Background: CRFR1 regulates the physiological response to stress and is implicated in the manifestation of depression.
Results: SAP97 interacts and co-localizes with CRFR1, suppresses CRFR1 endocytosis, and is required for CRFR1-mediated ERK1/2 phosphorylation.
Conclusion: SAP97 functionally regulates CRFR1 trafficking and signaling.
Significance: This is the first documentation of functional regulation of CRFR1 by a specific PDZ protein.
Keywords: Cyclic AMP (cAMP), Endocytosis, ERK, G Protein-coupled Receptors (GPCR), Receptor Endocytosis, Signal Transduction
Abstract
The corticotropin-releasing factor (CRF) receptor 1 (CRFR1) is a target for the treatment of psychiatric diseases such as depression, schizophrenia, anxiety disorder, and bipolar disorder. The carboxyl-terminal tail of the CRFR1 terminates in a PDZ-binding motif that provides a potential site for the interaction of PSD-95/Discs Large/Zona Occludens 1 (PDZ) domain-containing proteins. In this study, we found that CRFR1 interacts with synapse-associated protein 97 (SAP97; also known as DLG1) by co-immunoprecipitation in human embryonic 293 (HEK 293) cells and cortical brain lysates and that this interaction is dependent upon an intact PDZ-binding motif at the end of the CRFR1 carboxyl-terminal tail. Similarly, we demonstrated that SAP97 is recruited to the plasma membrane in HEK 293 cells expressing CRFR1 and that mutation of the CRFR1 PDZ-binding motif results in the redistribution of SAP97 into the cytoplasm. Overexpression of SAP97 antagonized agonist-stimulated CRFR1 internalization, whereas single hairpin (shRNA) knockdown of endogenous SAP97 in HEK 293 cells resulted in increased agonist-stimulated CRFR1 endocytosis. CRFR1 was internalized as a complex with SAP97 resulting in the redistribution of SAP97 to endocytic vesicles. Overexpression or shRNA knockdown of SAP97 did not significantly affect CRFR1-mediated cAMP formation, but SAP97 knockdown did significantly attenuate CRFR1-stimulated ERK1/2 phosphorylation in a PDZ interaction-independent manner. Taken together, our studies show that SAP97 interactions with CRFR1 attenuate CRFR1 endocytosis and that SAP97 is involved in coupling G protein-coupled receptors to the activation of the ERK1/2 signaling pathway.
Introduction
Corticotropin-releasing factor (CRF)2 is a neuropeptide that regulates the physiological response of the body to stress and initiates the hypothalamic-pituitary-adrenal axis stress response (1, 2). CRF mediates these responses by activating two distinct G protein-coupled receptors (GPCRs), CRF receptor 1 (CRFR1) and CRF receptor 2 (CRFR2) (3, 4). In comparison with CRFR2, CRFR1 exhibits a significantly higher affinity for CRF and shows far greater expression within the brain and in the pituitary (5, 6). The dysregulation of the hypothalamic-pituitary-adrenal axis is thought to be involved in the onset of psychiatric diseases, such as depression, and specific CRFR1 antagonists have recently been shown to demonstrate anxiolytic- and antidepressant-like effects (7, 8). GPCRs, like CRFR1, and their associated GPCR-interacting proteins (GIPs) have vast potential as pharmacological targets in the treatment of disease. Over 40% of modern pharmaceuticals target G protein-coupled receptors because of their widespread contributions to physiology (9). For these reasons, CRFR1 represents an excellent candidate as a pharmacological target for the treatment of mood disorders, such as depression, and thus a better understanding of the trafficking and signaling properties of CRFR1 may lead to the discovery of new pharmacological targets for mood regulation.
The physiological effects of GPCRs, like the CRFR1, are determined by the activation of receptor-mediated intracellular signaling pathways, and the intracellular trafficking of the receptor is responsible for determining the availability of receptor for agonist activation (10–12). CRFR1 has recently been shown to couple to, and activate, multiple different G proteins, including Gαs, Gαi, Gαq/11, Gαo, and Gαz (13, 14). However, CRFR1 is thought to preferentially couple to Gαs leading to adenylyl cyclase activation and cAMP production, which functions to stimulate protein kinase A (4, 15–19). In addition, agonist stimulation of the CRFR1 results in the desensitization of CRFR1 signaling, as a consequence of both second messenger-dependent protein kinase and G protein-coupled receptor kinase phosphorylation (20, 21). In particular, GRK6 promotes β-arrestin recruitment to CRFR1 to facilitate CRFR1 endocytosis (20). However, unlike what is observed for many GPCRs, CRFR1 stimulates the redistribution of β-arrestin2 to intracellular vesicles, but it does not co-localize with β-arrestin2 in the intracellular compartment (20).
Previous work from our laboratory has demonstrated a functional significance for the CRFR1 carboxyl-terminal class I ((S/T)Xφ-COOH, where φ represents any aliphatic amino acid residue) PSD-95/Discs Large/Zona Occludens-1 (PDZ)-binding motif in the heterologous sensitization of serotonin 2A receptor (5HT2AR) signaling, suggesting a novel role for PDZ domain-containing proteins in the regulation of CRFR1 function (22). Specifically, CRFR1 pre-activation selectively leads to increased 5-HT2AR signaling in response to subsequent 5-HT treatment, which is dependent upon both intact CRFR1 and 5-HT2AR carboxyl-terminal tail PDZ domain binding motifs. This CRFR1-dependent sensitization of 5-HT2AR signaling is also correlated with increased anxiety responses in mice (22). However, the identity of the specific PDZ protein(s) involved remains unknown.
We have investigated the capacity of the CRFR1 carboxyl-terminal tail to bind to a proteomic array of 96 class I PDZ domains (23, 24). We found that the CRFR1 carboxyl-terminal tail binds to a selective subset of class I PDZ domains on the array. In this study, we investigated the role of one of the positive CRFR1-interacting PDZ proteins identified in the screen that has been linked to the regulation of GPCR trafficking and signaling, synapse-associated protein 97 (SAP97; also known as DLG1). SAP97 has previously been identified as a GIP that binds to the 5-HT2AR, 5-HT2CR, β1-adrenergic receptor (β1AR), and somatostatin receptor subtype 1 and is reported to regulate β1AR recycling, as well as to couple somatostatin receptor subtype 1 activation to neurite outgrowth in development (25–27). We found that CRFR1 expression results in the PDZ-binding motif-dependent redistribution of SAP97 from the cytoplasm to the plasma membrane and that SAP97 functions to negatively regulate CRFR1 endocytosis and is required for CRFR1-mediated activation of extracellular signal-regulated kinase (ERK1/2) phosphorylation, without affecting CRFR1-stimulated cAMP formation.
EXPERIMENTAL PROCEDURES
Materials
Goat anti-glutathione S-transferase (GST) antibodies as well as ECL Western blotting detection reagents were purchased from GE Healthcare. Rabbit anti-phospho-p44/42 MAPK (Thr-202/Tyr-402) and rabbit anti-p44/42 MAPK antibodies were obtained from Cell Signaling Technology (Pickering, Ontario, Canada). Rabbit anti-GFP antibody was obtained from Invitrogen. Mouse anti-SAP97 antibody was obtained from Assay Designs/Enzo Life Sciences (Farmingdale, NY). Alexa Fluor 647 anti-mouse IgG and Alexa Fluor 633 goat anti-mouse IgG Zenon antibodies were purchased from Invitrogen. cAMP GLO assay was obtained from Promega (Madison, WI). Mouse anti-HA antibody and all other biochemical reagents were purchased from Sigma.
Plasmid Constructs
HA-tagged rat SAP97 isoform 2 (HA-CRFR1 and HA-CRFR1ΔTAV) constructs were described previously (20, 22). The YFP-SAP97 and SAP97 single hairpin RNA (shRNA) constructs were graciously provided by Dr. Suleiman W. Bahouth (Neuroscience Institute, University of Tennessee Health Sciences Center) (27). The YFP-SAP97 was subcloned into the pEGFP1 vector. For the human siRNA studies (HEK 293 cells), we used Silencer Validated siRNA (SAP97/DLG1) identification no. 146328 human NM_004087 GGAGAUCGUAUUAUAUCGGTT from Invitrogen. For the mouse siRNA studies (AtT20), we used Silencer Select siRNA (SAP97/DLG1) identification no. s232370 mouse NM_007862 AUGACAAGCGUAAAAAGAATT from Invitrogen. For the negative controls, we used Silencer Negative control 1 AM4635 AGUACUGCUUACGAUACGGTT from Invitrogen. The exchange proteins directly activated by cAMP biosensor was the gift of Drs. Ali Salahpour (University of Toronto) and Marc Caron (Duke University) (28). The CRFR1 carboxyl-terminal tail was cloned into pGEX-4 with ECOR1/NotI.
