Background: Dapper3 is a negative regulator of Wnt/β-catenin signaling in cancer, but its physiological functions are largely unknown.
Results: Disruption of Dapper3 aggravated renal fibrosis after UUO through up-regulating Dvl and Wnt/β-catenin signaling.
Conclusion: Dapper3 attenuates the fibrotic activity of Wnt signaling in the UUO model.
Significance: This study established the Dapper3 knock-out mouse model and unraveled functions of Dapper3 in renal fibrosis.
Keywords: Fibrosis, Gene Knockout, Renal Physiology, Signaling, Wnt Signaling, Dapper3, Dishevelled, Unilateral Ureteral Obstruction
Abstract
Wnt/β-catenin signaling plays key roles in embryonic development and tissue homeostasis. Dapper3/Dact3, one of the three members of the Dapper gene family, is transcriptionally repressed in colorectal cancer and may function as a negative regulator of Wnt/β-catenin signaling. To investigate its physiological functions, we generated a mouse strain harboring conditional null alleles of Dapper3 (Dapper3flox/flox), and homozygous Dapper3-deficient (Dapper3−/−) mice were produced after crossing with EIIa-cre transgenic mice. We found that Dapper3 is not essential for mouse embryogenesis, postnatal survival, and reproduction. However, adult Dapper3−/− mice exhibited a mild reduction in body weight compared with their wild-type littermates, suggesting a functional role of Dapper3 in postnatal growth. To investigate the role of Dapper3 in renal fibrosis, we employed the unilateral ureteral obstruction model. Dapper3 mRNA expression was up-regulated in kidney after unilateral ureteral obstruction. Loss of the Dapper3 gene enhanced myofibroblast activation and extracellular matrix overproduction in the obstructed kidney. Moreover, this aggravated fibrotic phenotype was accompanied with accumulation of Dishevelled2 and β-catenin proteins and activation of Wnt-targeted fibrotic genes. In primary renal tubular cells, Dapper3 inhibits Wnt-induced epithelial-to-mesenchymal transition. Consistently, Dapper3 interacted with and down-regulated Dishevelled2 protein and attenuated the Wnt-responsive Topflash reporter expression. These findings together suggest that Dapper3 antagonizes the fibrotic actions of Wnt signaling in kidney.
Introduction
Wnt/β-catenin signaling is crucial in cell fate determination during embryogenesis and tissue homeostasis maintenance after birth. The binding of Wnt ligands to their receptors Frizzled and low density lipoprotein receptor-related protein 5/6 (LRP5/6) leads to recruitment and phosphorylation of Dishevelled (Dvl)4 (1). Dvl, the hub of Wnt signaling connecting Frizzled and downstream components (2–4), is tightly regulated by Dapper1/Dact1, Inversin, NEDL1, Prickle-1, KLHL12, and pVHL-containing E3 ubiquitin ligase (5–9). Activated Dvl promotes the disassembly of the β-catenin degradation complex, resulting in the nuclear accumulation of β-catenin and transcriptional activation of Wnt target genes mediated by β-catenin/T cell factor complex (1, 10).
Dapper was first identified as a Dishevelled (Dvl/Dsh)-interacting protein by yeast two-hybrid screening in Xenopus (11). As an antagonist of both canonical and noncanonical Wnt signaling, it is required for notochord development of Xenopus embryos (11). The three members of the Dapper (gene symbol-Dact) family, i.e. Dapper1, Dapper2, and Dapper3, have been identified in zebrafish, mouse, and human (5, 12–15). Human Dapper1 can negatively modulate Wnt signaling by promoting Dvl degradation in the cytoplasm and disrupting the β-catenin·LEF1 complex in the nucleus (5, 16). Zebrafish and mouse Dapper2 can inhibit TGF-β/Nodal signaling during mesoderm induction by promoting lysosomal degradation of type I receptors ALK4 and ALK5 (17, 18). Dapper1 and Dapper2 knock-out mouse models have been generated. Dapper1−/− mice died in the perinatal period with multiple physiological defects including caudal vertebrae agenesis, anorectal malformation, renal dysplasia, loss of bladder, and genital tubercle (19, 20). Through regulating the protein level and cellular distribution of Dvl2 (20) or controlling Vangl2 activity (19) in the primitive streak region, Dapper1 plays a critical role in planar cell polarity signaling during mouse embryonic development. Missense heterozygote mutations of the Dapper1 gene in fetus with neural tube defects (NTD) were reported recently, implicating mutated Dapper1 as a risk factor for human NTD-related birth defects (21). Dapper2−/− mice displayed no apparent abnormalities but showed accelerated skin wound healing with enhanced TGF-β signaling activity, suggesting that Dapper2 functions in re-epithelialization of skin wounds by attenuating TGF-β signaling (22).
Human Dapper3 was reported to be transcriptionally repressed through bivalent histone modifications in colorectal cancer, and its protein product Dapper3 may function as a negative regulator of Wnt/β-catenin signaling (23). Mouse Dapper3 was broadly expressed during mouse embryogenesis and in adult tissues, especially enriched in adult brain and uterus (15). It shares 27% similarity to Dapper1 and 24% similarity to Dapper2 at the amino acid level. Among three members in the Dapper family, Dapper3 was the least understood, and its in vivo functions have not yet been reported.
Tubulointerstitial fibrosis, characterized by excess matrix accumulation and deposition, is considered as the final outcome of progressive kidney diseases and an indicator of end-stage renal diseases (24–28). The rodent model of unilateral ureteral obstruction (UUO) has been widely employed to study the molecular basis of tubulointerstitial disease (29). Wnt signaling is activated in the process of tubulointerstitial renal fibrosis with the first evidence of Wnt4 reactivation in collecting duct epithelium and interstitial myofibroblasts in the UUO model (30, 31). Wnt/β-catenin signaling is activated in tubular epithelial and interstitial cells after renal injury (32, 33). All members of the Wnt ligand family except for Wnt5b, Wnt8b, and Wnt9b, most of the Frizzled receptor genes, and all four Dickkopf (Dkk) members were found to be up-regulated in the fibrotic kidney after UUO (33). Major cellular events in tubulointerstitial fibrosis include infiltration of inflammatory cells, activation of fibroblasts, epithelial-to-mesenchymal transition (EMT), and production of extracellular matrix (34). Wnt signaling stabilizes both Snail and β-catenin proteins, both of which cooperatively control the EMT process (35). Plasminogen activator inhibitor-1 (PAI-1), a direct downstream target of Wnt/β-catenin signaling, is a critical player in the pathogenesis of chronic kidney diseases (36).
In this study we have generated mice harboring conditional or null allele of Dapper3. The mild growth retardation in adult Dapper3−/− mice implies the role of Dapper3 in postnatal growth. Dapper3−/− kidneys after UUO showed enhanced myofibroblast activation and extracellular matrix accumulation, leading to more pronounced renal fibrosis. These phenotypes were accompanied with up-regulation of Dvl2, β-catenin proteins, and Wnt-targeted fibrotic genes. The roles of Dapper3 in modulating Wnt signaling were also evidenced by the results that Dapper3 could interact with Dvl2, down-regulate Dvl2 proteins, and attenuate the expression of the Wnt-responsive Topflash reporter.
EXPERIMENTAL PROCEDURES
Generation of Dapper3+/flox and Dapper3−/− Mice
A 14-kb fragment consisting of Exon 2–4 of the Dapper3 gene was retrieved from the C57BL/6J-derived BAC. The 5′ loxP site was cloned upstream of Exon 2. The 3′ loxP site and an frt-neo-frt selection cassette were inserted downstream of Exon 3. The gene targeting vector was linearized with PvuI and electroporated into B6/BLU mouse ES cells. The G418-resistant ES clones were screened by long range PCR with Dapper3-ES-F 5′-tccaagacctgtaaagcttcca-3′ and Dapper3-ES-R 5′-aagggttattgaatatgatcgga-3′. The presence of both 5′ and 3′ loxP sites was additionally validated using PCR primers flanking the loxP site. Two positive co1onies were microinjected into different C57BL/6J blastocysts to generate chimeric mice.
To inactive the floxed Dapper3 allele, Dapper3+/flox mice were bred with EIIa-cre transgenic mice (The Jackson Laboratory). Depletion of Exon 2 and Exon 3 produced a stop codon TGA at the 142nd nucleotide of exon 4 and terminated translation in advance. Dapper3+/− mice were intercrossed to obtain homogenous Dapper3 knock-out mice. This study was carried out under the approval of the Animal Research Committee of Model Animal Research Center (MARC), Nanjing University, and Institutional Animal Care and Use Committee (IACUC) of Tsinghua University.
