Background: Ttll5/STAMP is a multifunctional protein in cells with unknown activity in animals.
Results: Targeted disruption of the Ttll5/Stamp gene in mice causes male infertility with reduced α-tubulin polyglutamylation and axoneme disruption in sperm.
Conclusion: Ttll5/Stamp deficiency differs from previously described defects in sperm maturation and function.
Significance: Ttll5/Stamp is a new gene involved in sperm maturation that may be relevant for human fertility.
Keywords: Fertilization, Gene Expression, Gene Knockout, Spermatogenesis, Tubulin, α-Tubulin Polyglutamylation, Ttll5/Stamp Targeted Mutation Mice, Disrupted Axoneme Formation, Gene Hypomorph, Male Infertility
Abstract
TTLL5/STAMP (tubulin tyrosine ligase-like family member 5) has multiple activities in cells. TTLL5 is one of 13 TTLLs, has polyglutamylation activity, augments the activity of p160 coactivators (SRC-1 and TIF2) in glucocorticoid receptor-regulated gene induction and repression, and displays steroid-independent growth activity with several cell types. To examine TTLL5/STAMP functions in whole animals, mice were prepared with an internal deletion that eliminated several activities of the Stamp gene. This mutation causes both reduced levels of STAMP mRNA and C-terminal truncation of STAMP protein. Homozygous targeted mutant (Stamptm/tm) mice appear normal except for marked decreases in male fertility associated with defects in progressive sperm motility. Abnormal axonemal structures with loss of tubulin doublets occur in most Stamptm/tm sperm tails in conjunction with substantial reduction in α-tubulin polyglutamylation, which closely correlates with the reduction in mutant STAMP mRNA. The axonemes in other structures appear unaffected. There is no obvious change in the organs for sperm development of WT versus Stamptm/tm males despite the levels of WT STAMP mRNA in testes being 20-fold higher than in any other organ examined. This defect in male fertility is unrelated to other Ttll genes or 24 genes previously identified as important for sperm function. Thus, STAMP appears to participate in a unique, tissue-selective TTLL-mediated pathway for α-tubulin polyglutamylation that is required for sperm maturation and motility and may be relevant for male fertility.
Introduction
TTLL5 (tubulin tyrosine ligase-like protein 5) is one of 13 members of the TTLL13 superfamily, all of which contain a core tubulin tyrosine ligase (TTL) domain of ∼190 amino acids (1–3). For STAMP/TTLL5, along with TTLL1, 2, 4, 6, 7, 9, 11, and 13, the TTL domain plus ∼150 amino acids on either side is involved in the progressive addition of glutamic acid residues to the γ-carboxyl group of one or more glutamic acids in the vicinity of the C terminus of polymerized tubulin (1–4). TTLL3, 8, and 10 are also considered to be polyglycylases (3, 5). Each TTLL member differs in its preference for side chain initiation or elongation and for substrate (α- or β-tubulin) with TTLL4, 5, and 7 being responsible for most initiation of polyglutamylation (2, 4, 6). TTLL5 has weak side chain elongation activity and strong initiating side chain polyglutamylation activity for α-tubulin, each of which requires added sequences of 100–150 amino acids on either side of the core TTL domain for full activity (3, 6). TTLL5 is encoded by a single-copy gene in the mouse genome that is well conserved among mammals.
The structure of murine TTLL5 is unusual in that, with 1328 amino acids, it contains a more than 400 residue longer C-terminal extension from the TTL domain than any of the other TTLL family members in mice (3). This C-terminal extension is responsible for the variety of transcriptional cofactor activities that prompted its alternative name of STAMP (7). Specifically, STAMP is a transcriptional cofactor that modulates all three properties of glucocorticoid receptor-regulated transcriptional activity: maximal activity or fold induction, the steroid concentration required for half-maximal induction (EC50), and the residual activity or partial agonist activity of antisteroids. In conjunction with the p160 coactivator TIF2, STAMP causes an additive, or more than additive, change in fold induction, EC50, and partial agonist activity of antisteroids for both endogenous and exogenous genes that are induced (7) or repressed (8) by glucocorticoid receptors. STAMP also specifically interacts with and modifies the transcriptional activity parameters of androgen and progesterone receptors but not either estrogen receptor α, another member of the classical steroid receptor family, or any other of the five nuclear receptors examined (7, 9). Thus, STAMP is a member of those cofactors that display preferential activity and binding with a subset of related transcription factors (10–13). The C-terminal extension of STAMP/TTLL5 contains not only the cofactor interaction domain needed for binding the coactivators TIF2 and SRC-1 but also the receptor interaction domains for glucocorticoid and progesterone receptor binding (7, 9). Furthermore, the ability of STAMP to alter the maximal activity, EC50, and partial agonist activity of antisteroids of glucocorticoid receptor-regulated gene transactivation was retained upon deletion of the TTL and polyglutamylation domains (7, 14).
Subsequent studies of STAMP uncovered yet additional activities. In human embryonic kidney 293 cells that were stably transfected with STAMP, the effect on maximal activity, EC50, and partial agonist activity of antisteroids was the opposite of that observed in monkey kidney CV-1 cells with transiently transfected STAMP (15). The 293 cells with stably transfected STAMP also displayed decreased cell growth that was independent of ligands for androgen, glucocorticoid, progesterone, or mineralocorticoid receptors. In interleukin-3-dependent mouse prolymphoid Ba/F3 cells, STAMP/TTLL5 has tumor suppressor-like activity (16). Interestingly, lowering STAMP levels with STAMP siRNA had both growth-promoting and growth-inhibiting effects among various ovarian cancer cell lines, whereas increased STAMP mRNA positively correlated with increasing stage numbers of human ovarian tumors (15). Conversely, STAMP/TTLL5 has recently been reported to be a candidate tumor suppressor for endometrial cancer (16). Thus, STAMP displays a variety of activities, some of which are steroid-independent, that are encoded by different regions of the full-length STAMP protein. This last conclusion is reinforced by the finding that different segments of glucocorticoid receptor, TIF2, and STAMP are responsible for modulating the maximal activity, EC50, and partial agonist activity of antisteroids of glucocorticoid receptor-regulated gene induction (14).
In view of the assorted activities of TTLL5/STAMP in vitro, it was of interest to know the physiological role(s) of TTLL5/STAMP in vivo. For this reason, we generated mice with a Ttll5/Stamp gene that would be functionally defective for modulating the properties of STAMP, i.e., steroid receptor regulated gene transcription, while retaining the TTL domain and its glutamylation activities. Surprisingly, this deletion also causes a decrease in all forms of TTLL5/STAMP mRNA. We now report that the homozygous targeted mutation mice (Stamptm/tm) have few phenotypic characteristics other than a sex-dependent effect on fertility. Female Stamptm/tm mice are normal, whereas male Stamptm/tm mice have dramatically reduced fertility. The infertility of the males is due to a unique combination of defects in sperm structure and motility that is unrelated to any of the genes previously identified as important for sperm function. Additional studies suggest that these unique defects in spermatogenesis and sperm motility are due not to the expected steroidogenic pathways but rather to structural defects in the sperm caused by reduced levels of the truncated mRNAs and resulting polyglutamylation activity.