Cell Culture and Transfection
Human embryonic kidney (HEK 293) cells were maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum. Cells were seeded on 10-cm dishes at 70–80% density 24 h prior to transfection. Transfection was performed using a modified calcium phosphate method, as described previously (29). Transfections were performed with 1 μg of each construct, with exception that 3 μg of plasmid cDNA was used for all shRNA constructs. Empty pcDNA3.1 vector was used to equalize the total amount of plasmid cDNA used to transfect cells. 18 h post-transfection, cells were washed with phosphate-buffered saline (PBS) and resuspended with trypsin, 0.25% EDTA.
For AtT20 cells, 3-ml aliquots of suspended AtT20 cells were transferred to T25 Nunc flasks; 30 μl of Lipofectamine was incubated with 250 μl of Opti-MEM for 5 min at room temperature and then added to 250 μl of Opti-MEM and 80 pmol of either control or SAP97 siRNA. After 6 h, cells were centrifuged at 800 × g, and transfection reagents were aspirated and replaced with 3 ml of media. Cells were then reseeded for experimentation. 72 h post-transfection, cells were spun down, and media were aspirated and replaced with 3 ml of HBSS for 1 h at 37 °C. Control and SAP97 siRNA samples were aliquoted into 1-ml samples and stimulated with 500 nm CRF for 0, 5, or 10 min. Cells were put on ice and spun down at 800 × g at 4 °C; HEPES-buffered saline solution (HBSS) was aspirated, and cells were lysed with 200 μl of lysis buffer (50 mm Tris, pH 8.0, 150 mm NaCl, and 0.1% Triton X-100) containing protease inhibitors (1 mm AEBSF, 10 μg/ml leupeptin, and 5 μg/ml aprotinin). All experiments were conducted ∼48 h after the initial transfection, with the exception of transfections involving SAP97 shRNA/siRNA, which were conducted 72 h after initial transfection to optimize the knockdown of endogenous SAP97, as confirmed by Western blotting.
PDZ Blot Overlay Assay
GST and GST-CRFR1 fusion proteins were generated by growing recombinant BL21 bacteria at 21 °C to an A600 of 0.6–1.0. Cultures were induced for 3 h with 1 mm IPTG, pelleted, resuspended in PBS containing protease inhibitors (1 mm AEBSF, 10 μg/ml leupeptin, and 5 μg/ml aprotinin), and lysed by mild sonication. The bacterial lysates were cleared of cellular debris by centrifugation and then applied to glutathione-Sepharose 4B overnight at 4 °C. GST and GST-CRFR1 fusion proteins bound to the matrix were washed extensively in PBS-containing 0.3% Triton X-100. 100 nm GST and GST-CRFR1 in blot buffer (2% nonfat dry milk, 0.1% Tween 20, 50 mm NaCl, 10 mm HEPES, pH 7.4) were incubated with gridded nylon membranes that were spotted with His/S-tagged PDZ domain fusion proteins (1 μg/bin) for 1 h at room temperature (23, 24). The arrays were then washed three times with blot buffer and incubated with a horseradish peroxidase-conjugated anti-GST antibody (1:3000). Interactions of the GST fusion proteins with the various PDZ domains were then visualized via chemiluminescence using the enhanced chemiluminescence kit from GE Healthcare.
Co-immunoprecipitation
Transfected HEK 293 cells were seeded onto 10-cm dishes the day before the experiment. Cells were serum-starved for 1 h in HBSS, and dishes were treated with either HBSS alone or with 100 nm CRF agonist in HBSS for 30 min at 37 °C. Cells were subsequently lysed in lysis buffer for 20 min on a rocking platform at 4 °C. Samples were collected into 1.5-ml Eppendorf tubes and centrifuged at 15,000 × g for 15 min at 4 °C to pellet insoluble material. A Bronsted-Lowry protein assay was performed, and 400 μg of protein was incubated for 1–2 h at 4 °C with protein G-Sepharose and mouse anti-HA antibody (1:50). After incubation, beads were washed three times with cold lysis buffer and incubated overnight at room temperature in 3× SDS Loading Buffer containing 2-mercaptoethanol. Samples were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted to identify co-immunoprecipitated GFP-SAP97 (rabbit anti-GFP, 1:1000). An additional Western blot was performed to examine HA-CRFR1, HA-CRFR1ΔTAV (mouse anti-HA, 1:1000), and GFP-SAP97 (rabbit anti-GFP, 1:1000) protein expression.
For the co-immunoprecipitation of endogenous proteins from cortical extracts, adult mouse brains were employed. Tissue was dissected and homogenized on ice in lysis buffer containing protease inhibitors. The particulate fraction was removed by centrifugation, and 2 mg of supernatant protein was incubated with 5 μl/sample of either goat polyclonal anti-CRFR1 (CRF-RI (V14) sc-12381) or CRFR2 (CRF-RII (C-15) sc-20550) antibody from Santa Cruz Biotechnology (Santa Cruz, CA) and protein G-Sepharose beads by 2 h of rotation at 4 °C. Afterward, the beads were washed two times with lysis buffer and one time with PBS, and proteins were eluted in SDS-PAGE loading buffer by warming the samples at 55 °C for 5 min. Eluted samples were subjected to SDS-PAGE, followed by electroblotting onto nitrocellulose membranes for immunoblotting with antibodies described in the figure legends.
Live HEK 293 Cell Immunofluorescent Confocal Microscopy
Following transfection, HEK 293 cells were re-seeded onto 35-mm glass bottom confocal dishes. Cells were serum-starved for 1 h at 37 °C in HBSS and then labeled with mouse anti-HA antibody (1:200) and Zenon Alexa Fluor 647 mouse IgG1 labeling kit (Invitrogen) at 4 °C for 30 min. The cells were washed with HBSS and warmed to 37 °C for live imaging using a heated stage. Confocal microscopy was performed on a Zeiss LSM-510 META laser scanning confocal microscope using a Zeiss ×63, 1.3 NA, oil immersion lens. Co-localization studies were performed using dual excitation (488 and 633 nm) and emission (band pass 505–550 nm and long pass 650 nm for YFP/GFP and Alexa Fluor 647, respectively) filter sets. The specificity of labeling and absence of signal crossover were established by examination of single-labeled samples. In receptor endocytosis experiments, the cells were additionally stimulated with 500 nm CRF agonist (Tocris), and specified cells were re-imaged at regular intervals for up to 60 min. Co-localization analysis was performed using Imaris 7.0 co-localization module (bit-plane) to determine the co-localization of the brightest 2% of pixels in each channel, as described previously (30).
Receptor Endocytosis
Following transfection, HEK 293 cells were re-seeded into 12-well plates. Cells were serum-starved for 1 h at 37 °C in HBSS and then stimulated for 30 min with or without 500 nm CRF in HBSS at 37 °C or for the times indicated in the figure legends. Cells were washed with cold HBSS and treated with mouse anti-HA antibody (1:500) for 45 min on ice. Cells were washed with cold HBSS and additionally treated with Alexa Fluor 647 donkey anti-mouse IgG (Invitrogen) (1:500) for 45 min on ice. Cells were washed with cold PBS and treated with 5 mm EDTA in PBS for 5 min on ice. Newly suspended HEK 293 cells were then transferred to flow cytometry tubes containing 4% formaldehyde in PBS. Samples were run on a FACSCalibur cytometer using BD CellQuest Pro software until 10,000 cells were counted. The geometric mean of fluorescence was determined using FlowJo analysis software, with less fluorescence corresponding to less CRFR1 on the membrane.
cAMP Assay
The cAMP GLO assay protocol was carried out as suggested by the manufacturer (Promega). Transfected HEK 293 cells were seeded into a 96-well plate (∼10,000 cells per well). Cells were incubated in induction buffer (HBSS with 500 μm isobutyl-1-methylxanthine) and increasing concentrations of CRF agonist for 30 min at 37 °C. Following stimulation, cells were solubilized with cAMP-GLO Lysis Buffer for 15 min with gentle shaking at 20–23 °C. cAMP-GLO detection solution containing protein kinase A was added for 20 min at 20–23 °C, followed by the addition of Kinase-Glo reagent for 10 min. Each solution was carefully transferred to a white opaque 96-well plate, and luminescence was measured using a Victor plate reader (PerkinElmer Life Sciences). SAP97 knockdown experiments were additionally performed using a bioluminescent resonance energy transfer-based biosensor (exchange proteins directly activated by cAMP) for cAMP, and the protocol was adapted from Barak et al. (28).
