar granule neurons: association of Iκβ phosphorylation with cell survival. J. Neurochem. 76, 1188–1198 [DOI] [PubMed] [Google Scholar]
- 25. Ghzili H., Grumolato L., Thouënnon E., Tanguy Y., Turquier V., Vaudry H., Anouar Y. (2008) Role of PACAP in the physiology and pathology of the sympathoadrenal system. Front. Neuroendocrinol. 29, 128–141 [DOI] [PubMed] [Google Scholar]
- 26. Emery A. C., Eiden L. E. (2012) Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 26, 3199–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. May V., Lutz E., MacKenzie C., Schutz K. C., Dozark K., Braas K. M. (2010) Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase and vesicle endocytosis for neuronal survival. J. Biol. Chem. 285, 9749–9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou C. J., Yada T., Kohno D., Kikuyama S., Suzuki R., Mizushima H., Shioda S. (2001) PACAP activates PKA, PKC and Ca2+ signaling cascades in rat neuroepithelial cells. Peptides 22, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 29. Shioda S., Ohtaki H., Nakamachi T., Dohi K., Watanabe J., Nakajo S., Arata S., Kitamura S., Okuda H., Takenoya F., Kitamura Y. (2006) Pleiotropic functions of PACAP in the CNS. Ann. N.Y. Acad. Sci. 1070, 550–560 [DOI] [PubMed] [Google Scholar]
- 30. Smale S. T. (2012) Dimer-specific regulatory mechanisms within the NF-κB family of transcription factors. Immunol. Rev. 246, 193–204 [DOI] [PubMed] [Google Scholar]
- 31. Verma I. M., Stevenson J. K., Schwarz E. M., Van Antwerp D., Miyamoto S. (1995) Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9, 2723–2735 [DOI] [PubMed] [Google Scholar]
- 32. Gutierrez H., Hale V. A., Dolcet X., Davies A. (2005) NF-κB signaling regulates the growth of neural processes in the developing PNS and CNS. Development 132, 1713–1726 [DOI] [PubMed] [Google Scholar]
- 33. Aubert N., Falluel-Morel A., Vaudry D., Xifro X., Rodriguez-Alvares J., Fisch C., de Jouffrey S., Lebigot J. F., Fournier A., Vaudry H., Gonzalez B. J. (2006) PACAP and C2-ceramide generate different AP-1 complexes through a MAP-kinase-dependent pathway: involvement of c-Fos in PACAP-induced Bcl-2 expression. J. Neurochem. 99, 1237–1250 [DOI] [PubMed] [Google Scholar]
- 34. Botia B., Basille M., Allais A., Raoult E., Falluel-Morel A., Galas L., Jolivel V., Wurtz O., Komuro H., Fournier A., Vaudry H., Burel D., Gonzalez B. J., Vaudry D. (2007) Neurotrophic effects of PACAP in the cerebellar cortex. Peptides 28, 1746–1752 [DOI] [PubMed] [Google Scholar]
- 35. Wajant H., Scheurich P. (2011) TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 278, 862–876 [DOI] [PubMed] [Google Scholar]
- 36. Haviv R., Stein R. (1998) The intracellular domain of p55 tumor necrosis factor receptor induces apoptosis which requires different caspases in naive and neuronal PC12 cells. J. Neurosci. Res. 52, 380–389 [DOI] [PubMed] [Google Scholar]
- 37. Beg A. A., Baltimore D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 [DOI] [PubMed] [Google Scholar]
- 38. Ghosh G., Wang V. Y., Huang D. B., Fusco A. (2012) NF-κB regulation: lessons from structures. Immunol. Rev. 246, 36–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bull P., Morley K. L., Hoekstra M. F., Hunter T., Verma I. M. (1990) The mouse c-Rel protein has an N-terminal regulatory domain and a C-terminal transcriptional transactivation domain. Mol. Cell. Biol. 10, 5473–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Azoitei N., Wirth T., Baumann B. (2005) Activation of the IκB kinase complex is sufficient for neuronal differentiation of PC12 cells. J. Neurochem. 93, 1487–1501 [DOI] [PubMed] [Google Scholar]