EXPERIMENTAL PROCEDURES
Stamp Mutant Mice
The Stamp mutant mice were commercially generated by inGenious Targeting Laboratory, Inc. (Stony Brook, NY). Briefly, Stamp locus backbone was isolated from C57BL/6 BAC clone (RPCI23), a single LoxP site was inserted upstream of the exon 23, and the LoxP/FRT-flanked neo cassette was inserted downstream of the exon 24. The targeting construct was introduced by electroporation into the iTL BA1 (C57BL/6 × 129/SvEv) hybrid embryonic stem cells and screened by G418. The recombinant ES cells were identified by PCR and injected into blastocysts derived from C57BL/6J mice. Chimeric mice that were able to transmit the recombinant allele through their germ lines were obtained, and the recombinant mice were crossed with EIIa-Cre transgenic mice (FVB/N; generously provided by Heiner Westphal, NICHD/NIH) to obtain the Stamp gene mutated mice. PCR-based genotyping was performed with tail DNA. The PCR primers were 5′-CTT TTG CCT TGC CTT TCT GT-3′ (G1), 5′-AAC CAC CAG TTC CTG TGT ACA TG-3′ (G2), 5′-TGG CAA CAG CAA CAC AAC T-3′ (G3), 5′-ATG TGC AAA TCC GTC TGA CA-3′ (E22), 5′-TCT CAA GCG AGC GTC AAG TA-3′ (E23), 5′-TTC ACC CCA TCT TCT TCC TC-3′ (E24), and 5′-CCT TTT GCC CCA CTA TCA GA-3′ (E25).
The mice were housed in polycarbonate cages and used after acclimation to an environmentally controlled room (temperature, 23 ± 2 °C; relative humidity, 50 ± 10%; frequent ventilation; and 12-h light cycle). All experimental procedures and animal uses were approved by the Ethics Committee of the NIDDK, National Institutes of Health. All male mice for sampling were fully matured (12–20 weeks old) and sacrificed by inhalation of carbon dioxide. For mating experiments, male (12–20 weeks old) and female mice (10–20 weeks old) were in the same cage for 4–14 days and then separated. The mated female mice were further observed for 20 days to check pregnancy status and the number of pups.
RNA Extraction and Real Time PCR
Total RNA was prepared with TRIzol reagent (Invitrogen) and reverse transcribed to first strand cDNA using the SuperScript III First-Strand Synthesis System for qRT-PCR (Invitrogen) according to the manufacturer's protocol. Stamp transcripts were quantitated using SyberGreen and the ABI 7900HT real time PCR system (Applied Biosystems, Carlsbad, CA). The GenBankTM accession number for Stamp cDNA is AY237126. The quantitation was normalized with glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The Stamp primers were 5′-ATG TGC AAA TCC GTC TGA CA-3′ and 5′-TTC ACC CCA TCT TCT TCC TC-3′. The Gapdh primers were 5′-TGT TCC TAC CCC CAA TGT GT-3′ and 5′-CCC TGT TGC TGT AGC CGT AT-3′. The primers for qRT-PCR quantitation of STAMP and assorted TTLLs are listed in Table 1. The primers for the 24 spermatogenesis-related genes of Fig. 4 are given in supplemental Table S1.
TABLE 1.
Primers for qRT-PCR quantitation of STAMP and TTLLs
Primers for the indicated positions of the listed genes are given in the 5′ to 3′ direction. The primers for STAMP spanning exons 23 and 24/25 detect only the full-length transcripts, whereas the exon 22 and 25 primers amplify the STAMP transcripts of both the wild type and TM mutant mice. Two different primer pairs were used for all TTLL genes except TTLL7.
| Gene | GenBankTM accession number | Region | Forward primer/reverse primer |
|---|---|---|---|
| STAMP | NM_001081423.2 | Exons 23 and 24/25 | GCGCAGGAGAATCCTTGCCCA |
| ACCTCCTCCAGTTCAGCCTCACT | |||
| STAMP | NM_001081423.2 | Exons 23 and 24/25 | CGGTTGGCACTTCTGGAGCG |
| TCCTCCAGTTCAGCCTCACTTGCT | |||
| STAMP | NM_001081423.2 | Exons 22 and 25 | ATGTGCAAATCCGTCTGACA |
| CCTTTTGCCCCACTATCAGA | |||
| TTLL1 | NM_178869.4 | Residues 1359–1479 | GATCGAGGTCAACGCGTCCCC |
| GCAGTCGGGAATCTCGCCGT | |||
| TTLL1 | NM_178869.4 | Residues 1253–1379 | TCGTGCAGTCTCTCAAGGCCG |
| GGGGACGCGTTGACCTCGAT | |||
| TTLL4 | NM_001014974.1 | Residues 2413–2562 | CCTGTCTCGCATGCAAAGCCGT |
| CGAGCAGATGCTGGTGGCTTCA | |||
| TTLL4 | NM_001014974.1 | Residues 3532–3661 | TCTCACCACCCAGTGGGAACAGA |
| TGGCAGAGACCACAGTGGGGC | |||
| TTLL6 | NM_172799.4 | Residues 1890–2010 | TGCCCAGCCCATAACGTCTGT |
| ACTCTCCGGGCTGCTGCACT | |||
| TTLL6 | NM_172799.4 | Residues 1430–1576 | AGCTGTATGCACGGCAGCTGAT |
| GTCTCTGTCGCTGGAGCCGC | |||
| TTLL7 | NM_027594.1 | Residues 1909–1991 | CCAACGTCAGCGTCCCGGTC |
| TGCCGCAAAGACTCGTTCCACT | |||
| TTLL11 | NM_028921.2 | Residues 1418–1487 | TGATCAGAGACACGCTGCGCC |
| TCCATCTGCTGAGACTGGATCTCCT | |||
| TTLL11 | NM_028921.2 | Residues 1384–1487 | CCCAGCCTGGTGGATGAAGAGGT |
| TCCATCTGCTGAGACTGGATCTCCT |
FIGURE 4.