ERK Phosphorylation
Following transfection, HEK 293 cells were re-seeded into 6-well plates. Cells were serum-starved for 1 h at 37 °C in HBSS and then stimulated with 500 nm CRF agonist for the duration of the described time points. Cells were lysed with lysis buffer (50 mm Tris, pH 8.0, 150 mm NaCl, and 0.1% Triton X-100) containing protease inhibitors (1 mm AEBSF, 10 μg/ml leupeptin, and 5 μg/ml aprotinin) for 20 min on a rocking platform at 4 °C. Samples were collected into 1.5-ml Eppendorf tubes and centrifuged at 15,000 × g for 15 min at 4 °C to pellet insoluble material. A Bronsted-Lowry protein assay was performed, and 50 μg of protein was incubated overnight at room temperature in 3× SDS Loading Buffer containing 2-mercaptoethanol. Samples were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted for ERK1/2 (rabbit anti-p44/42 MAPK, 1:1000), phospho-ERK1/2 (rabbit anti-phospho-p44/42 MAPK, 1:1000), SAP97 (mouse anti-SAP97, 1:1000), and HA-CRFR1 expression (mouse anti-HA, 1:1000), followed by a horseradish peroxidase-conjugated secondary anti-rabbit antibody (1:10,000) or anti-mouse antibody (1:10,000) where appropriate. Proteins were detected using chemiluminescence with the enhanced chemiluminescence kit from GE Healthcare.
Statistical Analysis
Densitometric data were normalized first for protein expression, and the maximum value was set to 100, with all other values displayed as the percentage thereof. One-way analysis of variance test was performed to determine significance, followed by a post hoc Tukey multiple comparison test or Bonferroni's multiple comparisons test to determine which means were significantly different (p < 0.05) from one another.
RESULTS
Proteomic Analysis of CRFR1-interacting PDZ Proteins
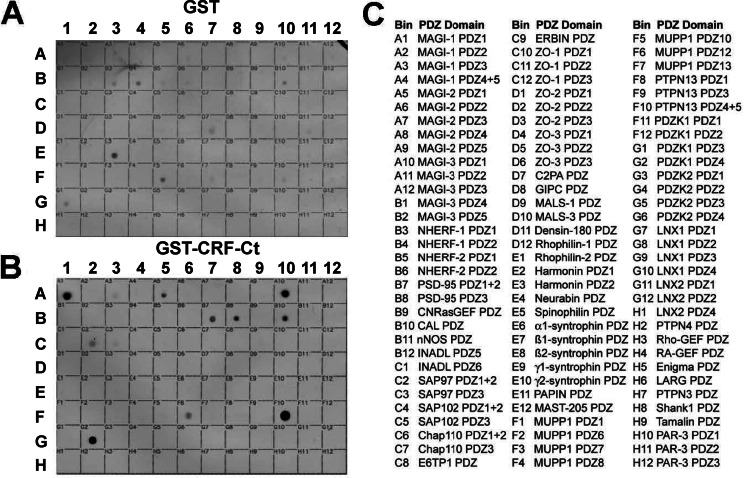
Previously, we demonstrated that the carboxyl-terminal tail class I CRFR1 PDZ-binding motif was essential for CRFR1-mediated sensitization of 5-HT2AR signaling (22). Therefore, we utilized an array of 96 class I PDZ domains spotted on a gridded nylon membrane, as described previously (23, 24), to identify potential CRFR1-interacting PDZ domain-containing proteins. The PDZ array was overlaid with 100 nm of either purified glutathione S-transferase GST-CRFR1 carboxyl-terminal tail or GST (as a control). As can be observed in Fig. 1, a subset of PDZ proteins on the array exhibited binding to the GST-CRFR1 carboxyl-terminal tail. Specifically, we found that the CRFR1 carboxyl-terminal tail selectively bound to a discrete group of PDZ domain-containing proteins as follows: MAGI-1 PDZ1; MAGI-2 PDZ1; MAGI-3 PDZ1; PSD95 PDZ 1 and 2; PSD95 PDZ3; CAL PDZ; SAP97 PDZ 1 and 2; PTPN13 PDZ 4 and 5; PDZK1 PDZ4, and MUPP1 PDZ12.
FIGURE 1.
CRFR1-CT binds to a specific subset of PDZ proteins. Equal amounts of purified His-tagged fusion proteins corresponding to a subset of PDZ domains were spotted on nylon membranes and overlaid with GST (A) versus GST-CRFR1-CT (B) to reveal specific CRFR1-CT binding to the spotted PDZ domains. C, identity of 96 distinct PDZ domains that were spotted on the nylon membranes. Data are representative of four independent experiments.
SAP97 Is Co-immunoprecipitated with CRFR1 in a PDZ-binding Motif-dependent Manner
SAP97 was one of the candidate CRFR1-binding proteins identified in the proteomic PDZ domain screen (Fig. 1). Because our previous data revealed that PDZ interactions were critical for cross-talk between CRFR1 and 5HT2AR (22), and SAP97 was previously identified as an interacting partner of 5HT2AR function (26), we focused on SAP97 in further experiments. First, we sought to confirm that SAP97 interacted with CRFR1 by co-immunoprecipitation. We found that GFP-SAP97 was co-immunoprecipitated with HA-CRFR1 from HEK 293 cells but that this interaction was not increased by agonist activation of HA-CRFR1 with 100 nm CRF (Fig. 2, A and B). The interaction was dependent upon an intact CRFR1 carboxyl-terminal PDZ-binding motif, as the deletion of the last three critical amino acids (ΔTAV) of the CRFR1 carboxyl-terminal tail prevented the co-immunoprecipitation of SAP97 with the subsequent HA-CRFR1-ΔTAV mutant (Fig. 2, A and B). Furthermore, we found that endogenous SAP97 could be co-immunoprecipitated with CRFR1 from cortical mouse brain lysates (Fig. 2C). Thus, an intact CRFR1 carboxyl-terminal PDZ-binding motif was required for SAP97 interactions with the receptor.
FIGURE 2.
GFP-SAP97 co-immunoprecipitates with HA-CRFR1 in a PDZ-binding motif-dependent and CRF agonist-independent manner. A, representative immunoblot of SAP97 co-immunoprecipitated (IP) with HA-CRFR1 but not HA-CRFR1ΔTAV. Transient transfections were performed in HEK 293 cells as labeled. Samples were run using SDS-PAGE and immunoblotted (IB) with rabbit anti-GFP. GFP-SAP97 co-immunoprecipitated with HA-CRFR1 but not HA-CRFR1ΔTAV, which lacks the PDZ-binding motif. B, effect of CRF treatment was quantified using densitometry and had no significant effect on the amount of GFP-SAP97 co-immunoprecipitated with HA-CRFR1. Data are representative of six independent experiments. C, representative immunoblot for endogenous SAP97 co-immunoprecipitated with endogenous CRFR1 but not CRFR2 from 2 mg of mouse cortical lysate. SAP97, CRFR1, and CRFR2 expression in 100 μg of cortical lysate are shown below. Data are representative of three independent experiments.