Genotyping of Dapper3 Knock-out Mice
Dapper3 conditional and deleted alleles were genotyped by PCR using genomic DNA derived from ES cells, tail tissue, or embryos. PCR primers used to identify Dapper3 allele are as follows: P1F, 5′-cctgatcatcctttcattgtcccacc-3′; P1R, 5′-catctgctcctgaaaccctatt-3′; P2R, 5′-tggcaagatcccttcaagtctcc-3′.
UUO
After general anesthesia, UUO was performed on both wild-type and knock-out (Dapper3−/−) male mice by ligating the left ureter using 4–0 silk after a midline abdominal incision. Sham-operated mice had their ureters exposed and manipulated but not ligated (37). At day 7 or day 14 after surgery, the perfused kidneys were removed for analysis. One piece of a kidney was fixed in 10% formalin followed by paraffin embedding for histologic and immunohistochemical studies. The remaining was frozen in liquid nitrogen and stored at −80 °C for extraction of RNA and proteins.
Histology and Immunohistochemistry
The 10% formalin-fixed kidneys were embedded with paraffin and sectioned at 4-μm intervals. Renal morphology was examined after they were stained with Masson's-modified trichrome stain (37). Picrosirius red staining was used for the histological assessment of collagen accumulation. Immunohistochemistry of paraffin sections was conducted with the Dako ChemMateTM EnVision System with primary antibodies for α-smooth muscle actin (α-SMA; 1:200) or β-catenin (1:200). In negative controls, primary antibodies were omitted or replaced by isotype-matched nonimmune IgG. To verify the phenotype, quantitative histology was performed, and the results were confirmed by independent pathologists blinded to groups of different genotype. Images were viewed and captured using a Nikon Labophot 2 microscope equipped with a Sony CCD-IRIS/RGB color video camera attached to a computerized imaging system and analyzed by Image Pro Plus 3.0 (ECLIPSE 80i/90i; Nikon, Tokyo, Japan). The relative α-SMA-positive area was calculated as a proportion of the α-SMA expressed area to total cortical area of the kidney section. For each kidney, 10 randomly selected fields were analyzed in a blinded manner.
Southern Blot Analysis
Genomic DNA from ES cells or mouse tissues was digested with XbaI. Approximately 10 μg of digested DNA was electrophoresed on 0.7% agarose gel, transferred to a nylon membrane, and fixed by UV cross-linking. The membrane was hybridized in ExpressHyb solution (Clontech, CA) for 2 h at 65 °C. The radioactively labeled blots were detected by exposure to Eastman Kodak Co. film at −80 °C for 72 h. The 5′ probe used to identify the wild-type (17.3 kb) and targeted allele (9.5 kb) from XbaI-digested genome was generated by primers 5F (5′-ccgctttacttctgagggtctg-3′) and 5R (5′-cctgaaggcacgacaacgac-3′).
Isolation and Culture of Mouse Embryonic Fibroblasts (MEFs)
The male and female Dapper3+/− mice were intercrossed, and the pregnant female mice were sacrificed at 13.5-days post coitus. After embryos were separated individually, and the brain, limbs, tail, and dark red organs of the embryos were cut away. The yolk membrane of every embryo was kept for genotyping. After washing several times with cold PBS, embryos were minced with surgical scissors in a minimal amount of trypsin and incubated at 37 °C for 10 min. Then DMEM with 10% FBS was added to separate tissues until free of any larger pieces of tissue. These cells were cultured at 37 °C with 5% CO2.
Immunoblotting Analysis
To detect nuclear β-catenin levels, the nuclear fraction was isolated from kidney homogenate as described (16). In other cases tissues or cells were lysed with lysis buffer (20 mm Tris-HCl, pH 7.4, 2 mm EDTA, 25 mm NaF, 1% Triton X-100) plus protease inhibitors (Roche Applied Science) for 30 min at 4 °C. After centrifugation at 12,000 × g for 10 min, the supernatant was analyzed by SDS-PAGE. Immunoblotting was performed with primary antibody, and the secondary anti-rabbit antibody was conjugated to horseradish peroxidase (GE Healthcare). Proteins were visualized by chemiluminescence. The Dapper3 polyclonal antibody was generated by immunizing rabbit with mouse Dapper3 (170–236 amino acids)-glutathione S-transferase fusion protein. The antibodies against collagen I, α-SMA, Dvl2, and β-catenin were purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA), Sigma, Cell Signaling Technology (Danvers, MA), and Santa Cruz Biotechnology (Dallas, TX), respectively.
RNA Isolation and Real Time RT-PCR
Total RNA was prepared with TRIzol reagent (Invitrogen), and cDNA was synthesized from 1 μg of RNA with Revertra Ace (Toyobo, Osaka, Japan). Real time RT-PCR was performed by the EvaGreen method with the Mx3000p Quantitative PCR system (Agilent Technologies, Santa Clara, CA). Gene expression was normalized against GAPDH mRNA. Each sample was measured in triplicate. The primers used were as follows: Col1a1, 5′-agacatgttcagctttgtggac-3′ and 5′-gcagctgacttcagggatg-3′; Col3a1, 5′-aggcaacagtggttctcctg-3′ and 5′-gacctcgtgctccagttagc-3′; Dapper3, 5′-agtcgcccgccttcagct-3′ and 5′-ccatcccgccccaactca-3′; Dvl2, 5′-gcttccacatggccatgggc-3′ and 5′-tggcactgctggtgagagtcacag-3′; Axin2, 5′-gcagcagatccgggaggatgaa-3′ and 5′-gattgacagccgggggtcttga-3′; c-Myc, 5′-tgagcccctagtgctgcat-3′ and 5′-agcccgactccgacctctt-3′; cyclin D1, 5′-gcgtaccctgacaccaatctc-3′ and 5′-ctcctcttcgcacttctgctc-3′; Acta2, 5′-gtcccagacatcagggagtaa-3′ and 5′-tcggatacttcagcgtcagga-3′; Fn1, 5′-cgtaaattgccccattgagtg-3′ and 5′-gagggtctgctaacatcactg-3′; Vim, 5′-aatgcttctctggcacgtct-3′ and 5′-gctcctggatctcttcatcg-3′; Mmp7, 5′-ctgccactgtcccaggaag-3′ and 5′-gggagagttttccagtcatgg-3′; PAI-1, 5′-cccgcctcctcatcctgcct-3′ and 5′-gccactgtgccgctctcgtt-3′; Snail, 5′-ggaagcccaactatagcgagc-3′ and 5′-cagttgaagatcttccgcgac-3′; Slug, 5′-catccttggggcgtgtaagtc-3′ and 5′-gcccagagaacgtagaataggtc-3′; pE1, 5′-atgatccgggccttctcgttc-3′; pE4, 5′-agtgggctaggtgtcaggaag-3′; Gapdh, 5′-catggccttccgtgttccta-3′ and 5′-cctgcttcaccaccttcttgat-3′.
Immunofluorescence
Primary renal tubular epithelial cells were isolated as previously described (37). Cells were grown on glass coverslips in 6-well plates and treated with 40 ng/ml Wnt3a (R&D Systems, Minneapolis, MN) for 72 h. Subsequently, the cells were fixed and stained for E-cadherin (Abcam, Cambridge, UK), α-SMA, and fibronectin (Santa Cruz Biotechnology, Dallas, TX). For co-localization assay, HeLa cells were transfected with FLAG-Dvl2 together with Myc-Dapper3 for 36 h and detected by indirect anti-FLAG or anti-Myc immunofluorescence. Nuclei were counterstained with DAPI. Images were taken with Leica TCS SP5 Confocal Laser Scanning Microscope.
Luciferase Reporter Assays
HEK293T cells were transfected with various plasmids as indicated. At 36 h post-transfection, the cells were harvested, and luciferase activities were measured by an aluminometer (Berthold Technologies, Bad Wildbad, Germany). Reporter activity was normalized to the co-transfected Renilla. Experiments were repeated in triplicate, and the data were represented in the mean ± S.D.
Statistical Analysis
For statistical analysis, χ2 test was use to determine whether genotype distribution of intercrossed offspring was in line with the expected Mendelian ratio. Independent Student's t test (two tailed) was performed for comparison of body weight, histology, protein, and mRNA data between wild-type and Dapper3-deficient mice. The p value less than 0.05 was regarded as statistically significant and is indicated by asterisk (*, p < 0.05; **, p < 0.01).