Glutamylation of Stamptm/tm sperm. A and C, immunoblot using anti-glutamylation antibodies (GT335 for α- and β-tubulins) and B3 (for α-tubulin) with sperm (A) and GT335 with testis (C). Lysate from Cos7 cells over expressing STAMP was used as a positive control (P*) and anti-mouse α-tubulin (lower panel in C) was used as a loading control. B, the amount of polyglutamylated α-tubulin from WT and TM sperm. The amounts were determined by densitometry, normalized with respect to α-tubulin expression as an internal control, and plotted as percentage of the average polyglutamylation in Stamp+/+ sperm. The bar graph gives the averages of two independent gels like A, each with three samples (± S.E.). *, p = 0.026. Another independent experiment with just GT335 gave similar results with the level of α- and β-tubulins in TM mice being reduced by 49% (p = 0.025; data not shown). D, immunocytochemistry of Stamp+/+ (WT) and Stamptm/tm (TM) sperm using GT335 antibody. S, normal sperm; C, coiled sperm; H, head only; T, tail only. The antibody staining intensity of individual Stamp+/+ and Stamptm/tm sperm (lower panels) was determined for the mid-piece (#1), upper (#2), and lower (#3) tail. E, polyglutamylation varies along length of sperm tail. The binding of GT335 (red) to the sperm mid-piece (#1), upper tail (#2), and lower tail (#3) was normalized to DAPI staining (blue) of the sperm nucleus to adjust for differences in brightness of each image. The mean ± S.E. of antibody staining to seven normal and mutant sperm was compared by setting the average value for normal sperm as 100%. *, p ≤ 0.0041.
Western Blot Analysis
Western blots were prepared, probed with mouse anti-polyglutamylated tubulin monoclonal antibody (GT335; a gift from Carsten Janke, CNRS, Montpellier, France and Adipogen, San Diego, CA), mouse anti-polyglutamylated α-tubulin monoclonal antibody (ab11324; Abcam, Cambridge, MA), rabbit anti-STAMP polyclonal antibody against amino acids 525–544 (7), rabbit β-actin polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or rabbit anti-α-tubulin antibody (Cell Signaling Technology Inc., Boston, MA), and visualized by ECL detection reagents as described by the manufacturer (GE Healthcare).
Immunocytochemical Staining and Histomorphometry
Freshly isolated sperm from epididymis was fixed by 4% paraformaldehyde and incubated with GT335 antibody in TBS containing 5% BSA and further probed with anti-mouse IgG-Cy3 (Sigma-Aldrich). The slides were mounted using VECTASHIELD with DAPI (Vector Laboratories, Inc., Burlingame, CA) and analyzed under fluorescence microscopy. Male reproductive organs were fixed in 4% paraformaldehyde and sent to Histoserve Inc. (Germantown, MD) for hematoxylin and eosin staining. The following four antisense (A) or sense (S) oligonucleotide probes were used together for in situ hybridization: STAMP 22S forward, ATG TGC AAA TCC GTC TGA CA; STAMP 22A reverse, TGT CAG ACG GAT TTG CAC AT; STAMP 23S forward, TCT CAA GCG AGC GTC AAG TA; STAMP 23A reverse, TAC TTG ACG CTC GCT TGA GA; STAMP 24S forward, GAG GAA GAA GAT GGG GTG AA; STAMP 24A reverse, TTC ACC CCA TCT TCT TCC TC; STAMP 25S forward, TCT GAT AGT GGG GCA AAA GG; and STAMP 25A reverse, CCT TTT GCC CCA CTA TCA GA. All probes, 5′-labeled with digoxin, were generated from IDTDNA Inc. (Coralville, IA) and sent to Histoserve for in situ hybridization with their protocol. All slides were analyzed under a light microscope.
Computer-assisted Sperm Analysis
Caudal epididymal sperm were analyzed on a HTM-IVOS (Version 10.8) motility analyzer (Hamilton Thorne Biosciences, Beverly, MA) with the following settings: phase contrast; frame rate, 60 Hz; minimum contrast, 30; low and high static size gates, 1–4; low and high intensity gates, 0.7–1.52; low and high static elongation gates, 10 and 100; default cell size, 13 pixels; default cell intensity, 75; and magnification, 0.78. Sperm were loaded into a prewarmed, 80-μm 2X-CEL® dual sided sperm analysis chamber (Hamilton Thorne Biosciences) and observed under 4× magnification. After incubation (15 min, 37 °C) in M16 medium (Specialty Media, Chemicon International, Phillipsburg, NJ), sperm motility, viability, velocity (VSL, straight line velocity; VCL, curvilinear velocity) and other movement characteristics (LIN, linearity; STR, straightness) were determined. Motility and progressive motility were defined as the percentage of sperm with an average path velocity (VAP) >10 or >50 μm/s, respectively. The exclusion of fluorescent Viadent, a vital dye, was used to assess viability.
Epididymal sperm were collected separately from caput and cauda epididymides in M16 medium (Specialty Media) and fixed on glass slides for digital imaging using a Zeiss Axioplan 2 microscope. Random fields were selected, and detached sperm heads and intact sperm were counted (>500) from three independent biological samples.
Electron Microscopy
Caudal epididymal sperm were fixed in 2% glutaraldehyde buffered in sodium cacodylate, pH 7.4, for 2 h at 4 °C followed by post-fixation in 1% osmium tetroxide in water for 1 h at 4 °C. After extensive washing, the cells were pelleted by centrifugation and embedded in melted (37 °C) agarose. Samples were dehydrated through a graded series of ethanol and processed for embedding in LR-White resin (17). Ultrathin sections were counterstained with uranyl acetate followed by lead citrate and imaged in a Phillips (FEI Company) CM120 transmission electron microscope.
The following criteria were used to number the peripheral nine microtubule doublets in the 9 + 2 arrangement in sperm axonema. Twenty high definition images, in which the orientation of the dynein arms could be identified, were selected for study. Orienting the image so that the dynein side arms point to the right gave each axonemal cross-section a defined polarity and clockwise orientation in the direction of doublet number 1–9. Doublet number 1 lies perpendicular to the line between the two central microtubules. Support for this assignment comes from the fact that more peripheral dense fibers associated with doublets 1, 5, and 6 usually are distinctly larger than the others, have the dumbbell shape of the infinity symbol (∞), and are the last to disappear toward the end of the principal piece (18). Of 20 images analyzed, doublet number 4 was missing in 19, or 95%. In more severely affected axonema, it was not possible to confidently determine the number(s) of the missing doublet(s).
Serum Collection and Biochemical Analysis
Blood was collected from each mouse after CO2 inhalation, incubated at 37 °C for 30 min, and centrifuged at 2000 rpm for 15 min. Serum testosterone levels were determined by testosterone assay kits (KGE010, R&D Systems, Minneapolis, MN; and TE187S-100, Calbiochem, Spring Valley, CA) according to the manufacturers' instructions.
Microarray Assay
RNA samples were isolated from the testes of 3 WT and 3 Stamptm/tm mutant mice of mixed genetic background and purified using TRIzol (Invitrogen). Total RNA was further purified using DNase and RNeasy MinElute RNA cleanup kits (Qiagen) according to the manufacturer's recommendations, and the RNA quality and integrity were tested using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Target labeling and hybridization to exon chips were carried out in the NIDDK microarray core using GeneChip® mouse exon 1.0 ST arrays (Affymetrix, Inc., Santa Clara, CA). The microarray signals were normalized using the RMA algorithm. The significantly expressed genes were selected based on analysis of variance by Partek Pro software (Partek, St. Charles, MO). The analysis of variance gene lists by p value of 0.05 and absolute value of fold change of 1.5 including up and down-regulated genes were used in the gene ontology analysis by the commercial gene pathway analysis web tool.