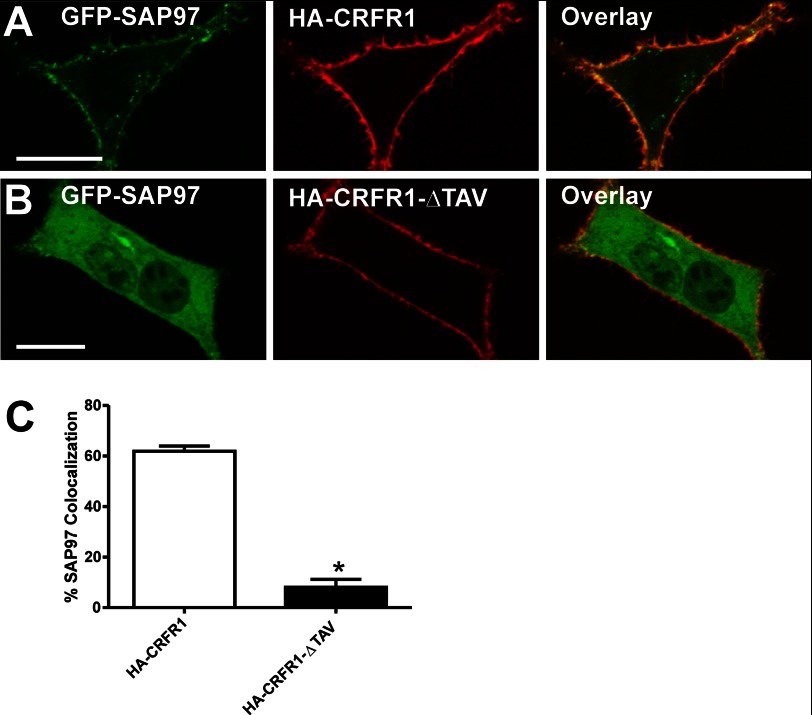
SAP97 Recruitment to the Plasma Membrane Is Dependent on the CRFR1 PDZ-binding Motif
When expressed alone in HEK 293 cells, GFP-SAP97 was diffusely localized throughout the cytoplasm and did not exhibit localization to the plasma membrane (data not shown). However, when GFP-SAP97 (green) was co-expressed with HA-CRFR1 (red), the GFP-SAP97 was predominantly localized with the receptor at the plasma membrane (Fig. 3A). When the CRFR1 PDZ motif was deleted from the carboxyl terminus of the receptor (ΔTAV), the resulting HA-CRFR1-ΔTAV mutant did not promote the plasma membrane localization of GFP-SAP97 (Fig. 3B). We found that 62 ± 2% of SAP97 was co-localized with HA-CRFR1 at the cell surface, whereas only 8 ± 3% of SAP97 was co-localized with the HA-CRFR1-ΔTAV mutant (Fig. 3C). Thus, these data in combination with the co-immunoprecipitation data indicated that SAP97 interacts with CRFR1 in a cellular context and that this interaction is dependent upon the CRFR1 PDZ-binding motif.
FIGURE 3.
GFP-SAP97 co-localizes at the membrane with HA-CRFR1 in a PDZ-binding motif-dependent manner. A, representative confocal microscopy image demonstrating the co-localization of GFP-SAP97 (green) and cell surface HA-CRFR1 (red) labeled with Zenon Alexa Fluor 633-conjugated mouse HA antibody in live HEK 293 cells. B, representative confocal microscopy image demonstrating the co-localization of GFP-SAP97 (green) and cell surface HA-CRFR1-ΔTAV (red) labeled with Zenon Alexa Fluor 633-conjugated mouse HA antibody in live HEK 293 cells. C, quantification of CRFR and GFP-SAP97 co-localization. Data are representative of 37 (HA-CRFR1) and 17 (HA-CRFR1-ΔTAV) cells. *, p < 0.05.
SAP97 Antagonizes CRFR1 Endocytosis in a PDZ Motif-dependent Manner
PDZ interactions have been reported to regulate the endocytosis and trafficking of a number of GPCRs (31, 32). Therefore, we initially examined the effect of overexpressing GFP-SAP97 on the endocytosis of wild-type CRFR1 and the CRFR1 mutant lacking a PDZ-binding motif (ΔTAV). In cells expressing only wild-type CRFR1, agonist treatment for 30 min with 500 nm CRF at 37 °C resulted in a 24 ± 4% loss of cell surface HA-CRFR1 as measured by flow cytometry (Fig. 4A). However, co-expression of GFP-SAP97 led to a significant attenuation of HA-CRFR1 endocytosis (Fig. 4A). Unexpectedly, deletion of the CRFR1 PDZ-binding motif resulted in an HA-CRFR1-ΔTAV mutant that was impaired in its endocytosis when compared with the internalization of the wild-type receptor (Fig. 4A). GFP-SAP97 overexpression did not further antagonize the internalization of the HA-CRFR1-ΔTAV mutant (Fig. 4A). To examine the role of endogenous SAP97 in the regulation of agonist-stimulated CRFR1 endocytosis in HEK 293 cells, we transfected the cells with either scrambled shRNA or an shRNA SAP97 construct that was previously shown to knock down SAP97 expression and tested CRFR1 internalization (27). As shown in Fig. 4B, the SAP97 shRNA construct effectively knocked down the expression of endogenous SAP97 protein expression in HEK 293 cells 72 h post-transfection. Consequently, all subsequent shRNA experiments were performed 72 h after HEK 293 cell transfection. We found that shRNA knockdown of SAP97 significantly increased the maximal extent of HA-CRFR1 endocytosis following 30 and 60 min of agonist treatment with 500 nm CRF (Fig. 4C). In contrast, knockdown of endogenous SAP97 expression did not influence the extent of HA-CRFR1-ΔTAV mutant internalization (Fig. 4D). Thus, taken together these data indicated that SAP97 antagonizes agonist-stimulated internalization of CRFR1 but that the CRFR1 PDZ-binding motif is required for effective internalization of the receptor.
FIGURE 4.
SAP97 antagonizes HA-CRFR1 endocytosis. A, agonist-stimulated internalization of either HA-CRFR1 or HA-CRFR1-ΔTAV in cells co-transfected with either GFP or GFP-SAP97. The internalization of HA-tagged receptors labeled with Alexa Fluor-conjugated mouse anti-NA antibody was measured in cells treated with 500 nm CRF for 30 min and compared with vehicle-treated control cells. The data represent the mean ± S.E. of nine independent experiments. *, p < 0.05 versus control CRFR1 internalization. B, representative immunoblot of endogenous SAP97 protein expression in HEK 293 cells transfected with 3 μg of plasmid cDNA encoding either scrambled (SCR) or SAP97 shRNA at 48 and 72 h initial transfection. IB, immunoblot. C, agonist-stimulated (500 nm CRF) internalization of HA-CRFR1 in cells co-transfected with scrambled (SCR) and SAP shRNA at 5, 15, 30, and 60 min. The data represent the mean ± S.E. of five independent experiments. *, p < 0.05 versus SCR shRNA-treated cells. D, agonist-stimulated (500 nm CRF) internalization of HA-CRFR1-ΔTAV in cells co-transfected with scrambled (SCR) and SAP97 shRNA at 30 and 60 min. The data represent the mean ± S.E. of four independent experiments.
SAP97 Co-localizes with CRFR1 during Receptor Endocytosis
The overexpression of GFP-SAP97 antagonized HA-CRFR1 endocytosis but did not completely block the internalization of the receptor. Therefore, we examined whether internalized HA-CRFR1 was either internalized as a complex with GFP-SAP97 or whether a population of HA-CRFR1 was internalized independently of GFP-SAP97. To do this, HEK 293 cells were transfected with both HA-CRFR1 and GFP-SAP97, and the HA-CRFR1 was labeled with Alexa Fluor 647-conjugated monoclonal HA mouse antibody (1:200 dilution) for 45 min on ice. Live labeled cells were then imaged by laser scanning confocal microscopy. Each cell was allowed to warm to 37 °C, imaged prior to the addition of 500 nm CRF, and then consecutively imaged every 5 min for 30 min. We found that, prior to agonist treatment, Alexa Fluor 647-conjugated mouse monoclonal HA antibody-labeled CRFR1 was co-localized with GFP-SAP97 at the cell surface (Fig. 5A). Upon CRF treatment, we observed limited internalization of HA-CRFR1 at 30 min of stimulation with agonist, but the HA-CRFR1 that was internalized was co-localized with GFP-SAP97 in endocytic vesicles (Fig. 5B). This indicated that endocytosed CRFR1 redistributed GFP-SAP97 into the endosomal compartment, despite the role for SAP97 in antagonizing CRFR1 endocytosis.
FIGURE 5.
GFP-SAP97 exhibits limited endocytosis with HA-CRFR1. Live cell microscopic imaging of GFP-SAP97 (green) and HA-CRFR1 (red) labeled with Alexa Fluor 633-conjugated mouse anti-HA antibody prior (A) to and following (B) 500 nm activation for 30 min in a live HEK 293 cell is shown. Image is representative of 20 cells.