RESULTS
Generation of Dapper3 Conditional Knock-out and Null Allele Mice
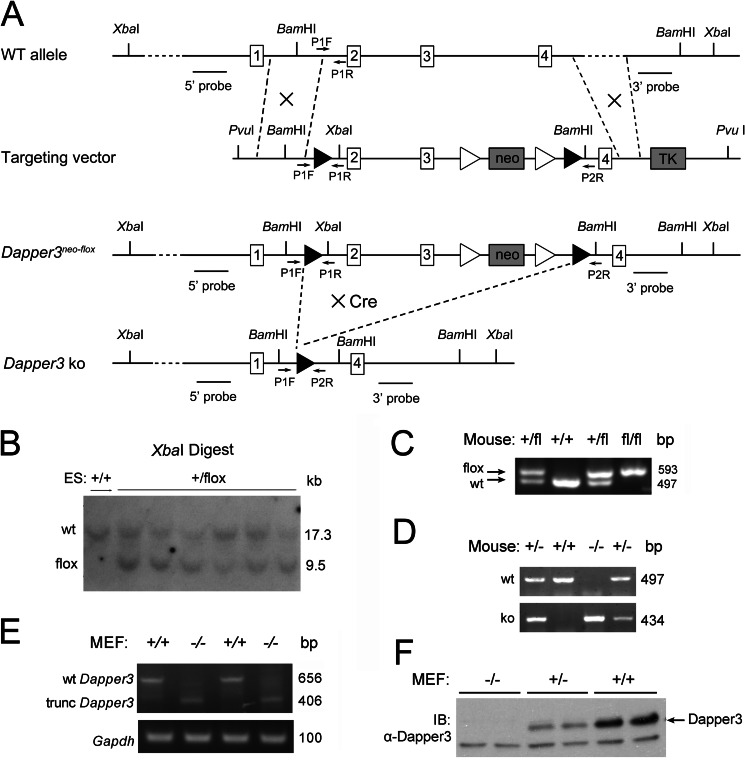
To investigate the physiological functions of Dapper3, we generated conditional Dapper3 knock-out mice with the Cre/loxP homologous recombination system. The Exon 2 and 3 of the Dapper3 gene were flanked with the loxP sites for Cre-mediated deletion to produce a stop codon at the 142nd nucleotide of Exon 4 (Fig. 1A). The long-range PCR identified six B6/BLU ES cells colonies with a successful recombination event. They were further verified by Southern blotting with XbaI (Fig. 1B)- or BamHI-digested genomic DNA (data not shown). The presence of both the 5′ and 3′ loxP sites were confirmed using PCR primers flanking them. The intercrossed offspring of Dapper3+/flox F1 mice could be distinguished by PCR genotyping (Fig. 1C). Dapper3+/flox mice and EIIa-cre transgenic mice were mated to produce Dapper3+/−. The heterozygous Dapper3+/− mice were intercrossed to produce Dapper3−/− embryos (Fig. 1D). To confirm the genotypes, we examined the Dapper3 mRNA and protein expression in MEFs. As expected, RT-PCR with primers in Exon 1 and Exon 4 revealed a 656-bp fragment in Dapper3+/+ MEFs and a 406-bp truncated fragment in Dapper3−/− MEFs (Fig. 1E). Western blot analysis confirmed the absence of a full-length Dapper3 protein in MEFs (Fig. 1F). These data together demonstrate that we have successfully generated Dapper3 conditional knock-out and null allele mice.
FIGURE 1.
Generation of mice harboring Dapper3neo-flox or Dapper3 null allele. A, shown is a schematic diagram of the wild-type Dapper3 allele, targeting vector, targeted Dapper3neo-flox, and Dapper3 null allele. Boxes with numbers represent exons. Arrows mark the position of the PCR primers used for genotyping. The location of the 5′ outside and 3′ outside probes for Southern blot analysis are indicated. Solid triangles denote loxP sites; open triangles denote frt sites. Neo and TK are positive and negative selection genes, respectively. Besides restriction site PvuI, two additional sites for Southern blot were introduced in the targeting vector: a BamHI site between 5′ loxP site and Exon 2 and an XbaI site between 3′ loxP site and Exon 4. The 5′ homologous arm of the targeting vector is 5.7 kb, and the 3′ arm is 4.5 kb. Dapper3neo-flox allele is created through homologous recombination between wild-type allele and targeting vector. Dapper3 null allele was conversed from Dapper3neo-flox allele by crossing to transgenic cre mice. B, Southern blot analysis of XbaI digested genome DNA from positive ES cell clones is shown. A 5′ outside probe was used to identify the wild-type (17.3 kb) and targeted allele (9.5 kb) from XbaI-digested genome DNA. The Dapper3+/flox ES cell clones show both 17.3-kb and 9.5-kb bands as expected. wt, wild type; fl, flox. C, PCR genotyping of mice with Dapper3neo-flox allele is shown. The primer set P1F-P1R was used to distinguish the targeted allele (593 bp) from the wild-type allele (497 bp). +/+, wild-type; +/fl, Dapper3+/flox; fl/fl, Dapper3flox/flox. D, generation of mouse embryos harboring Dapper3 null allele is shown. The combination of primer sets P1F-P1R and P1F-P2R could identify three genotypes; only one 497-bp band represents the Dapper3+/+ embryo; only one 434-bp band indicates Dapper3−/− embryo; embryos with both the 497-bp and the 434-bp bands are Dapper3+/−. E, genotyping of MEFs cells by RT-PCR is shown. The primer set pE1-pE4 was used to amplify the wild-type Dapper3 (656 bp) or the truncated Dapper3 (406 bp). Gapdh (100 bp) is used as an internal control. F, immunoblotting (IB) of mouse Dapper3 protein from Dapper3+/+, Dapper3+/−, and Dapper3−/− MEFs cells is shown. The arrow indicates the position of Dapper3. The band below is a none-specific protein.
Adult Dapper3−/− Mice Showed Mild Growth Retardation
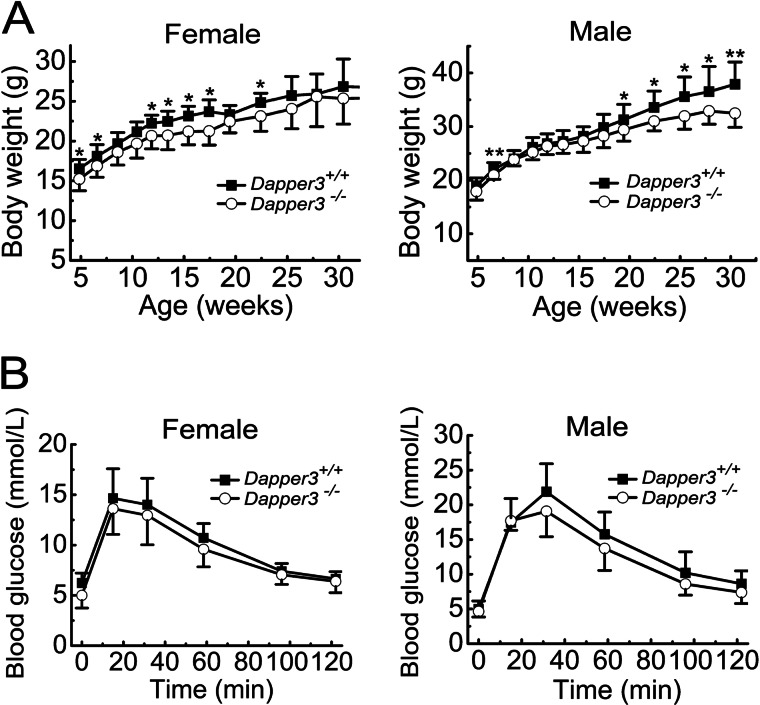
Dapper3−/− mice are viable and fertile. Of 156 offspring from Dapper3+/− intercross, 45 (29.0%) were wild type, 81 (52.1%) were heterozygous, and 30 (18.9%) were homozygous for the Dapper3 allele, accordant with the Mendelian ratio by χ2 analysis (p = 0.178). To investigate whether Dapper3−/− knock-out has influence on mouse postnatal growth, Dapper3−/− and wild-type littermates were weighed at an average interval of 2 weeks for 6 months, and their growth curves were drawn (Fig. 2A). Female Dapper3−/− mice exhibited a small reduction (6.5% on average) in body weight compared with their wild-type littermates. The significant difference was observed in some of early points of growth, reaching the largest at about 17 weeks old. However, after that, no significant difference could be seen between Dapper3−/− and wild-type females. In contrast, Dapper3−/− males showed a significant lag in weight gain compared with wild-type mice from 17-weeks-old on, and this difference increased gradually with age from 5.0% reduction to 8.6%. It is unlikely due to the skeleton growth defects as there is no significant difference in body length between wild-type and knock-out mice (data not shown). The mild growth retardation in Dapper3−/− mice, especially in males, implies that Dapper3 may play a role in normal growth of adult mice.