Statistical Analysis
The values of n independent experiments were analyzed for statistical significance by the two-tailed Student's t test using InStat 2.03 for Macintosh (GraphPad Software, San Diego, CA). When the difference between the S.D. values of two populations is significantly different, then the Mann-Whitney test or the alternate Welch t test is used.
RESULTS
Testicular Ttll5/Stamp mRNA Expression and Generation of a Functionally STAMP-deficient Mouse
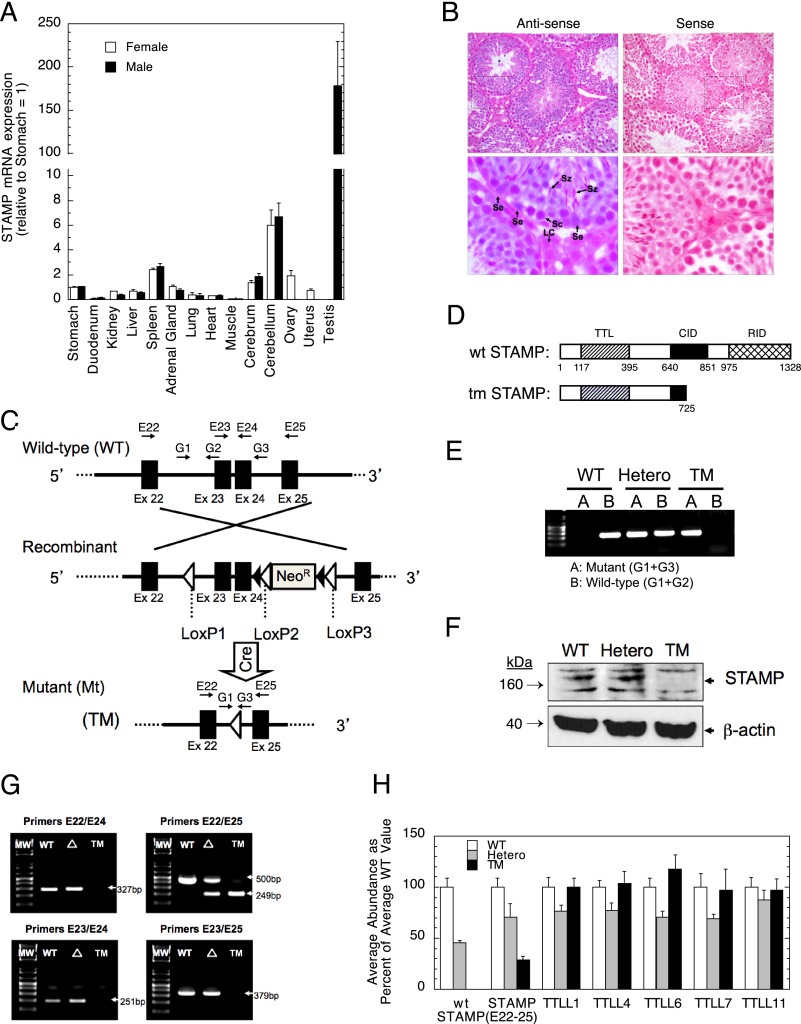
Determination of Ttll5/Stamp gene transcripts in various mouse organs by qRT-PCR revealed much higher levels of Ttll5/Stamp mRNA in the testis than any other surveyed organ (Fig. 1A). For more precise identification of Ttll5/Stamp-expressing cell types, we performed in situ hybridization assays. Ttll5/Stamp transcripts were detected mainly in spermatocytes, elongating spermatids, and Sertoli cells, but not in Leydig cells (Fig. 1B).
FIGURE 1.
Expression of Stamp/Ttll5 and establishment of mutant mouse line. A, qRT-PCR of STAMP transcripts in total RNA isolated from 14 mouse tissues (means ± S.E. of triplicate experiments from three male and female mice). B, localization of Stamp transcripts in Stamp+/+ testis using in situ hybridization. Hybridization of anti-Stamp oligonucleotide probes is indicated by the bluish purple color. The nuclei were stained by Nuclear Fast Red. Magnification for the top row is 200-fold. The areas within the dashed rectangles were computer-enlarged an additional 4-fold in the corresponding lower panels. Se, Sertoli cells; LC, Leydig cells; Sz, elongating spermatids; Sc, spermatocytes. C, schematic representation (not to scale) of the normal (WT) Stamp locus, the recombination locus containing LoxP/FRT flanked neomycin resistance gene (Neor), and the mutant (TM) after crossing with EIIa-Cre mice transgenic mice. The arrows indicate primer (G1–G3 and E22–E25) locations for genotyping. Solid boxes, exons 22–25; open triangles, LoxP sites; solid triangles, FRT. D, cartoon of normal and the predicted truncation of STAMP protein. The indicated domains in normal (WT) STAMP are TTL, cofactor interaction domain (CID), and receptor interaction domain (RID), with numbering corresponding to the amino acid boundaries of the domains. E, PCR-based genotyping of Stamp+/+ (WT: only lane B, ∼260 bp), Stamp+/tm (Hetero: both lanes A and B), and Stamptm/tm (TM: only lane A, ∼250 bp). F, immunoblot in the regions of 140–190 and 40 kDa of testicular STAMP protein using β-actin as a loading control. G, STAMP mRNA species in different genotypes. STAMP cDNA was prepared from mRNA in Stamp+/+ (WT), Stamp+/tm (Δ), and Stamptm/tm (TM) mouse testes and then amplified by PCR using the indicated primers (defined in Fig. 1C) to detect species with and without exons 23 and 24. The amplification efficiencies of the products by primer set E22/E25 were the same (104 ± 7%, S.D.) for each group of mice. Only the heterozygotes (Δ) contain both forms. MW, molecular weight markers. H, quantitation of STAMP and TTLL mRNA transcripts in Stamp+/+ (WT/WT), Stamp+/tm (WT/TM), and Stamptm/tm (TM/TM) mouse testes by qRT-PCR. mRNAs from three animals each of WT, heterozygous, and homozygous mutant mice was quantitated in triplicate in two independent qRT-PCR assays using the primer sets of Table 1. For most genes, two different primers sets were used. The results for each primer set were normalized to the average value for WT mice and then averaged (± S.E., n = 12) to give the final average expression level for each mouse phenotype. The value for WT STAMP in the TM mice is 0.01 ± 0.00 and is not visible. The data for STAMP (E22–25) were obtained using primers in exons 22 and 25 and thus detected both full-length STAMP and mutant STAMP lacking exons 23 and 24.