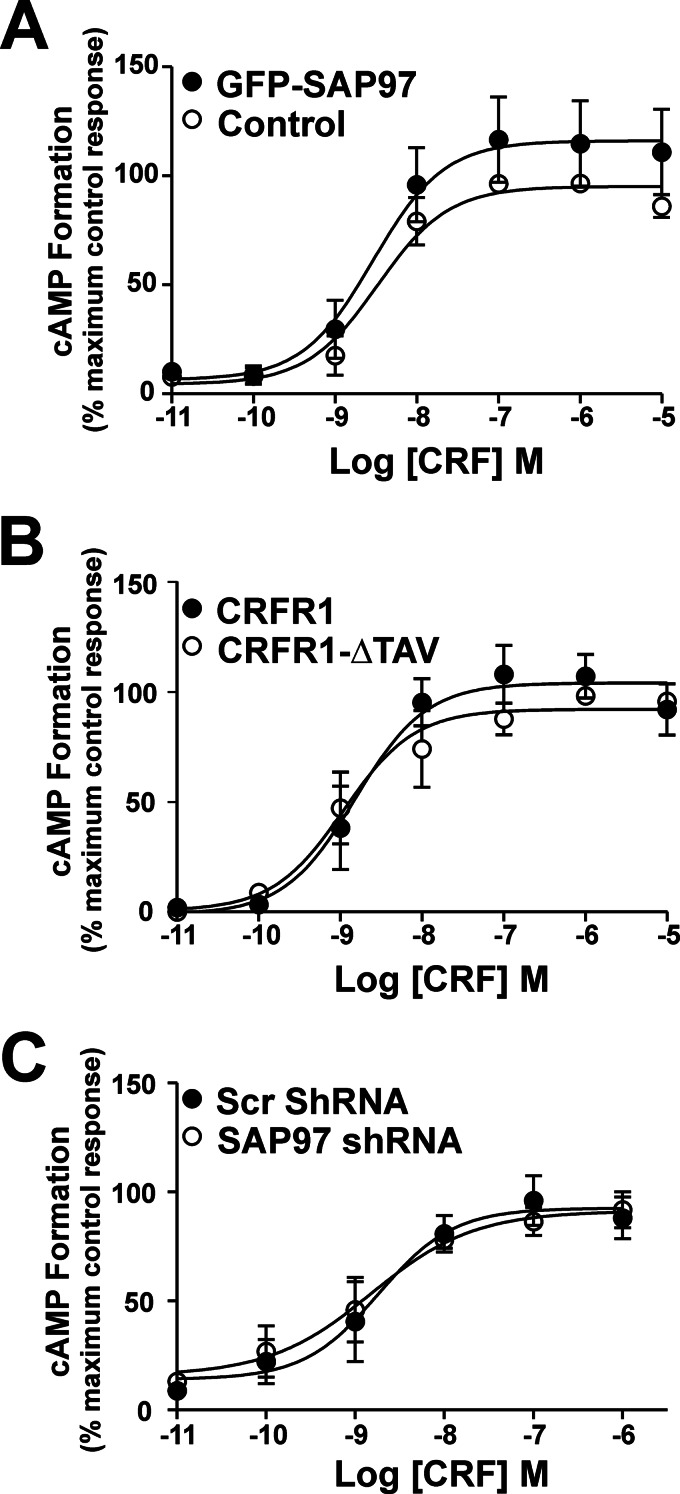
SAP97 Does Not Regulate CRFR1-mediated cAMP Signaling
Because SAP97 overexpression antagonized CRFR1 internalization and SAP97 down-regulation enhanced CRFR1 endocytosis, we sought to determine whether SAP97 and/or the CRFR1 PDZ-binding motif contributed to the regulation of CRFR1-mediated cAMP formation. In cells transfected with HA-CRFR1 with and without GFP-SAP97 and treated with increasing doses of CRF, there was no significant change in the maximum efficacy for CRF-stimulated cAMP formation (Fig. 6A). Similarly, deletion of the CRFR1 PDZ-binding motif had no effect on the maximum efficacy for CRF-stimulated cAMP formation in response to the activation of either the wild-type CRFR1 or the CRFR1-ΔTAV mutant (Fig. 6B). Consistent with what was observed following GFP-SAP97 overexpression, SAP97 shRNA knockdown did not result in an increase in the maximum efficacy for CRF-stimulated cAMP formation by the CRFR1 (Fig. 6C). Thus, SAP97 did not appear to contribute to the regulation of CRFR1-stimulated cAMP production.
FIGURE 6.
SAP97 does not regulate CRFR1-mediated cAMP formation. A, CRFR1-mediated cAMP formation, as assessed by a BRET-based biosensor assay, following co-transfection with either GFP (control) or GFP-SAP97. The data represent the mean ± S.E. of seven independent experiments. B, CRFR1- and CRFR1-ΔTAV-mediated cAMP formation, as assessed by a BRET-based biosensor assay. The data represent the mean ± S.E. of four independent experiments. C, CRFR1-mediated cAMP formation as assessed by a cAMP GLO assay following co-transfection with either scrambled (SCR) or SAP97 shRNA. The data represent the mean ± S.E. of five independent experiments.
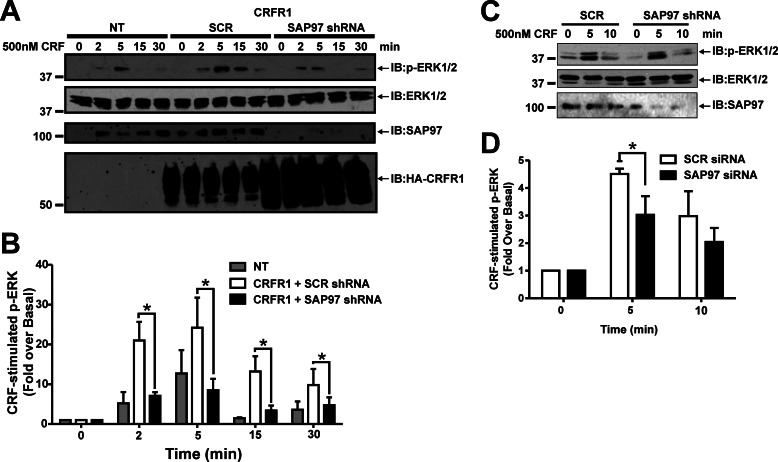
CRFR1-mediated ERK1/2 Phosphorylation Is Dependent on Endogenous SAP97 Expression
Previous work has demonstrated CRFR1 can activate the MAPK signaling pathway, as evidenced by CRFR1-mediated ERK1/2 phosphorylation (33). Therefore, we examined whether endogenous SAP97 expression was required for CRFR1-mediated ERK1/2 phosphorylation. HEK 293 cells were transiently transfected with and without HA-CRFR1 along with either scrambled shRNA or SAP97 shRNA to knock down SAP97 expression, and ERK1/2 phosphorylation in response to 500 nm CRF for 0, 2, 5, 15, and 30 min was determined by densitometric analysis of immunoblots (Fig. 7B). We found that the treatment of nontransfected HEK 293 cells with 500 nm CRF led to an increase in detectable ERK1/2 phosphorylation at 5 min, which was likely due to endogenous CRFR2 that is expressed in these cells (Fig. 7, A and B) (20). However, in cells transfected with HA-CRFR1 and scrambled shRNA, 500 nm CRF treatment resulted in a more robust and sustained activation of ERK1/2 phosphorylation (Fig. 7, A and B). Knockdown of SAP97 protein expression led to an attenuation of CRFR1-mediated ERK1/2 phosphorylation following 500 nm CRF treatment to levels that were comparable with those observed in nontransfected cells (Fig. 7, A and B). The overexpression of GFP-SAP97 had no significant effect on ERK1/2 phosphorylation (data not shown). To examine whether SAP97 regulates ERK1/2 phosphorylation in response to the activation of endogenous CRFR1 activation, identical experiments were performed in AtT20 cells that express endogenous CRFR1 (34). AtT20 cells grow in suspension, thereby complicating both transfection efficiency and the determinations of ERK1/2 phosphorylation in this cell type. Nevertheless, following siRNA knockdown of endogenous SAP97, we observed a small but significant attenuation of ERK1/2 phosphorylation following 5 min of CRF stimulation (Fig. 7, C and D).
FIGURE 7.