FIGURE 2.
Mild growth retardation in adult Dapper3−/− mice. A, shown are growth curves of female (n = 7) or male (n = 9) Dapper3+/+ and Dapper3−/− mice. *, p < 0.05; **, p < 0.01. The Dapper3+/+ and Dapper3−/− mice from 5–30 weeks old were weighed at an average interval of 2 weeks. B, intraperitoneal glucose tolerance tests during 120 min in both female and male mice are shown. The tests were conducted on 8-month-old female and 6-month-old male mice (n = 7).
The difference in body weight prompted us to examine whether it is due to defects of glucose and lipid metabolism. Glucose tolerance tests showed that both wild-type and knock-out mice could quickly clear the redundant glucose from blood (Fig. 2B). Although the glucose values in Dapper3−/− mice are lower at each time point, there is no statistical significance. Measurement of several parameters in mouse serum revealed that the Dapper3−/− mice have no serious problem in lipid metabolism as well as functions of liver and kidney (Table 1).
TABLE 1.
Serum biochemistry parameters of male Dapper3+/+ and Dapper3−/− mice
These mice were 7 months old. Data are the mean ± S.D. (n = 9). There was no statistically significant difference in all parameters between WT and KO groups. BUN, blood urea nitrogen; CREA, creatinine; UA, uric acid; ALB, albumin; ALP, alkalinephosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CHOL, cholesterol; TG, triglyceride.
| Parameter | BUN | CREA | UA | ALB | ALP | AST | ALT | CHOL | TG |
|---|---|---|---|---|---|---|---|---|---|
| mmol/liter | mg/dl | μmol/liter | g/liter | IU/liter | IU/liter | IU/liter | mmol/liter | mmol/liter | |
| Dapper3+/+ | 9.844 ± 1.966 | 0.411 ± 0.060 | 156.333 ± 8.327 | 16.333 ± 1.658 | 61.222 ± 11.883 | 48.714 ± 14.233 | 47.286 ± 11.354 | 2.132 ± 0.340 | 0.807 ± 0.218 |
| Dapper3−/− | 9.640 ± 1.405 | 0.440 ± 0.070 | 140.333 ± 6.506 | 16.556 ± 1.236 | 64.444 ± 10.899 | 49.429 ± 4.826 | 48.889 ± 17.794 | 2.214 ± 0.538 | 0.774 ± 0.313 |
| p value | 0.798 | 0.350 | 0.138 | 0.751 | 0.557 | 0.902 | 0.839 | 0.701 | 0.797 |
Renal Fibrosis Is Aggravated in Dapper3−/− Mice after UUO
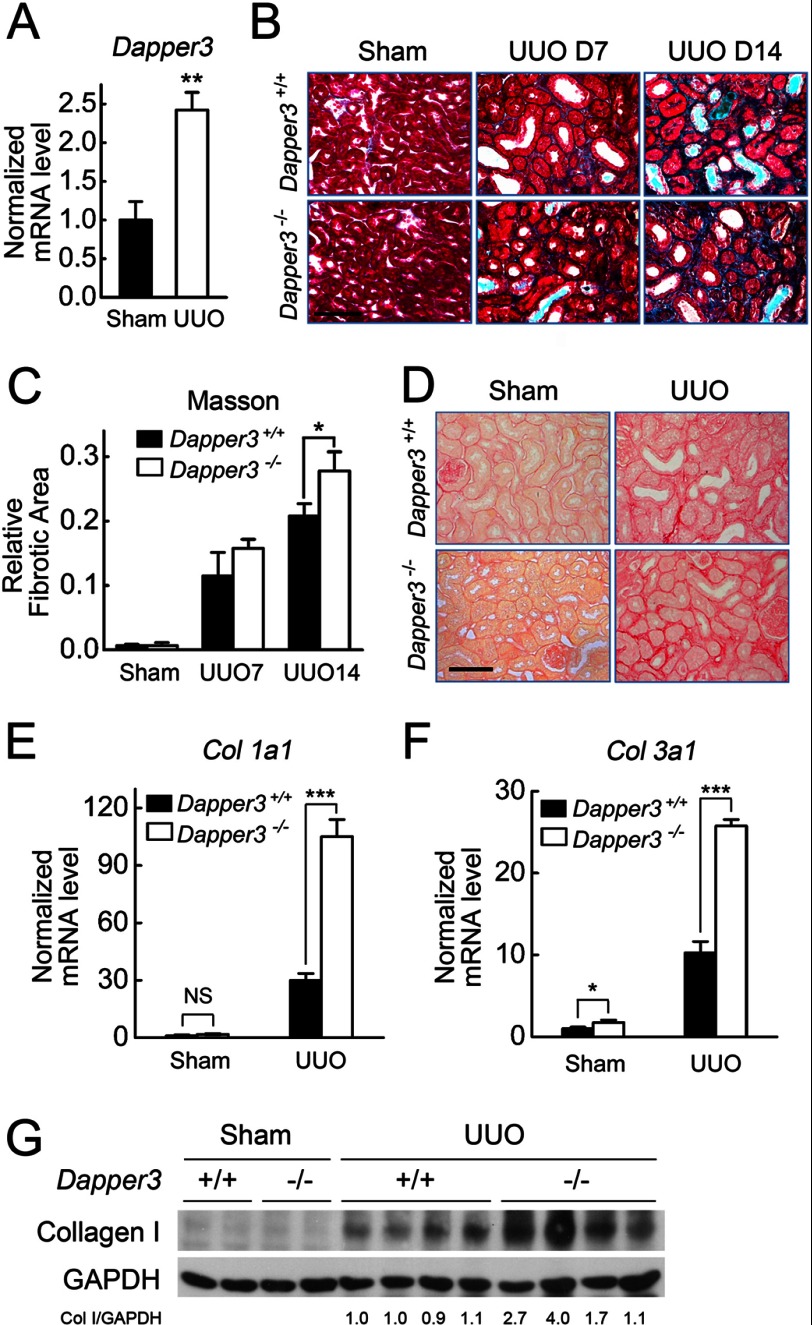
To investigate the role of Dapper3 in physiological/pathological conditions, we employed UUO-induced kidney fibrosis model, as we and others have reported that the Wnt/Snail pathway is involved in kidney fibrosis (32, 33, 37). We found Dapper3 mRNA was up-regulated after UUO (Fig. 3A). Importantly, interstitial fibrotic area was significantly increased in Dapper3−/− kidneys 7 days after UUO compared with that in wild-type kidneys, as shown by Masson's trichrome staining (Fig. 3, B and C). UUO led to a marked increase in collagen accumulation and deposition in wild-type mice, which was detected by a collagen-specific Picrosirius red staining, and disruption of the Dapper3 gene further enhanced the collagen deposition visualized by greater red staining area (Fig. 3D). Consistently, the expressions of Col1a1 and Col3a1, encoding type I and type III collagen, respectively, were significantly increased in Dapper3−/−-obstructed kidneys (Fig. 3, E and F). The collagen I protein level was significantly higher in the Dapper3−/−-obstructed kidneys than that in wild-type kidneys at day 14 of UUO (Fig. 3G). These results strongly suggest that disruption of Dapper3 expression promotes UUO-induced renal fibrosis.
FIGURE 3.
Renal fibrosis is aggravated in Dapper3−/− mice after UUO. A, Dapper3 mRNA levels from the kidneys of Sham-operation and UUO mice for 14 days were detected by real-time RT-PCR. B, Masson's trichrome staining of wild-type and Dapper3−/− kidney sections from Sham-operated, 7-day, and 14-day UUO groups is shown. Scale bars, 100 μm. C, relative fibrotic area statistics of Masson's trichrome staining in B is shown. *, p < 0.05. n = 3. D, Picrosirius red staining of kidney sections of wild-type and Dapper3−/−mice after UUO for 7 days is shown. Scale bars, 100 μm. E and F, shown are relative mRNA levels of Col1a1 (E) and Col3a1 (F) from wild-type and Dapper3−/− kidney after Sham-operation or UUO for 14 days. NS, not significant. G, shown is a Western blot of collagen I (col I) protein in contralateral and 14-day UUO samples. GAPDH is used as a loading control. Quantification of collagen I protein levels relative to GAPDH is shown below each band.