A targeted mutant (TM) Stamp gene was prepared by inserting LoxP sites on either side of exons 23 and 24, with a Neomycin resistance gene between two LoxP sites in the intron between exons 24 and 25 (Fig. 1C). We chose to target STAMP activities because the ∼200-amino acid size of just the TTLL domain of Ttll5 is encoded by 10 exons spanning 82 kb, which is too much to remove by targeted deletion techniques. Exons 23 (residues 726–774) and 24 (residues 775 through the first two nucleotides of codon 809) were selected for deletion for four reasons. First, elimination of exons 23 and 24 has not been reported as a naturally occurring splicing variant. Second, removal of exons 23 and 24 gets rid of ∼40% of the amino acids near the C-terminal end of the cofactor interaction domain region, which is essential for the binding of TIF2. Third, excision of exons 23 and 24 causes a frame shift so that translation prematurely stops after 7 triplets (Fig. 1D; the seven new residues are too small to be visible and not indicated in the diagram of TM STAMP). Fourth, deletion of exons 23 and 24 eradicates all methionine codons for the next 395 residues. A start of translation at this downstream methionine codon would give a small protein of 124 amino acids that is missing almost all of the biologically relevant sequences for STAMP activity. Thus, by removing all of the region required for binding steroid receptors and most of the region for TIF2 and SRC-1 association while retaining the TTLL domain, this deletion allow us to selectively probe Stamp participation in steroid receptor versus TTLL functions. For this reason, we call the resulting mice Stamp targeted mutant, or Stamptm, mice.
Floxed mice carrying the conditional Stamp deletion were mated with EIIA-Cre mice to give the functional Stamp deficiency in a mixed genetic background (129/SvEv, C57BL/6, and FVB/N). The mutated genotypes were confirmed by PCR-based genotyping (Fig. 1E). Full-length STAMP protein (apparently 160 kDa) was detected by Western blotting in the testes of wild type (Stamp+/+) and heterozygous (Stamp+/tm) mice but not in the testes of homozygous mutant (Stamptm/tm) mice (Fig. 1F). This antibody only weakly identifies the full-length STAMP protein and was unable to reproducibly detect the predicted truncated protein in Stamp+/tm and Stamptm/tm mice (data not shown). Therefore, PCR and qRT-PCR were used to further analyze the STAMP species and other TTLLs in the testes of age-matched wild type, heterozygous, and homozygous mutant mice.
PCR amplification of cDNA from the testes of different genotypes revealed that, as expected, WT animals contain only the full-length STAMP gene, TM/TM mice have only the message lacking exons 23 and 24 (STAMP [E22–25]), and heterozygous mice (Hetero) possess both transcripts (Fig. 1G). The STAMP species detected with primers in exons 22 and 25 (STAMP [E22–25]) would all contain the TTL domain. This species, as documented by others (3, 6), would retain polyglutamylation initiating activity and is predicted from earlier work with truncated STAMP species (7) to be stable. The relative abundance of these species with initiating activity for polyglutamylation is ∼100:71:28 for WT:heterozygous:TM/TM mice, respectively. Thus, Stamptm/tm mice could be functional hypomorphs. Finally, the levels of several other TTLLs were unchanged in the testes of TM versus wild type mice. TTLL7 is another species with initiating activity, and its level is also the same in TM versus WT mice (Fig. 1H).
Stamptm/tm mice were born from heterozygotic parents at the expected Mendelian ratio overall, indicating that the Stamptm/tm gene is not lethal during embryonic development. This was unexpected in view of the report that depletion of the TTLL5 (STAMP)-related gene in Caenorhabditis elegans (C55A6.2) by siRNA causes embryonic lethality in 44% of the cases and sterility (2). Stamptm/tm mice do not differ significantly from their littermates (Stamp+/+ and Stamp+/tm) with respect to development or life span up to more than 1 year of age. Initially it appeared that the Stamptm/tm males were slightly smaller. However, subsequent litter-matched comparisons from heterozygous matings (which were confounded by imperfect Mendelian ratios of gender and genotype that often prevented direct comparisons) failed to support this observation (data not shown). Therefore, these studies were discontinued. The genotypes were indistinguishable with respect to behavior in standard cage environments. These results suggested that one in vivo function of STAMP could be in spermatogenesis, in which case STAMP might participate in male reproductive function.
Male Reproductive Function Is Defective in Stamptm/tm Mice
Both Stamp+/+ and Stamptm/tm male mice exhibited normal libido when presented with a female mouse in mating cages. Evidence of ejaculation in the form of vaginal plugs was usually found within a few days. However, the successful pregnancy and delivery rates and the pup numbers per litter are dramatically lower in the mating cages with Stamptm/tm compared with Stamp+/+ male mice (Table 2). In contrast, female Stamptm/tm mice do not exhibit any reproductive abnormalities when mated with Stamp+/+ male mice. Although the litter frequency and size of Stamptm/tm matings with WT and heterozygous females is markedly reduced, there were no obvious developmental or physiological problems with the infrequent offspring of the Stamptm/tm males when continuously observed for more than 1 year. This suggests that the defects of Stamptm/tm males may involve steps leading to conception and not those following fertilization.
TABLE 2.
Fertility test using Stamptm/tm mice of heterogeneous genetic background
The mating rate is the number of successful pregnancies after co-caging of male and female for 2–4 weeks, with the number in parentheses indicating the percentage of co-caging that results in live pups. Errors in average pups/litter is ± S.E. for n ≥ 3 and range for n = 2.

a This mouse, the only TM/TM male to give a litter of >4 pups, was regenotyped and reconfirmed to be TM/TM.
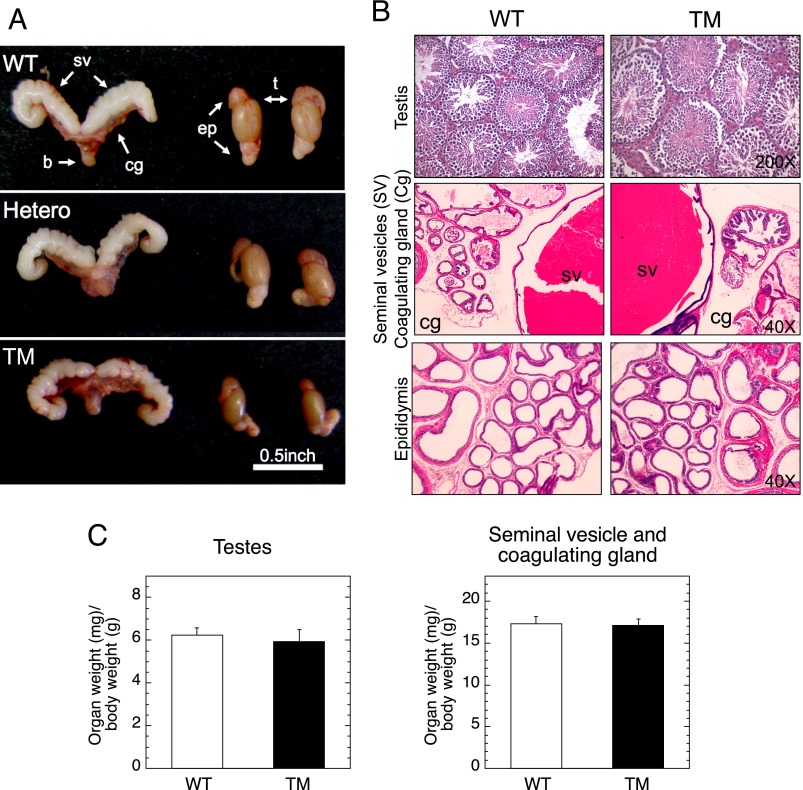
To determine the cause of infertility with Stamptm/tm males, we first compared the gross appearances of the male reproductive organs (seminal vesicle, coagulating gland, testis, and epididymis) between genotypes (Fig. 2A). The organs of Stamptm/tm mice are not noticeably different from those of Stamp+/+ and Stamp+/tm mice. Furthermore, the histological structures are indistinguishable between genotypes (Fig. 2B). Seminiferous tubules, their cell components, and interstitial cells are all normal in the Stamptm/tm testes. In addition, the relative weights (organ weight/body weight) of testis, seminal vesicle, and coagulating gland display no significant differences (Fig. 2C). We conclude that the absence of fully functional STAMP protein does not affect the development of male sex organs or their structure. Furthermore, the mutant Stamp does not appear to alter sexual behavior or sexual performance.