Knockdown of endogenous SAP97 suppresses HA-CRFR1-mediated ERK1/2 phosphorylation. A, representative immunoblot showing ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, 5, 15, and 30 min in nontransfected (NT) HEK 293 cells and HEK 293 cells transfected with HA-CRFR1 and either scrambled (SCR) or SAP97 shRNA. Shown are the corresponding immunoblots for total ERK1/2, SAP97, and HA-CRFR1 protein expression. B, densitometric analysis of ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, 5, 15, and 30 min in nontransfected (NT) HEK 293 cells, and HEK 293 cells transfected with HA-CRFR1 and either scrambled (SCR) or SAP97 shRNA. The data represent the mean ± S.E. of four independent experiments. *, p < 0.05 versus SCR shRNA-treated cells. C, representative immunoblot (IB) showing ERK1/2 phosphorylation in response 500 nm CRF treatment for 0, 5, and 10 min in AtT20 cells transfected with either scrambled (SCR) or SAP97 siRNA. Shown are the corresponding immunoblots for total ERK1/2 and SAP97 protein expression. D, densitometric analysis of ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 5, and 10 min in AtT20 cells transfected with either scrambled (SCR) or SAP97 siRNA. The data represent the mean ± S.E. of four independent experiments. *, p < 0.05 versus SCR shRNA-treated cells.
To further examine the specificity of SAP97 with the CRFR1 PDZ-binding motif, we assessed whether deleting the motif altered CRFR1-stimulated ERK1/2 phosphorylation in the presence and absence of SAP97 expression. Surprisingly, we found that the deletion of the CRFR1 PDZ-binding motif did not prevent ERK1/2 phosphorylation following agonist activation of CRFR1-ΔTAV mutant (Fig. 8, A and B). Moreover, we found that shRNA knockdown of SAP97 reduced CRFR1-ΔTAV mutant ERK1/2 phosphorylation to the same extent as was observed for the wild-type CRFR1 (Fig. 8, A and B). To determine whether this effect of SAP97 was specific to CRFR1-mediated ERK1/2 activation, we assessed whether SAP97 knockdown attenuated ERK1/2 phosphorylation following the stimulation of CRFR2, a GPCR that does not encode a PDZ-binding motif. Again, we found that SAP97 knockdown resulted in a significant reduction of CRFR2-mediated ERK1/2 phosphorylation (Fig. 8, C and D). Taken together, these data suggested that SAP97 is required for the activation of ERK1/2 signaling by the CRFR1, without modulating cAMP production, but does so via a mechanism that is independent of interactions with a PDZ-binding motif.
FIGURE 8.
Knockdown of endogenous SAP97 suppresses HA-CRFR1-ΔTAV- and HA-CRFR2-mediated ERK1/2 phosphorylation. A, representative immunoblot (IB) showing ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, and 5 min in HEK 293 cells transfected with HA-CRFR1-ΔTAV and either scrambled (SCR) or SAP97 shRNA. Shown are the corresponding immunoblots for total ERK1/2, SAP97, and HA-CRFR1-ΔTAV protein expression. B, densitometric analysis of ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, and 5 min in HEK 293 cells transfected with HA-CRFR1-ΔTAV and either scrambled (SCR) or SAP97 shRNA. The data represent the mean ± S.E. of four independent experiments. *, p < 0.05 versus SCR shRNA-treated cells. C, representative immunoblot showing ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, 5, 15, and 30 min in nontransfected (NT) HEK 293 cells and HEK 293 cells transfected with HA-CRFR2 and either scrambled (SCR) or SAP97 shRNA. Shown are the corresponding immunoblots for total ERK1/2, SAP97, and HA-CRFR2 protein expression. D, densitometric analysis of ERK1/2 phosphorylation in response to 500 nm CRF treatment for 0, 2, 5, 15, and 30 min in nontransfected (NT) HEK 293 cells and HEK 293 cells transfected with HA-CRFR2 and either scrambled (SCR) or SAP97 shRNA. The data represent the mean ± S.E. of three independent experiments. *, p < 0.05 versus SCR shRNA-treated cells.
DISCUSSION
In a previous study, we found that PDZ protein interactions with CRFR1 play an important role in regulating CRFR1-dependent sensitization of 5-HT2AR signaling (22), prompting us to search for candidate PDZ proteins that interact with CRFR1. We found that a subset of class I PDZ proteins, including SAP97, interact with the CRFR1 C-tail on a proteomic PDZ domain array. We further demonstrated that SAP97 interacts with the carboxyl-terminal CRFR1 PDZ-binding motif resulting in recruitment of SAP97 to the cell surface as well as antagonism of CRFR1 endocytosis and promotion of CRFR1-stimulated ERK1/2 signaling but not cAMP production. CRFR1 represents the fifth GPCR to which SAP97 has been demonstrated to interact (25–27). SAP97 was previously shown to regulate the recycling of the β1AR and neurite outgrowth in response to somatostatin receptor activation (25, 27). In contrast to the positive effects of SAP97 on CRFR1-stimulated signaling to ERK1/2 observed in this study, the interaction of the PDZ domain-containing protein MAGI-3 with the β1AR was shown to antagonize β1AR-dependent activation of ERK1/2 (24). Our studies indicate that SAP97 may be required for the activation of ERK1/2 phosphorylation by many GPCRs independent of PDZ domain interactions as a loss of SAP97 expression resulted in significantly reduced ERK1/2 phosphorylation following the activation of CRFR1-ΔTAV mutant and CRFR2 that does not encode a PDZ-binding motif.
Previous to our current studies with CRFR1, SAP97 had been demonstrated to bind to the β1AR to regulate the recycling of the receptor (22, 27). Interestingly, both receptors share a similar class I carboxyl-terminal PDZ motif, defined by (S/T)XΦ, where Φ represents any hydrophobic residue (35, 36). Gardner et al. (27) provided evidence that SAP97 may link the β1AR to the A-kinase anchoring protein 79/150 (AKAP79/150). This interaction was hypothesized to facilitate protein kinase A-mediated phosphorylation of Ser-312 of the β1AR third intracellular loop, thereby promoting receptor recycling and resensitization (27, 37). In support of this hypothesis, knockdown of endogenous SAP97 suppresses β1AR phosphorylation and recycling. Based on these previous data with the β1AR (27), it might seem tempting to speculate that SAP97 may play a generalized role in regulating the activity of GPCRs that encode class I PDZ-binding motifs. However, PDZ protein interactions with a variety of GPCRs have demonstrated PDZ protein-specific functions with respect to the regulation of GPCR activity. There are several examples to support this assertion. First, PSD-95 was reported to suppress 5HT2AR receptor internalization (38), but yet it promotes the internalization of the 5HT2CR (39). Second, MAGI-2 overexpression was found to have no effect on β1AR-mediated cAMP signaling and to promote agonist-induced β1AR internalization (40), but it was found to reduce vasoactive intestinal polypeptide type-1 receptor-mediated cAMP signaling and suppress agonist-induced receptor internalization (41). Finally, although MUPP1 enhances GABAB receptor signaling, it functions to uncouple the melatonin-1 receptor from Gαi (42, 43). Similarly, this study demonstrates that SAP97 regulates CRFR1 trafficking, because overexpression of GFP-SAP97 prevents CRFR1 endocytosis and knockdown of endogenous SAP97 promotes CRFR1 endocytosis. However, because of the antagonism of CRFR1 endocytosis in the presence of SAP97, it is unlikely that SAP97 subserves the same recycling function for CRFR1 as it does for the β1AR. For this reason, discussions of PDZ protein functions in the regulation of GPCR activity should likely be prefaced by the GPCR with which a given PDZ protein is interacting.
Previous research has identified a number of proteins that appear to form complexes with CRFR1 at various stages of receptor activation and trafficking. It has been demonstrated that CRFR1 activation leads to β-arrestin1 and β-arrestin2 recruitment to the membrane where they co-localize and directly interact with CRFR1 (20, 44, 45). Additionally, CRFR1 has been shown to internalize to both Rab5-positive early endosomes and Rab4-positive recycling endosomes (20). Previous work on the parathyroid hormone 1 receptor (PTH1R) and another PDZ domain-containing protein, NHERF1, demonstrated that the β-arrestin2 interaction with the PTH1R was prevented by the receptor's interaction with NHERF1 (46). Additionally, NHERF1 inhibited the uncoupling of PTH1R from Gαs (46). It is plausible that SAP97 and/or one of the other CRFR1-interacting PDZ scaffolds identified in our screens could similarly dictate which proteins are associating with CRFR1 during different states of receptor activation and trafficking, thereby regulating the trafficking and signaling of CRFR1. Future studies will look to examine what role SAP97 and other CRFR1-interacting PDZ scaffolds might play in preventing CRFR1 recruitment of β-arrestin1/2. However, quantitative studies of potential SAP97-mediated antagonism of β-arrestin translocation to CRFR1 are technically challenging due to the fact that any carboxyl-terminal fusion of bioluminescent reporter proteins will disrupt PDZ protein interactions with the receptor.