Dapper3 Deficiency Enhances Myofibroblast Activation and EMT in Obstructive Nephropathy
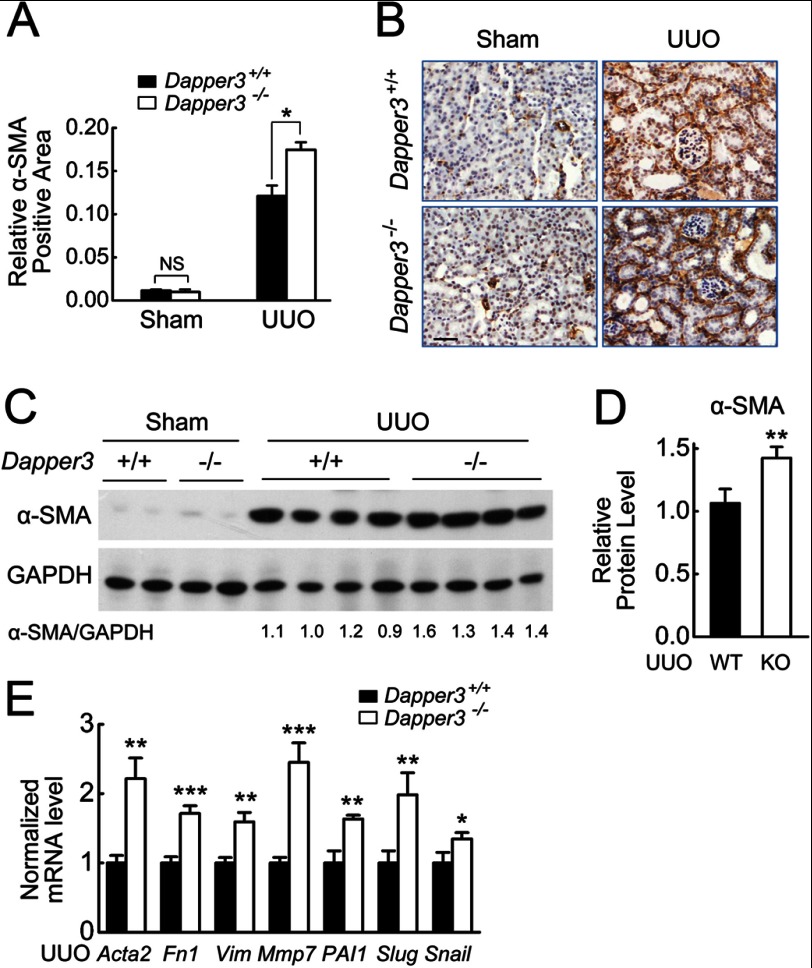
As myofibroblasts are the principal cells responsible for interstitial matrix accumulation and deposition (38), we next investigated the effects of Dapper3 deficiency on fibroblast activation after UUO. The mRNA expression of α-SMA, the molecular marker of myofibroblasts (39), was dramatically induced by UUO in both Dapper3+/+ and Dapper3−/− kidneys, and a significantly stronger expression of α-SMA was observed in the Dapper3−/− kidneys (Fig. 4, A and B), whereas no difference was detected in the sham-operated sections. Similarly, the α-SMA protein levels were significantly higher in the Dapper3−/−-obstructed kidneys than that in wild-type controls at Day 7 of UUO (Fig. 4, C and D). Accordantly, myofibroblast marker genes (α-SMA coding gene Acta2, vimentin), interstitial matrix related genes (Fn1, Col1a1, Col3a1, Mmp7), and EMT marker genes (PAI1, snail, slug) (34) were all significantly increased at the mRNA levels in Dapper3−/−-obstructive kidneys compared to that in wild type after UUO (Figs. 4E and 3, E and F). Collectively, these results suggest that disruption of the Dapper3 gene accelerates myofibroblast activation, EMT, and matrix production in obstructed kidney, leading to the enhanced fibrotic phenotypes in the UUO model.
FIGURE 4.
Dapper3 deficiency enhances myofibroblast activation and EMT in kidneys after UUO. A, shown are representative diagrams of α-SMA immunostaining in WT and Dapper3−/− kidneys 7 days after Sham-operation or UUO. Scale bars, 100 μm. NS, not significant. B, relative α-SMA-positive area was calculated from immunostaining pictures in A (see “Experimental Procedures” for the details). *, p < 0.05. n = 3. C and D, Western blots (C) and quantification analysis (D) of α-SMA protein levels in contralateral and 7-day UUO samples are shown. GAPDH is used as an internal control. **, p < 0.01. n = 4. E, effects of Dapper3 knock-out on various fibrotic gene expression are shown. wild-type and Dapper3−/− mice were subjected to UUO, and after 14 days the relative transcript levels of genes involved in renal fibrosis were measured.
Promotion of Dvl and β-Catenin Accumulation as Well as Wnt Target Gene Expression in Dapper3−/−-obstructed Kidneys
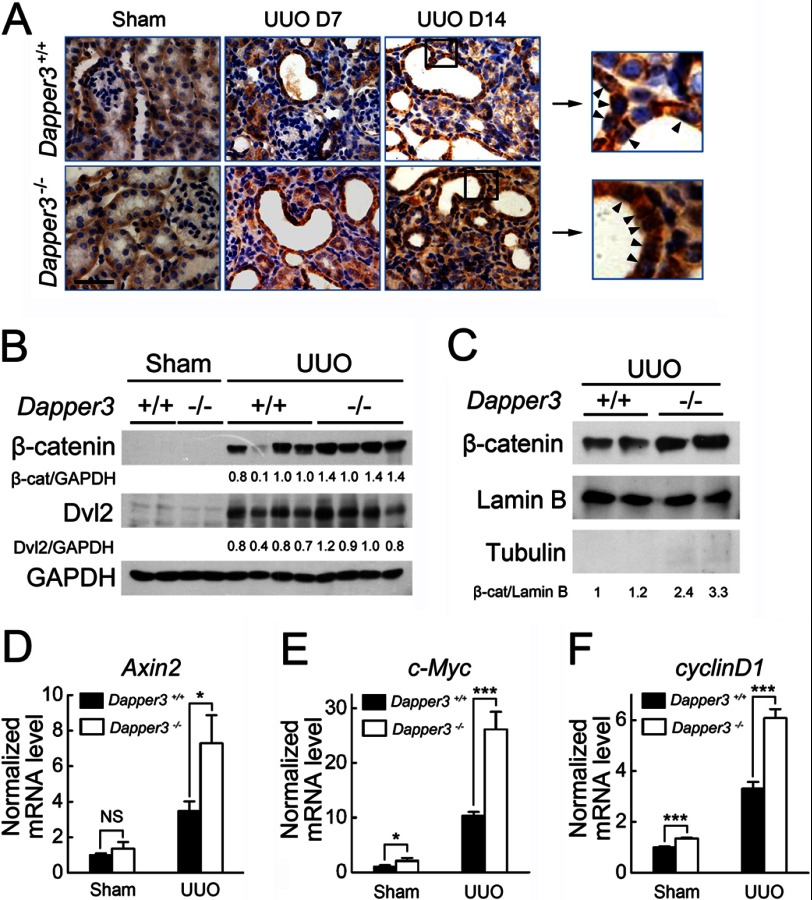
Wnt signaling plays a critical role in kidney fibrosis (32, 33). To explore the molecular mechanism underlying the fibrosis phenotype in the Dapper3−/−-obstructed kidneys, we examined the influence of Dapper3 on renal β-catenin abundance after obstructive injury. As shown in Fig. 5A, UUO caused a marked induction and time-dependent increase of β-catenin protein levels predominantly in renal tubules, and a stronger β-catenin induction was observed in Dapper3−/− kidneys than Dapper3+/+ controls at both 7 and 14 days after UUO. In addition to being found at the cell-cell adhesions, β-catenin was also localized in the cytoplasm and the nuclei of tubular epithelial cells, and much more nuclear β-catenin was observed in Dapper3−/− samples (Fig. 5A, arrowheads), indicating enhanced Wnt signaling. A Western blot also confirmed that both the total β-catenin protein level (Fig. 5B) and nuclear β-catenin (Fig. 5C) was significantly up-regulated in Dapper3−/−-obstructed kidneys. Dvl2, another core Wnt pathway component upstream of β-catenin, was not found to be significantly increased in Dapper3−/− kidneys without injury, perhaps due to the low expression of endogenous protein. However, greater increases in Dvl2 protein levels were noticed in Dapper3−/− kidney than in wild-type kidneys after UUO (Fig. 5B). As the Dvl2 mRNA level was unchanged (data not shown), these data suggest that Dapper3 may regulate Dvl2 at the protein level in renal fibrosis. Supporting this note, classical Wnt target gene Axin2, c-Myc, and cyclin D1 were all up-regulated in Dapper3−/− fibrotic kidney (Fig. 5, D and F). All these results indicate that loss of Dapper3 aggravates ureteral obstruction-induced renal fibrosis through up-regulating Wnt/β-catenin signaling.