FIGURE 2.
Male reproductive organs in Stamptm/tm mice. A, gross appearance of seminal vesicles (sv), coagulating gland (cg), testis (t), epididymis (ep), and bladder (b) of 12-week-old Stamp+/+ (WT), Stamp+/tm (Hetero), and Stamptm/tm (TM) male mice. B, histology of testis, seminal vesicle, coagulating gland, and epididymis in Stamp+/+ (WT) and Stamptm/tm (TM) male mice. C, relative organ weights (mg of testis or seminal vesicle of coagulating gland) were normalized to grams of body weight and graphed (average ± S.E., n = 3 for WT and n = 5 for TM). For testis, the average of each pair from one animal = one value.
To distinguish between effects of STAMP protein on fertility versus embryonic development, we performed in vitro fertility tests using the sperm from Stamp+/+ and Stamptm/tm male mice. As seen in Table 3, the success rate for production of two-cell embryos with Stamp+/+ sperm was 93.1%, whereas only 2.9% of the eggs exposed to Stamptm/tm sperm progressed to the two-cell stage. These data argue that the deficiency of STAMP in male mice directly reduces fertilization rates as opposed to impeding normal embryonic development.
TABLE 3.
In vitro fertility test of Stamptm/tm male mice
Eggs were harvested for use from healthy wild type C57BL6Tac female mice.
| Genotype | No. of eggs | Two-cell embryo | Fertility |
|---|---|---|---|
| % | |||
| Stamp+/+ | 393 | 366 | 93.1 |
| Stamptm/tm | 348 | 10 | 2.9 |
| Positive controla | 262 | 220 | 84.0 |
a Positive control is C57BL6Tac male mouse.
In view of the heterogeneous genetic composition of the above Stamptm/tm male (and female) mice and reports of phenotypic differences of mutant mice of mixed versus homogenous backgrounds (19), we asked whether the same male infertility would be observed in a >99% pure genetic background. After seven generations of backcrossing of Stamp+/tm females with C57BL/6 Stamp+/+ males, Stamptm/tm mice with >99.2% C57BL/6 genetic background were obtained. Again, the Stamptm/tm males displayed a severely reduced level of fertility (successful mating rate of Stamptm/tm = 2/15 (13%) with 0.2 ± 0.14 (S.E.) pups/mating versus 17/17 (100%) mating rate and 6.0 ± 0.5 (S.E.) pups/mating for Stamp+/+ mice) that is indistinguishable from that of the above mixed background Stamptm/tm males. Serum testosterone levels in Stamp+/+ and Stamptm/tm males vary considerably but are not significantly different (data not shown). We conclude that the infertility of Stamptm/tm males that are functionally devoid of STAMP is independent of the genetic background of the mouse and has minimal effects on circulating testosterone levels.
Effects of Stamp Protein on Sperm Morphology and Function
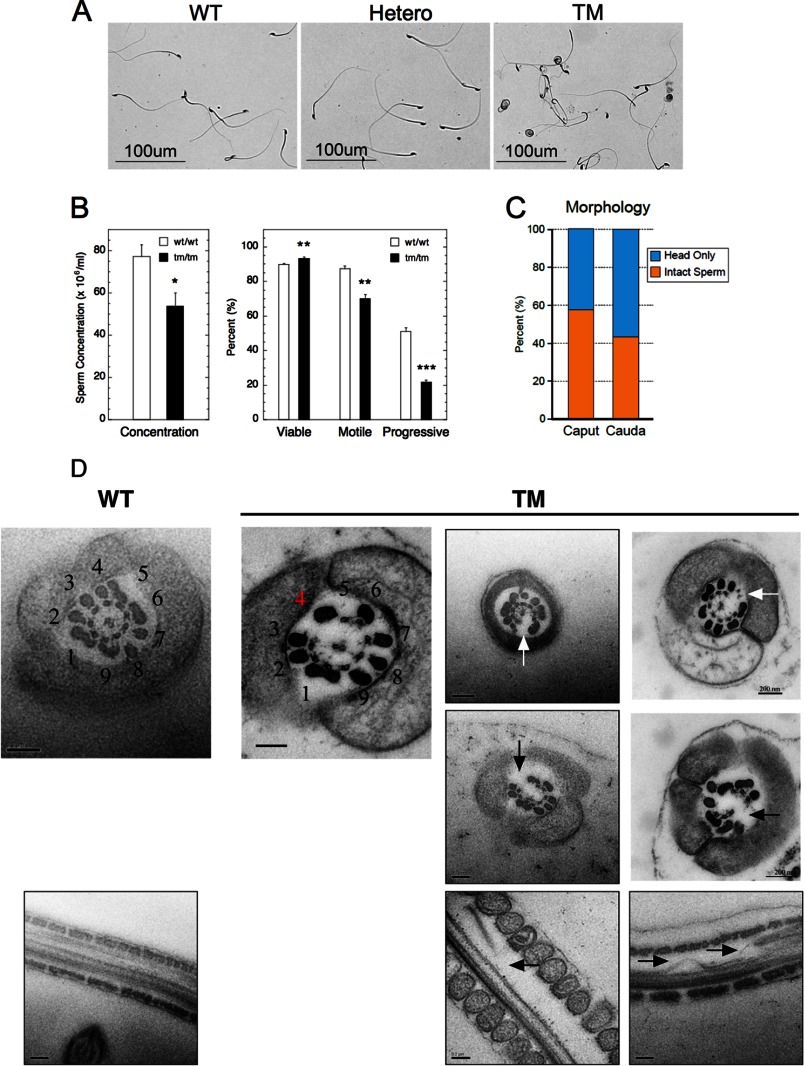
In an effort to understand the causes of male infertility in Stamptm/tm mice despite the presence of normal appearing male sex organs, we examined the caudal epididymal sperm from Stamp+/+, Stamp+/tm, or Stamptm/tm mice under a light microscope (Fig. 3A). Normally, sperm mature and become motile as they transit the epididymis from the beginning (caput) to the end (cauda). Most of the caudal sperm from Stamp+/+ and Stamp+/tm mice have the normal shape. However, the Stamptm/tm sperm are highly irregular. Most Stamptm/tm sperm have detached heads and tails. Many of the tails are tightly coiled, whether or not they are still attached to a head. No detached heads or coiled tails are observed in the sperm of Stamp+/+ or Stamp+/tm mice. Thus, the phenotype of the Stamp mutant sperm does not display a gene dosage effect. The concentration of sperm isolated from Stamptm/tm epididymides was ∼70% of that of normal mice (54 versus 77 × 106/ml). Computer-assisted sperm analysis documented no decrease in viability (90 versus 93% but significant decreases in motility (87 versus 70%) and especially progressive motility (51 versus 22%) in Stamp+/+ compared with Stamptm/tm sperm (Fig. 3B). The differences in progressive motility are even more dramatic under conditions of in vitro fertilization, where WT sperm are very mobile with vigorous activity in the vicinity of oocytes, whereas mutant sperm are almost immobile and display little motion around oocytes (supplemental Fig. S1; see supplemental Movie S1). Dismembered sperm were rarely observed in the controls, as seen in Fig. 3A, but were present in >50% of Stamptm/tm sperm as measured by the percentage of isolated sperm heads. When looking earlier upstream in the epididymis (i.e., in the caput), we saw no significant difference between the number of disjointed sperm in the caput compared with the cauda epididymides (Fig. 3C). This suggests that sperm disruption occurs earlier in spermatogenesis. These results indicate that the heads of Stamptm/tm sperm are readily disrupted from their tails and that the remaining intact sperm have poor progressive motility, resulting in the observed infertility.
FIGURE 3.