Neither the overexpression of GFP-SAP97 nor the shRNA knockdown of endogenous SAP97 in our studies had a significant effect on the EC50 or maximal CRFR1-mediated cAMP accumulation. Additionally, deletion of the CRFR1 PDZ-binding motif had no significant effect on CRFR1-mediated cAMP accumulation. These results are consistent with previous research on the β1AR, where mutation of the PDZ-binding motif had no effect on β1AR-mediated cAMP accumulation (27). Interestingly, PSD-95 has similar structural domains to SAP97 and has similarly been shown to suppress β1AR endocytosis and have no effect on β1AR-mediated cAMP accumulation (47).
Previous work has demonstrated that CRFR1 can signal through the MAPK pathway, as evidenced by CRFR1-mediated ERK1/2 phosphorylation (33). Interestingly, shRNA knockdown of endogenous SAP97 significantly reduced ERK1/2 phosphorylation to levels comparable with cells lacking the overexpressed HA-CRFR1. Work from our laboratory has demonstrated that HEK 293 cells express endogenous functional CRFR2, as evidenced by stimulation of cAMP production at the high CRF concentrations necessary to activate CRFR2 (20). Thus, the observed CRF-induced increases in ERK1/2 phosphorylation in nontransfected control cells in the present studies were expected. Knockdown of SAP97 would not be expected to alter CRFR2-mediated ERK1/2 phosphorylation, as the receptor does not possess a PDZ-binding motif at its carboxyl-terminal tail. However, we found that SAP97 knockdown significantly impaired ERK1/2 phosphorylation in cells either overexpressing CRFR2 or a CRFR1-ΔTAV mutant suggesting that SAP97 may not only play a role in scaffolding ERK1/2 protein complexes but may function to regulate GPCR-mediated ERK1/2 signaling independently of receptor interactions. Thus, our studies suggest a novel role for SAP97 in GPCR-mediated signaling, but further studies will be required to understand the precise role SAP97 plays in the regulation of ERK1/2 phosphorylation by GPCRs.
Although this is the first evidence of a role for SAP97 in the GPCR-mediated activation of ERK1/2 phosphorylation, previous research has demonstrated an ability of members of the MAPK family to phosphorylate SAP97, including ERK2 specifically (48). This phosphorylation of SAP97 by MAPK family members was shown to lead to its dissociation from the guanylate kinase-associated protein and, consequently, the cytoskeleton (48). Additionally, PSD-95 has been shown to be phosphorylated by MAPKs, including ERK2, in response to mitogens (49). It is plausible that SAP97-dependent CRFR1-mediated ERK1/2 phosphorylation may participate in a negative feedback loop whereby “activated” pERK1/2 could slowly phosphorylate SAP97, leading to dissociation of a signaling complex that would lead to attenuated receptor-mediated ERK1/2 phosphorylation. The dissociation of this signaling complex could also disrupt cytoskeletal interactions and allow for relocalization of proteins through trafficking mechanisms. The mechanism by which SAP97 facilitates CRFR1-mediated activation of ERK1/2 remains to be determined. However, the demonstration that SAP97 knockdown significantly attenuates CRFR1-mediated ERK1/2 phosphorylation indicates that other GIPs scaffolded by GPCR protein interaction motifs, in addition to β-arrestins, play a role in dictating the intracellular signaling pathways activated by GPCRs. Thus, it may be that in addition to ligand-dependent stabilization of distinct “biased” GPCR activation states (50–52), the association of intracellular GIPs with GPCRs bias GPCR signal transduction. It is likely that these two events are not mutually exclusive but function interdependently, thereby complicating the outcome of ligand-biased screening approaches for biased GPCR ligands.
In conclusion, we have presented the first evidence for SAP97 as a regulator of CRFR1 trafficking and signaling. Specifically, we have found that SAP97 interacts with the CRFR1 PDZ-binding motif to antagonize CRFR1 endocytosis without affecting cAMP production, whereas the regulation of ERK1/2 phosphorylation by the receptor does not require PDZ motif interactions. This suggests that SAP97 may function to bias CRFR1 signaling toward the ERK1/2 pathway. This provides the potential for GIPs other than β-arrestins to mediate biased signaling of GPCRs. This interaction may be a pharmaceutical target for the regulation of CRFR1 function and therefore has implications in the potential treatment of mood disorders, such as depression.
This work was supported by Canadian Institutes of Health Research Grant MOP-62738 (to S. S. G. F.).
- CRF
- corticotropin-releasing factor
- CRFR1
- CRF receptor 1
- CRFR2
- CRF receptor 2
- β1AR
- β1-adrenergic receptor
- GIP
- GPCR-interacting protein
- GPCR
- G protein-coupled receptor
- PTH1R
- parathyroid hormone 1 receptor
- PDZ
- PSD-95/Discs Large/Zona occludens
- 5-HT2AR
- 5-HT2A receptor
- 5-HT2CR
- 5-HT2C receptor
- HBSS
- HEPES-buffered saline solution
- AEBSF
- 4-(2-aminoethyl)benzenesulfonyl fluoride.
REFERENCES
- 1. Vale W., Spiess J., Rivier C., Rivier J. (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 2. Leonard B. E. (2005) The HPA and immune axes in stress: the involvement of the serotonergic system. Eur. Psychiatry 20, S302–S306 [DOI] [PubMed] [Google Scholar]
- 3. Chalmers D. T., Lovenberg T. W., Grigoriadis D. E., Behan D. P., De Souza E. B. (1996) Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol. Sci. 17, 166–172 [DOI] [PubMed] [Google Scholar]
- 4. Dautzenberg F. M., Hauger R. L. (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol. Sci. 23, 71–77 [DOI] [PubMed] [Google Scholar]
- 5. Chalmers D. T., Lovenberg T. W., De Souza E. B. (1995) Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 15, 6340–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palchaudhuri M. R., Wille S., Mevenkamp G., Spiess J., Fuchs E., Dautzenberg F. M. (1998) Corticotropin-releasing factor receptor type 1 from Tupaia belangeri–cloning, functional expression, and tissue distribution. Eur. J. Biochem. 258, 78–84 [DOI] [PubMed] [Google Scholar]
- 7. Overstreet D. H., Griebel G. (2004) Antidepressant-like effects of CRF1 receptor antagonist SSR125543 in an animal model of depression. Eur. J. Pharmacol. 497, 49–53 [DOI] [PubMed] [Google Scholar]
- 8. Chaki S., Nakazato A., Kennis L., Nakamura M., Mackie C., Sugiura M., Vinken P., Ashton D., Langlois X., Steckler T. (2004) Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 485, 145–158 [DOI] [PubMed] [Google Scholar]
- 9. Marshall F. H. (2001) Heterodimerization of G-protein-coupled receptors in the CNS. Curr. Opin. Pharmacol. 1, 40–44 [DOI] [PubMed] [Google Scholar]
- 10. Ferguson S. S., Zhang J., Barak L. S., Caron M. G. (1998) Molecular mechanisms of G protein-coupled receptor desensitization and resensitization. Life Sci. 62, 1561–1565 [DOI] [PubMed] [Google Scholar]
- 11. von Zastrow M. (2001) Role of endocytosis in signalling and regulation of G-protein-coupled receptors. Biochem. Soc. Trans. 29, 500–504 [DOI] [PubMed] [Google Scholar]
- 12. Brady A. E., Limbird L. E. (2002) G protein-coupled receptor interacting proteins: emerging roles in localization and signal transduction. Cell. Signal. 14, 297–309 [DOI] [PubMed] [Google Scholar]
- 13. Grammatopoulos D. K., Randeva H. S., Levine M. A., Kanellopoulou K. A., Hillhouse E. W. (2001) Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J. Neurochem. 76, 509–519 [DOI] [PubMed] [Google Scholar]
- 14. Gutknecht E., Van der Linden I., Van Kolen K., Verhoeven K. F., Vauquelin G., Dautzenberg F. M. (2009) Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol. Pharmacol. 75, 648–657 [DOI] [PubMed] [Google Scholar]
- 15. Chen F. M., Bilezikjian L. M., Perrin M. H., Rivier J., Vale W. (1986) Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 381, 49–57 [DOI] [PubMed] [Google Scholar]
- 16. Dautzenberg F. M., Higelin J., Teichert U. (2000) Functional characterization of corticotropin-releasing factor type 1 receptor endogenously expressed in human embryonic kidney 293 cells. Eur. J. Pharmacol. 390, 51–59 [DOI] [PubMed] [Google Scholar]