FIGURE 5.
Enhanced activation of the Wnt/β-catenin canonical pathway in Dapper3−/− obstructive kidneys. A, shown is immunohistochemical staining of β-catenin in wild-type and Dapper3−/− kidneys in different groups: Sham controls, 7 days after UUO, and 14 days after UUO. Two representative areas with β-catenin staining in 14-day UUO group are enlarged in the right boxes. Nuclear localization of β-catenin in tubular epithelial cells (arrowheads) is increased in Dapper3−/− kidneys after UUO than that in wild-type ones. Scale bars, 100 μm. n = 3. B, Western blot analysis shows a further increase in renal β-catenin and Dvl2 levels 7 days after obstructive injury in Dapper3−/− group. Quantification of β-catenin and Dvl2 protein levels relative to GAPDH is shown below each band. n = 4. C, a Western blot of β-catenin in the nuclear extracts from kidney tissues shows increased nuclear β-catenin levels in Dapper3−/− group at day 14 after obstructive injury. Quantification of β-catenin protein levels relative to the nucleus marker lamin B is shown below each band. Tubulin is used as the cytosolic marker. D--F, relative mRNA levels of Axin2 (D), c-Myc (E), and cyclin D1 (F) genes in wild-type and Dapper3−/− mice 14 days after Sham-operation or UUO are shown. **, p < 0.01.
Dapper3 Inhibits Wnt-induced EMT in Renal Tubular Cells
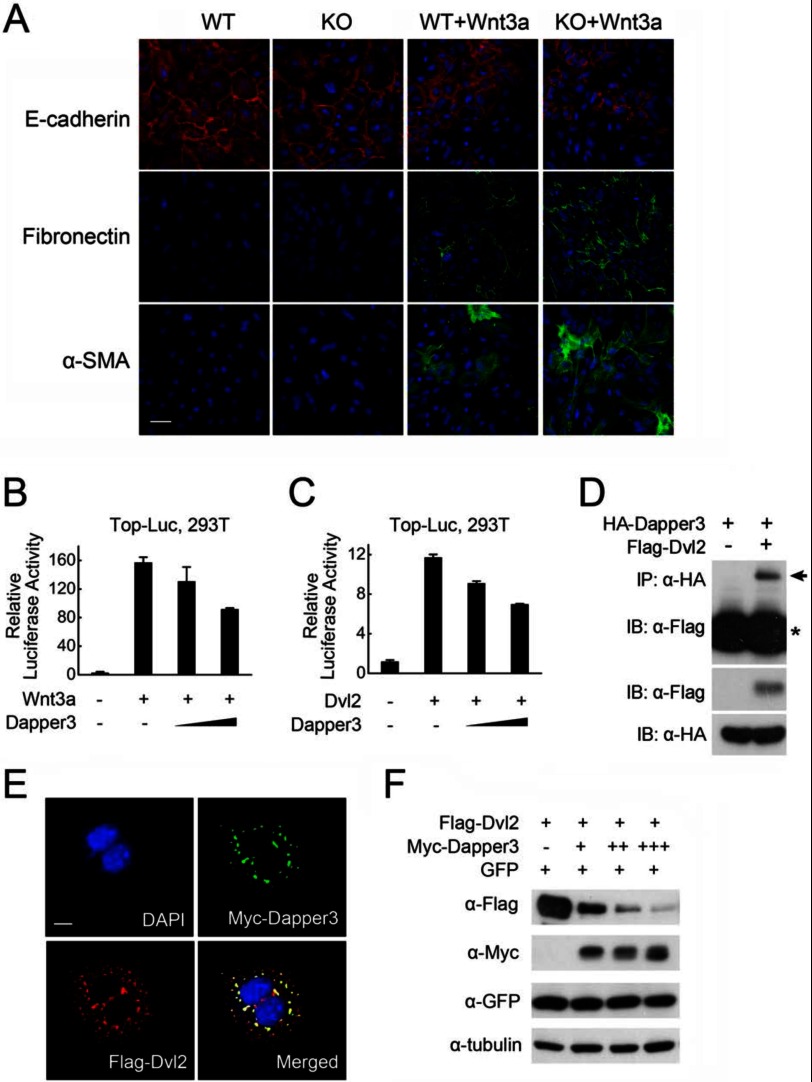
EMT of tubular epithelium could contribute to the pool of matrix-producing cells in renal fibrosis, in which Wnt signaling serves as an important promoter. We then examined the role of Dapper3 in EMT in primary cultured renal tubular cells. Treatment of cells with Wnt3a significantly decreased the level of the epithelial marker E-cadherin and increased the level of mesenchymal cell markers, fibronectin, and α-SMA (Fig. 6A). Moreover, in cells from Dapper3 KO mice, Wnt-induced changes of EMT markers were more prominent compared with those in wild-type cells, suggesting that loss of Dapper3 enhanced Wnt-induced EMT in renal tubular cells.
FIGURE 6.
Dapper3 inhibits Wnt signaling. A, shown is immunofluorescence staining of E-cadherin, α-SMA, and fibronectin in renal tubular cells. Primary renal tubular epithelial cells from Dapper3 wild-type and KO mice were treated with 40 ng/ml Wnt3a for 72 h and stained for E-cadherin (red), α-SMA (green), and fibronectin (green). Nuclei were counter-stained with DAPI (blue). Scale bars, 50 μm. B and C, Dapper3 inhibits Wnt3a (B)- or Dvl2 (C)-activated expression of TopFlash-luciferase reporter in a dose-dependent manner. HEK293T cells were co-transfected with reporter plasmid (0.1 μg), and the constructs encoding Wnt3a (0.1 μg) or Dvl2 (0.1 μg) with or without Dapper3 (50–200 ng). At 36 h post-transfection, the cells were harvested for luciferase assay. Experiments were repeated in triplicate. D, Dapper3 interacts with Dvl2. HEK293T cells were transfected with HA-Dapper3 and FLAG-Dvl2 as indicated. At 36 h post-transfection, the cells were harvested for anti-HA immunoprecipitation (IP) and anti-FLAG immunoblotting (IB, upper panel, arrow). Protein expression was confirmed by immunoblotting with the total cell lysates (middle and lower panels). The asterisk indicates IgG heavy chain. E, subcellular co-localization of exogenous Dapper3 and Dvl2 is shown. HeLa cells were transfected with FLAG-Dvl2 together with Myc-Dapper3. Subcellular localization of Dvl2 (red) or Myc-Dapper3 (green) was detected by indirect anti-FLAG or anti-Myc immunofluorescence. Their co-localization was shown in the merged images (yellow), and the nuclei were counter-stained with DAPI (blue). Scale bars, 10 μm. F, Dapper3 could effectively reduce the protein level of Dvl2. HEK293T cells were co-transfected with FLAG-Dvl2 (0.2 μg) and gradient Myc-Dapper3 (0.3–1.0 μg) along with GFP (0.2 μg) as a control. At 36 h post-transfection, the cells were harvested for immunoblotting.