Abnormal sperm in Stamptm/tm mice. A, morphology of Stamp+/+ (WT), Stamp+/tm (Hetero), and Stamptm/tm (TM) male mice (12 weeks old) was determined by light microscopy. Sperm was isolated from epididymis and stained with 0.5% trypan blue. B, epididymal spermatozoa were analyzed by computer-assisted sperm analysis to determine concentration, viability, and motility. Viability was defined by the ability to exclude fluorescent Viadent; motility was defined by the ratio of the motile to the total sperm concentration; and progressive motility was defined by sperm moving faster than the minimum path velocity (VAP). The data are expressed as the means ± S.E. of eight independently obtained biological samples of >99% C57/Bl6 mice (each >500 sperm). *, p < 0.05; **, p < 0.005; ***, p < 0.0005. C, sperm isolated from caput or cauda epididymides of Stamptm/tm mice were imaged by light microscopy. The percentage of disrupted sperm was determined from the number of detached sperm heads and intact sperm in three independent (>500 sperm) biological samples. Using a Student's t test, there was no statistically significant difference between Stamptm/tm sperm isolated from the caput and cauda epididymis. D, disrupted axoneme in Stamptm/tm sperm tails. Electron microscopy detected the canonical arrangement of nine peripheral microtubule doublets, and the larger associated, more peripheral outer dense bodies or fibers, surrounding a central doublet (9 + 2) that were intact in transverse and longitudinal sections in 100% of normal sperm tails. The top left panel is from WT sperm, with the numbering of the nine microtubule doublets. In Stamptm/tm sperm, doublet 4 is missing from the 9 + 2 formation in virtually all of the axonema (see top left panel of TM pictures for numbering, with the red number 4 indicating the position of missing double 4 and white arrows for other examples). Severe disruption of microtubule polymers (arrows in bottom row) was observed in transverse sections. Occasionally, more severe defects were observed (middle row). The more peripheral dense fibers were generally indistinguishable from normal controls except that the outer dense fiber paired with doublet 4 usually disappears with doublet 4. The samples were independently obtained from three biological samples. Scale bars, 0.2 μm.
Caudal epididymal sperm of both mice were imaged by transmission electron microscopy in an effort to detect changes in organelle details that could account for the above abnormalities in the Stamptm/tm sperm. The canonical 9 + 2 microtubule axonema with intact microtubule polymers were observed in 100% of the cross-sections of normal sperm tails (Fig. 3D, top left panel). Interestingly, mutant mice are missing a single doublet, doublet 4, from the 9 + 2 formation in 95% of the axonema analyzed (see “Experimental Procedures” and supplemental Fig. S2) to give eight doublets with an open ring structure (Fig. 3D, top row of TM panels). When doublet 4 is missing, the associated dense fiber is also gone. The more peripherally located dense fibers appear normal. Occasionally, more severely affected axonema were observed with fewer dimers and gross disruption of microtubule polymers (Fig. 3D, middle row). Because of the dramatic reorganization in these axonema, it was not possible to identify the missing doublets. In three surveys of 112–201 tails (total = 506), 84.9 ± 5.6% (S.D.) of the axonema were deformed. These results suggest that the irregular axonema formation in Stamptm/tm sperm causes the detached head and coiled tail that result in the poor motility and greatly reduced fertility.
Absence of Stamp Protein Does Not Alter Expression of Genes Known to Affect Spermatogenesis
Twenty-four genes have been described to influence spermatogenesis in mice (20). We determined the levels of each of these genes in Stamp+/+, Stamp+/tm, and Stamptm/tm testes (supplemental Fig. S3). No differences in the expression of any of these genes were observed in testes with two, one, and no copies of the wild type Stamp gene. This indicates that STAMP deficiency does not cause sperm malformation via altered expression levels of any of these genes that are known to affect spermatogenesis. This conclusion is consistent with the appearance of the Stamptm/tm sperm, which is unlike that caused by any other gene for spermatogenesis of which we are aware.
Absence of STAMP Protein Does Not Alter Expression of Genes Known to Affect Axoneme Formation/Function
In an effort to explore the potential genomic basis for male infertility in Stamptm/tm mice, we performed an exon-based microarray analysis comprised of more than 750,000 unique probe sets constituting over 28,000 genes using RNA from the testes of wild type or Stamptm/tm males. Using the stringencies described under “Experimental Procedures,” 33 genes other than Stamp (see below) were identified as being significantly differentially regulated, and only 4 genes were identified using a 2-fold cutoff (supplemental Table S2). None of these genes are listed as affecting either sperm axonemal structure or sperm motility (21) or as participating in spermatozoa maturation, motility, or fertilization in a 2008 review (22). Several of these genes (Skiv2l2, Scn10a, and Egr1) have subsequently been reported to be relevant to spermatogenesis and reproductive function (23–25), but none have been shown to be involved with axoneme formation or to result in the phenotype seen in the Stamptm/tm mice following disruption/overexpression (26, 27). Interestingly, 6 of the 33 affected genes are known to be regulated by androgen receptors. These genes are indicated by bold lettering with underlining in supplemental Table S2.