- 17. Grammatopoulos D. K., Chrousos G. P. (2002) Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol. Metab. 13, 436–444 [DOI] [PubMed] [Google Scholar]
- 18. Hauger R. L., Risbrough V., Brauns O., Dautzenberg F. M. (2006) Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol. Disord. Drug Targets 5, 453–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arzt E., Holsboer F. (2006) CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol. Sci. 27, 531–538 [DOI] [PubMed] [Google Scholar]
- 20. Holmes K. D., Babwah A. V., Dale L. B., Poulter M. O., Ferguson S. S. (2006) Differential regulation of corticotropin releasing factor 1α receptor endocytosis and trafficking by β-arrestins and Rab GTPases. J. Neurochem. 96, 934–949 [DOI] [PubMed] [Google Scholar]
- 21. Oakley R. H., Olivares-Reyes J. A., Hudson C. C., Flores-Vega F., Dautzenberg F. M., Hauger R. L. (2007) Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and β-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R209–R222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magalhaes A. C., Holmes K. D., Dale L. B., Comps-Agrar L., Lee D., Yadav P. N., Drysdale L., Poulter M. O., Roth B. L., Pin J. P., Anisman H., Ferguson S. S. (2010) CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat. Neurosci. 13, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fam S. R., Paquet M., Castleberry A. M., Oller H., Lee C. J., Traynelis S. F., Smith Y., Yun C. C., Hall R. A. (2005) P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc. Natl. Acad. Sci. U.S.A. 102, 8042–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He J., Bellini M., Inuzuka H., Xu J., Xiong Y., Yang X., Castleberry A. M., Hall R. A. (2006) Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J. Biol. Chem. 281, 2820–2827 [DOI] [PubMed] [Google Scholar]
- 25. Cai C., Li H., Kangasniemi A., Pihlajamaa T., Von Ossowski L., Kerkelä K., Schulz S., Rivera C., Keinänen K. (2008) Somatostatin receptor subtype 1 is a PDZ ligand for synapse-associated protein 97 and a potential regulator of growth cone dynamics. Neuroscience 157, 833–843 [DOI] [PubMed] [Google Scholar]
- 26. Bécamel C., Gavarini S., Chanrion B., Alonso G., Galéotti N., Dumuis A., Bockaert J., Marin P. (2004) The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 279, 20257–20266 [DOI] [PubMed] [Google Scholar]
- 27. Gardner L. A., Naren A. P., Bahouth S. W. (2007) Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human β1-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J. Biol. Chem. 282, 5085–5099 [DOI] [PubMed] [Google Scholar]
- 28. Barak L. S., Salahpour A., Zhang X., Masri B., Sotnikova T. D., Ramsey A. J., Violin J. D., Lefkowitz R. J., Caron M. G., Gainetdinov R. R. (2008) Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol. Pharmacol. 74, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferguson S. S., Caron M. G. (2004) Green fluorescent protein-tagged β-arrestin translocation as a measure of G protein-coupled receptor activation. Methods Mol. Biol. 237, 121–126 [DOI] [PubMed] [Google Scholar]
- 30. Lorenzen A., Samosh J., Vandewark K., Anborgh P. H., Seah C., Magalhaes A. C., Cregan S. P., Ferguson S. S., Pasternak S. H. (2010) Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol. Brain. 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ritter S. L., Hall R. A. (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magalhaes A. C., Dunn H., Ferguson S. S. (2012) Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 165, 1717–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kageyama K., Hanada K., Moriyama T., Imaizumi T., Satoh K., Suda T. (2007) Differential regulation of CREB and ERK phosphorylation through corticotropin-releasing factor receptors type 1 and 2 in AtT-20 and A7r5 cells. Mol. Cell. Endocrinol. 263, 90–102 [DOI] [PubMed] [Google Scholar]
- 34. Westendorf J. M., Schonbrunn A. (1985) Peptide specificity for stimulation of corticotropin secretion: activation of overlapping pathways by the vasoactive intestinal peptide family and corticotropin-releasing factor. Endocrinology 116, 2528–2535 [DOI] [PubMed] [Google Scholar]
- 35. Songyang Z., Fanning A. S., Fu C., Xu J., Marfatia S. M., Chishti A. H., Crompton A., Chan A. C., Anderson J. M., Cantley L. C. (1997) Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275, 73–77 [DOI] [PubMed] [Google Scholar]
- 36. Beuming T., Skrabanek L., Niv M. Y., Mukherjee P., Weinstein H. (2005) PDZBase: a protein-protein interaction database for PDZ-domains. Bioinformatics 21, 827–828 [DOI] [PubMed] [Google Scholar]
- 37. Gardner L. A., Delos Santos N. M., Matta S. G., Whitt M. A., Bahouth S. W. (2004) Role of the cyclic AMP-dependent protein kinase in homologous resensitization of the β1-adrenergic receptor. J. Biol. Chem. 279, 21135–21143 [DOI] [PubMed] [Google Scholar]
- 38. Xia Z., Gray J. A., Compton-Toth B. A., Roth B. L. (2003) A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J. Biol. Chem. 278, 21901–21908 [DOI] [PubMed] [Google Scholar]
- 39. Gavarini S., Bécamel C., Altier C., Lory P., Poncet J., Wijnholds J., Bockaert J., Marin P. (2006) Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol. Biol. Cell 17, 4619–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu J., Paquet M., Lau A. G., Wood J. D., Ross C. A., Hall R. A. (2001) β1-Adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2). Differential regulation of receptor internalization by MAGI-2 and PSD-95. J. Biol. Chem. 276, 41310–41317 [DOI] [PubMed] [Google Scholar]
- 41. Gee H. Y., Kim Y. W., Jo M. J., Namkung W., Kim J. Y., Park H. W., Kim K. S., Kim H., Baba A., Yang J., Kim E., Kim K. H., Lee M. G. (2009) Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology 137, 607–617 [DOI] [PubMed] [Google Scholar]
- 42. Balasubramanian S., Fam S. R., Hall R. A. (2007) GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J. Biol. Chem. 282, 4162–4171 [DOI] [PubMed] [Google Scholar]
- 43. Guillaume J. L., Daulat A. M., Maurice P., Levoye A., Migaud M., Brydon L., Malpaux B., Borg-Capra C., Jockers R. (2008) The PDZ protein Mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J. Biol. Chem. 283, 16762–16771 [DOI] [PubMed] [Google Scholar]
- 44. Rasmussen T. N., Novak I., Nielsen S. M. (2004) Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur. J. Biochem. 271, 4366–4374 [DOI] [PubMed] [Google Scholar]
- 45. Perry S. J., Junger S., Kohout T. A., Hoare S. R., Struthers R. S., Grigoriadis D. E., Maki R. A. (2005) Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J. Biol. Chem. 280, 11560–11568 [DOI] [PubMed] [Google Scholar]
- 46. Wang B., Yang Y., Abou-Samra A. B., Friedman P. A. (2009) NHERF1 regulates parathyroid hormone receptor desensitization: interference with β-arrestin binding. Mol. Pharmacol. 75, 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu L. A., Tang Y., Miller W. E., Cong M., Lau A. G., Lefkowitz R. J., Hall R. A. (2000) β1-Adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of β1-adrenergic receptor interaction with N-methyl-d-aspartate receptors. J. Biol. Chem. 275, 38659–38666 [DOI] [PubMed] [Google Scholar]
- 48. Sabio G., Arthur J. S., Kuma Y., Peggie M., Carr J., Murray-Tait V., Centeno F., Goedert M., Morrice N. A., Cuenda A. (2005) p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 24, 1134–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sabio G., Reuver S., Feijoo C., Hasegawa M., Thomas G. M., Centeno F., Kuhlendahl S., Leal-Ortiz S., Goedert M., Garner C., Cuenda A. (2004) Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38γ and ERK1/ERK2. Biochem. J. 380, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luttrell L. M., Gesty-Palmer D. (2010) Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 62, 305–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galandrin S., Oligny-Longpré G., Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 28, 423–430 [DOI] [PubMed] [Google Scholar]
- 52. Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]