Dapper3 Down-regulates Dvl2 Protein Levels and Wnt Signaling Activity
The above observations suggest that Dapper3 is a negative regulator of Wnt/β-catenin signaling. To further investigate it, we examined the effect of Dapper3 on Wnt/β-catenin signaling in HEK293T cells. As shown in Fig. 6, B and C, Dapper3 interfered with the Wnt3a- and Dvl2-induced expression of the Wnt-responsive Topflash-luciferase reporter in a dose-dependent manner. As Dapper3 inhibited Wnt signaling activity at the level of Dvl, we next studied the relationship of these two proteins. Indeed, FLAG-Dvl2 was found to interact with HA-Dapper3 (Fig. 6D), which was further consolidated by immunofluorescence analysis showing that Dapper3 was colocalized with Dvl2 in the cytoplasm (Fig. 6E). Our previous work demonstrated that Dapper1 could promote Dvl degradation (5). To test whether Dapper3 has the similar effect, HEK293T cells were co-transfected with FLAG-Dvl2 and Myc-Dapper3 along with GFP as a control. As shown in Fig. 6F, Dapper3 expression reduced Dvl2 protein levels in a dose-dependent manner. These results together indicate that Dapper3 negatively regulates Wnt/β-catenin signaling through interacting and down-regulating Dvl2 in the cytoplasm.
DISCUSSION
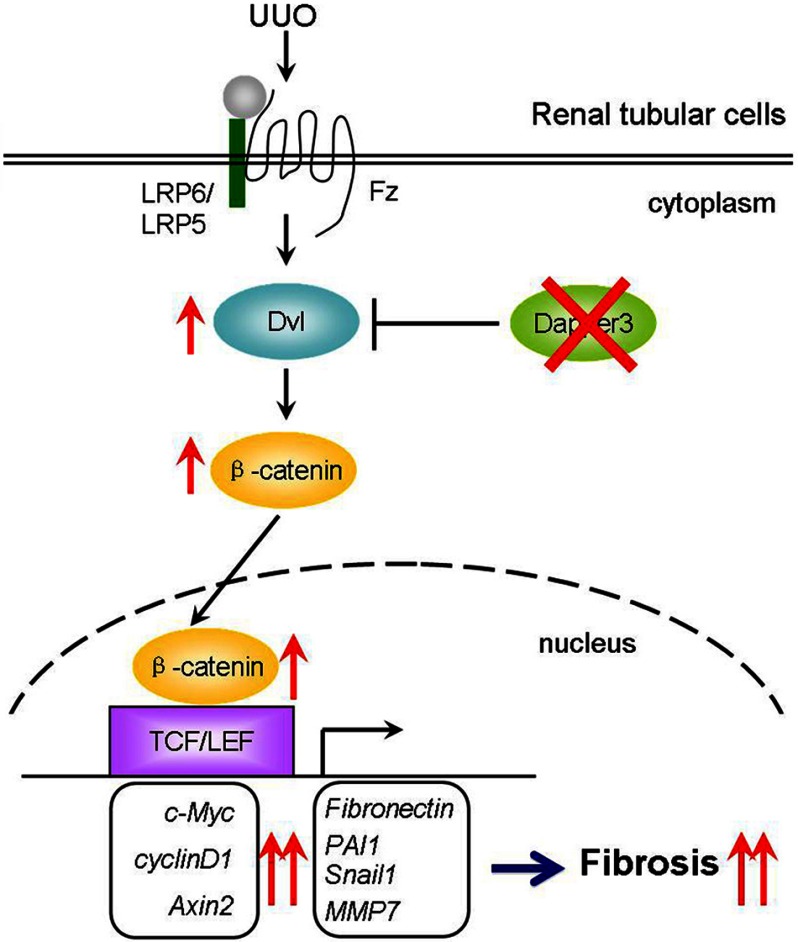
Our results indicate an important role of Dapper3 in UUO-induced renal fibrosis by regulating the Wnt/b-catenin signaling pathway (Fig. 7). Wnt/β-catenin signaling is activated by UUO and contributes to renal fibrosis. Dapper3 negatively regulates Wnt signaling by inducing Dvl degradation. Dapper3 knock-out leads to the up-regulation of Dvl and thus enhanced Wnt/β-catenin signaling, leading to further activation of fibrotic related target genes and aggravated renal fibrosis.
FIGURE 7.
Schematic diagram illustrates the role of Dapper3 in UUO-induced renal fibrosis by regulating Wnt/β-catenin signaling. The Wnt/β-catenin pathway is activated by UUO and contributes to renal fibrosis. Dapper3 induces Dvl degradation and thus attenuates Wnt signaling. Ablation of Dapper3 leads to increased protein levels of Dvl and Wnt/β-catenin signaling, further activating fibrotic gene expression and causing aggravated renal fibrosis. TCF, T cell factor; LEF, lymphoid enhancer-binding factor; Fz, Frizzled.
All the three members of the Dapper family have been targeted for gene deletion, but the phenotypes of the knock-out mice are very distinct. Dapper1 knock-out mice are perinatal lethal with multiple urogenital defects, whereas Dapper3 was not essential for mouse embryogenesis. The mild growth retardation in adult Dapper3−/− mice implies a possible function in mouse postnatal growth, but there were no obvious defects either in skeleton growth defect, glucose and lipid metabolism, or in motor ability on the treadmill (data not shown). Therefore, the exact role of Dapper3 in this process requires further investigation. During the course of this study, we observed a young Dapper3−/− mouse suffering from polycystic kidney disease and renal fibrosis, whereas all the other Dapper3−/− mice did not exhibit renal dysfunction or pathological changes spontaneously. However, a 2.4-fold induction of Dapper3 mRNA expression level was observed in kidneys after UUO, implying a potential role of Dappper3 in UUO-induced renal fibrosis. Consistent with this hypothesis, Dapper3 knock-out mice exhibited more severe kidney fibrosis after UUO, as shown by the increase in collagen accumulation and induction of α-SMA and other fibrotic genes.
Wnt signaling plays a critical role in organogenesis and tissue homeostasis of kidney, and its over-activation can cause fibrosis and progressive renal failure (40). In Dapper3−/− kidney, Wnt signaling was more activated than wild-type controls upon UUO. Our data provided multiple supports for this conclusion, including increased protein levels of Dvl and β-catenin, enhanced nuclear localization of β-catenin, and stronger induction of the Wnt targets Axin2, c-Myc, and cyclin D1 in Dapper3−/− mice. Among these Wnt targets, c-Myc was most enhanced by UUO injury (33) and was further stimulated by loss of Dapper3.
Wnt signaling is also a key mediator during the fibrosis-related EMT process. The EMT marker genes PAI1, Snail, and Slug were all significantly increased in Dapper3−/− kidneys compared with wild-type kidneys after UUO. In primary cultured renal tubular cells, Wnt3a treatment decreased the level of the epithelial marker E-cadherin and increased the mesenchymal markers fibronectin and α-SMA, and Dapper3 ablation further amplified this phenomenon. These data indicate that loss of Dapper3 promotes renal fibrosis through deregulating Wnt-regulated EMT event. In Dapper3−/−-obstructed kidneys, we found the expression levels of the interstitial matrix related genes fibronectin, Mmp7, and PAI1 were all raised. Fibronectin expression has been shown to be controlled by β-catenin (41), MMP-7 is regulated by Wnt4 and Wnt1 in the kidney (42), and PAI-1 was identified as a direct downstream target of Wnt/β-catenin signaling moderating the fibrotic action of Wnt signaling (36). Therefore, it is conceivable that Dapper3 may antagonize the fibrotic actions of Wnt signaling through repressing its target genes.
Mechanistically, Dapper3 may function as a negative regulator of Wnt/β-catenin signaling as Dapper1 does. Dapper3 could reduce Topflash-luciferase expression induced by both Wnt3a and Dvl2 in a dose-dependent manner. Consistently, Dapper3 promoted Dvl2 degradation. Our findings were in agreement with the inhibitory effect of Dapper3 on Wnt signaling in colorectal cancer (23).
Targeting Wnt/β-catenin signaling might be an effective strategy to hinder the progression of renal interstitial fibrosis (32, 33). The identification of the novel antifibrotic factor Dapper3 may yield new opportunity for therapeutic interventions of renal fibrosis.
Acknowledgments
We are grateful to Xu Li, Hannah Collins, and Qin Zhang for critical comments on the manuscript. We appreciate valuable discussions with the members of the Chen laboratory.
This work was supported by grants from the National Natural Science Foundation of China (30930050, 31221064, 31090363) and the 973 Program (2011CB943803).