- 41. Pizzi M., Sarnico I., Boroni F., Benarese M., Steimberg N., Mazzoleni G., Dietz G. P., Bähr M., Liou H. C., Spano P. F. (2005) NF-κB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid β-peptide toxicity. Cell Death Differ. 12, 761–772 [DOI] [PubMed] [Google Scholar]
- 42. Chen C., Edelstein L. C., Gélinas C. (2000) The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol. Cell. Biol. 20, 2687–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossmann M., O'Reilly L. A., Gugasyan R., Strasser A., Adams J. M., Gerondakis S. (2000) The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 19, 6351–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rácz B., Gasz B., Gallyas F., Jr., Kiss P., Tamás A., Szántó Z., Lubics A., Lengvári I., Tóth G., Hegyi O., Roth E., Reglodi D. (2008) PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul. Pept. 145, 105–115 [DOI] [PubMed] [Google Scholar]
- 45. Gerlo S., Kooijman R., Beck I. M., Kolmus K., Spooren A., Haegeman G. (2011) Cyclic AMP: a selective modulator of NF-κB action. Cell Mol. Life Sci. 68, 3823–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lazarovici P., Jiang H., Fink D., Jr. (1998) The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 Cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21ras G protein, and pp60c-src cytoplasmic tyrosine kinase. Mol. Pharmacol. 54, 547–558 [DOI] [PubMed] [Google Scholar]
- 47. Hamelink C., Tjurmina O., Damadzic R., Young W. S., Weihe E., Lee H. W., Eiden L. E. (2002) Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc. Natl. Acad. Sci. U.S.A. 99, 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cañadillas S., Canalejo R., Rodriguez-Ortiz M. E., Martinez-Moreno J. M., Estepa J. C., Zafra R., Perez J., Muñoz-Castañeda J. R., Canalejo A., Rodriguez M., Almaden Y. (2010) Up-regulation of parathyroid VDR expression by extracellular calcium is mediated by ERK1/2-MAPK signaling pathway. Am. J. Physiol. Renal Physiol. 298, F1197–1204 [DOI] [PubMed] [Google Scholar]
- 49. Zocco D., McMorrow J. P., Murphy E. P. (2010) Histamine modulation of peripheral CRH receptor type 1α expression is dependent on Ca2+ signalling and NF-κB/p65 transcriptional activity. Mol. Immunol. 47, 1426–1437 [DOI] [PubMed] [Google Scholar]
- 50. Naumann M., Nieters A., Hatada E. N., Scheidereit C. (1993) NF-κB precursor p100 inhibits nuclear translocation and DNA binding of NF-κB/rel-factors. Oncogene 8, 2275–2281 [PubMed] [Google Scholar]
- 51. Xiao G., Harhaj E. W., Sun S. C. (2001) NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7, 401–409 [DOI] [PubMed] [Google Scholar]
- 52. Xiao G., Fong A., Sun S. C. (2004) Induction of p100 processing by NF-κB-inducing kinase involves docking IκB Kinase (IKK) to p100 and IKK-mediated phosphorylation. J. Biol. Chem. 279, 30099–30105 [DOI] [PubMed] [Google Scholar]
- 53. Senftleben U., Cao Y., Xiao G., Greten F. R., Krähn G., Bonizzi G., Chen Y., Hu Y., Fong A., Sun S. C., Karin M. (2001) Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science 293, 1495–1499 [DOI] [PubMed] [Google Scholar]
- 54. Dejardin E. (2006) The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 72, 1161–1179 [DOI] [PubMed] [Google Scholar]
- 55. Foehr E. D., Bohuslav J., Chen L. F., DeNoronha C., Geleziunas R., Lin X., O'Mahony A., Greene W. C. (2000) The NF-κB-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J. Biol. Chem. 275, 34021–34024 [DOI] [PubMed] [Google Scholar]