- Dvl
- Dishevelled
- Dact
- Dishevelled-associated antagonist of β-catenin
- UUO
- unilateral ureteral obstruction
- EMT
- epithelial-to-mesenchymal transition
- PAI-1
- plasminogen activator inhibitor-1
- Mmp7
- matrix metallopeptidase 7
- α-SMA
- α-smooth muscle actin
- Fn
- fibronectin
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2. Gao C., Chen Y. G. (2010) Dishevelled. The hub of Wnt signaling. Cell. Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 3. Wharton K. A., Jr. (2003) Runnin' with the Dvl. Proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253, 1–17 [DOI] [PubMed] [Google Scholar]
- 4. Malbon C. C., Wang H. Y. (2006) Dishevelled. A mobile scaffold catalyzing development. Curr. Top. Dev. Biol. 72, 153–166 [DOI] [PubMed] [Google Scholar]
- 5. Zhang L., Gao X., Wen J., Ning Y., Chen Y. G. (2006) Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J. Biol. Chem. 281, 8607–8612 [DOI] [PubMed] [Google Scholar]
- 6. Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Krönig C., Schermer B., Benzing T., Cabello O. A., Jenny A. (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 37, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan D. W., Chan C. Y., Yam J. W., Ching Y. P., Ng I. O. (2006) Prickle-1 negatively regulates Wnt/β-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology 131, 1218–1227 [DOI] [PubMed] [Google Scholar]
- 8. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 9. Gao C., Cao W., Bao L., Zuo W., Xie G., Cai T., Fu W., Zhang J., Wu W., Zhang X. (2010) Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 10. Angers S., Moon R. T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 11. Cheyette B. N., Waxman J. S., Miller J. R., Takemaru K., Sheldahl L. C., Khlebtsova N., Fox E. P., Earnest T., Moon R. T. (2002) Dapper, a dishevelled-associated antagonist of β-catenin and JNK signaling, is required for notochord formation. Dev. Cell 2, 449–461 [DOI] [PubMed] [Google Scholar]
- 12. Katoh M. (2003) Identification and characterization of human DAPPER1 and DAPPER2 genes in silico. Int. J. Oncol. 22, 907–913 [PubMed] [Google Scholar]
- 13. Yau T. O., Chan C. Y., Chan K. L., Lee M. F., Wong C. M., Fan S. T., Ng I. O. (2005) HDPR1, a novel inhibitor of the WNT/β-catenin signaling, is frequently down-regulated in hepatocellular carcinoma. Involvement of methylation-mediated gene silencing. Oncogene 24, 1607–1614 [DOI] [PubMed] [Google Scholar]
- 14. Waxman J. S., Hocking A. M., Stoick C. L., Moon R. T. (2004) Zebrafish dapper1 and dapper2 play distinct roles in Wnt-mediated developmental processes. Development 131, 5909–5921 [DOI] [PubMed] [Google Scholar]
- 15. Fisher D. A., Kivimäe S., Hoshino J., Suriben R., Martin P. M., Baxter N., Cheyette B. N. (2006) Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev. Dyn. 235, 2620–2630 [DOI] [PubMed] [Google Scholar]
- 16. Gao X., Wen J., Zhang L., Li X., Ning Y., Meng A., Chen Y. G. (2008) Dapper1 is a nucleocytoplasmic shuttling protein that negatively modulates Wnt signaling in the nucleus. J. Biol. Chem. 283, 35679–35688 [DOI] [PubMed] [Google Scholar]
- 17. Zhang L., Zhou H., Su Y., Sun Z., Zhang H., Zhang L., Zhang Y., Ning Y., Chen Y. G., Meng A. (2004) Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science 306, 114–117 [DOI] [PubMed] [Google Scholar]
- 18. Su Y., Zhang L., Gao X., Meng F., Wen J., Zhou H., Meng A., Chen Y. G. (2007) The evolutionally conserved activity of Dapper2 in antagonizing TGF-β signaling. FASEB J. 21, 682–690 [DOI] [PubMed] [Google Scholar]
- 19. Suriben R., Kivimäe S., Fisher D. A., Moon R. T., Cheyette B. N. (2009) Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat. Genet. 41, 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen J., Chiang Y. J., Gao C., Xue H., Xu J., Ning Y., Hodes R. J., Gao X., Chen Y. G. (2010) Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J. Biol. Chem. 285, 11023–11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y., Ding Y., Lei Y.-P., Yang X.-Y., Xie G.-M., Wen J., Cai C.-Q., Li H., Chen Y., Zhang T., Wu B.-L., Jin L., Chen Y.-G., Wang H.-Y. (2012) Identification of novel rare mutations of DACT1 in human neural tube defects. Hum. Mutat. 33, 1450–1455 [DOI] [PubMed] [Google Scholar]
- 22. Meng F., Cheng X., Yang L., Hou N., Yang X., Meng A. (2008) Accelerated re-epithelialization in Dpr2-deficient mice is associated with enhanced response to TGF-β signaling. J. Cell Sci. 121, 2904–2912 [DOI] [PubMed] [Google Scholar]
- 23. Jiang X., Tan J., Li J., Kivimäe S., Yang X., Zhuang L., Lee P. L., Chan M. T., Stanton L. W., Liu E. T., Cheyette B. N., Yu Q. (2008) DACT3 is an epigenetic regulator of Wnt/β-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 13, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schainuck L. I., Striker G. E., Cutler R. E., Benditt E. P. (1970) Structural-functional correlations in renal disease. Part II: the correlations. Hum. Pathol. 1, 631–641 [DOI] [PubMed] [Google Scholar]
- 25. Striker G. E., Schainuck L. I., Cutler R. E., Benditt E. P. (1970) Structural-functional correlations in renal disease. Part I: A method for assaying and classifying histopathologic changes in renal disease. Hum. Pathol. 1, 615–630 [DOI] [PubMed] [Google Scholar]
- 26. Risdon R., Sloper J., De Wardener H. (1968) Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 292, 363–366 [DOI] [PubMed] [Google Scholar]
- 27. Nath K. (1992) Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17 [DOI] [PubMed] [Google Scholar]
- 28. Mackensen-Haen S., Bader R., Grund K. E., Bohle A. (1981) Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules, and impairment of the glomerular filtration rate. Clin. Nephrol. 15, 167–171 [PubMed] [Google Scholar]
- 29. Diamond J. R., Ricardo S. D., Klahr S. (1998) Mechanisms of interstitial fibrosis in obstructive nephropathy. Semin. Nephrol. 18, 594–602 [PubMed] [Google Scholar]
- 30. Nguyen H. T., Thomson A. A., Kogan B. A., Baskin L. S., Cunha G. R. (1999) Expression of the Wnt gene family during late nephrogenesis and complete ureteral obstruction. Lab. Invest. 79, 647–658 [PubMed] [Google Scholar]
- 31. Surendran K., McCaul S. P., Simon T. C. (2002) A role for Wnt-4 in renal fibrosis. Am. J. Physiol. Renal Physiol. 282, F431–F441 [DOI] [PubMed] [Google Scholar]
- 32. Surendran K., Schiavi S., Hruska K. A. (2005) Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 [DOI] [PubMed] [Google Scholar]
- 33. He W., Dai C., Li Y., Zeng G., Monga S. P., Liu Y. (2009) Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y. (2011) Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yook J. I., Li X. Y., Ota I., Fearon E. R., Weiss S. J. (2005) Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 280, 11740–11748 [DOI] [PubMed] [Google Scholar]
- 36. He W., Tan R., Dai C., Li Y., Wang D., Hao S., Kahn M., Liu Y. (2010) Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J. Biol. Chem. 285, 24665–24675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng J., Truong L. D., Wu X., Kuhl D., Lang F., Du J. (2010) Serum-and glucocorticoid-regulated kinase 1 is up-regulated following unilateral ureteral obstruction causing epithelial-mesenchymal transition. Kidney Int. 78, 668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grande M. T., López-Novoa J. M. (2009) Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol 5, 319–328 [DOI] [PubMed] [Google Scholar]
- 39. Strutz F., Zeisberg M. (2006) Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 17, 2992–2998 [DOI] [PubMed] [Google Scholar]
- 40. Nelson P. J., von Toerne C., Gröne H.-J. (2011) Wnt-signaling pathways in progressive renal fibrosis. Expert Opin. Ther. Targets 15, 1073–1083 [DOI] [PubMed] [Google Scholar]
- 41. Gradl D., Kühl M., Wedlich D. (1999) The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol. Cell. Biol. 19, 5576–5587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Surendran K., Simon T. C., Liapis H., McGuire J. K. (2004) Matrilysin (MMP-7) expression in renal tubular damage. Association with Wnt4. Kidney Int. 65, 2212–2222 [DOI] [PubMed] [Google Scholar]