An advantage of the use of an exon-based array is that, in addition to whole gene expression data, alternative splicing data are also available. In our case, 904 exons were found to significantly differ between the WT and Stamptm/tm mouse testes (supplemental Table S3). In all cases, each gene is detected by at least two exons. Importantly, all 30 of the 34 Stamp exons that were probed are reduced. Excluding exons 22 and 23 (which are reduced by a factor of >77), all of the other exons are reduced by a factor of 2.41 ± 0.06 (S.E., n = 31) in the mutant mice (supplemental Table S4). Thus, the deletion of exons 22 and 23 somehow decreases the abundance of all other forms of STAMP mRNA. These exon array data further suggest the possibility that STAMP influences gene splicing.
Of the 13 members of the Ttll family, of which Stamp is Ttll5, all but Ttlls 2, 7, 10, and 11 were monitored by ≥14 probes each on our exon array. None of the exons other than for Stamp were significantly different in the WT and Stamptm/tm mouse testes, with the average fold change of all exons for each other Ttll gene being ≤±1.06. In combination with the data of Fig. 1H, which confirm the results of the microarrays, these data argue that of the three initiating TTLLs (4, 5, and 7), only the amount of STAMP/TTLL5 is altered in the testes of the TM mice and that most other TTLLs retain wild type levels of expression in the testes of Stamptm/tm mice.
Interestingly, none of the affected genes are currently known to be involved with axoneme formation. A preliminary GeneGo pathway analysis of the exon microarray data at the level of genes and of exons is consistent with a role for STAMP in responses to stimuli (but not steroids) and cellular processes, respectively (data not shown). None of the top 10 pathways for either data set are sperm-related.
STAMP Deficiency Causes Reduced Polyglutamylation
One of the activities observed for STAMP is to modify the amount of α-tubulin polyglutamylation (3). We therefore examined the levels of polyglutamylated proteins, including α-tubulin, in Stamp+/+, Stamp+/tm, and Stamptm/tm sperm using both the anti-polyglutamylation antibody, GT335, from Janke et al. (2) that detects α- and β-tubulins and an α-tubulin-specific antibody, B3 (28). The level of polyglutamylated tubulins (α plus β or just α; Fig. 4A) was normalized to the amount of total α-tubulin in each sample and plotted in Fig. 4B. The amount of polyglutamylated α- and β-tubulins is reduced by Stamp deficiency by ∼40%. The reduction of polyglutamylated α-tubulin was even greater (76 ± 9%, p = 0.026). Interestingly, the amount of polyglutamylated tubulins in whole testes is very similar for all genotypes (Fig. 4C). These data suggest that, although STAMP is the major initiating glutamylase in sperm, Stamptm/tm male mice are functionally hypomorphic. Furthermore, other initiating glutamylases than STAMP (i.e., Ttll4 and 7) are apparently responsible for a greater percentage of total tubulin polyglutamylation in testes than in sperm. The fact that the electrophoretic mobility of α-tubulin in Fig. 4 (A and B) is not significantly altered by the observed level of polyglutamylation was unexpected but consistent with the reports of others (3).
We next asked whether sperm fragility in the Stamptm/tm mice could be related to the reduced polyglutamylation of α-tubulin. Sperm from Stamp+/+ and Stamptm/tm mice were therefore analyzed by immunocytochemistry with the GT335 antibody, which is seen in Fig. 4 (A and B) to be sufficient to detect differences in α-tubulin polyglutamylation. As shown in Fig. 4D, sperm from Stamp+/+ mice were evenly stained on the body and tail parts but not in the head. In contrast, those Stamptm/tm sperm that are not tightly coiled were not stained evenly. Sperm tails were divided into three parts (mid-piece (part 1), upper tail (part 2), and lower tail (part 3)) for more precise analysis. As seen on Fig. 4E, the intensities of the mid-piece (part 1) of both Stamp+/+ and Stamptm/tm sperm are not appreciably different. However, the magnitude of polyglutamylation in the more distal tails parts (parts 2 and 3) of Stamptm/tm sperm is significantly lower than that of Stamp+/+ sperm. These data support the postulate that STAMP deficiency induces incomplete polyglutamylation in the spermatozoa, resulting in a structural defect of sperm axonema, composed of α-tubulin, that is linked with infertility. A similar analysis of the tightly coiled tails without heads was not possible because of an inability to discern the different regions of these compacted tails.
Other Tubulin-dependent Organs (Retina and Cochlea) Are Normal
In view of the abnormal physiology of sperm tails and their axonema, we enquired whether other organelles dependent upon α-tubulin might be affected. For example, TTLL family members are known to be important regulators of cilia structure and motility across many species including Drosophila, C. elegans, Danio rerio, and higher organisms (2, 5, 29–31) and thus could also affect axonemal structures in respiratory epithelia, cochlear hair cells, and photoreceptors. In one study, the defects became more pronounced at 7 months of age (31). Interestingly, no differences in retinal photoreceptor or cochlear hair cell development were observed in either 3- or 7-month-old Stamptm/tm male mice (supplemental Fig. S4).
DISCUSSION
This report examines the consequences in mice of disrupting one domain of the Ttll5/Stamp gene (that required for STAMP activity with steroid receptors) while preserving another domain (for TTLL activity). These mice are therefore called STAMP targeted mutants. The deletion of exons 23 and 24 generates an unnatural in-frame translation termination midway through the protein, thereby eliminating the presence of the full-length protein in Western blots (Fig. 1F). In mice that are homozygous for the disrupted Stamp gene, the only phenotypic change that has been observed is that the males, but not the females, have dramatically reduced fertility (e.g., Tables 2 and 3). This infertility appears to result from severe defects in sperm formation that cause a high frequency of coiling of the tails and detachment of the heads along with decreased motility in the remaining intact sperm (Fig. 3, A–C). These defects are associated with, and are most likely caused by, abnormal axonema formation in the tails of 85% of the Stamptm/tm sperm (Fig. 3D). A plausible molecular cause for the abnormal axonema is the reduced levels of polyglutamylated α-tubulin (Fig. 4), which is a major component of the axonema.
The choice to delete exons 23 and 24 to give a truncated protein was dictated by two considerations. First, the large size of the TTLL domain (82 kb) in exons 9–18 precluded its deletion. Second, deletion of exons 23 and 24 introduced a premature stop codon, thereby preventing the translation of the downstream regions encoding protein sequences required for the binding of STAMP to and augmenting the transactivation of both glucocorticoid and progesterone receptors (7, 9). The current sex-linked defect in Stamptm/tm male mice suggests that it may be related to the previously reported very strong activities of STAMP with androgen receptors (7). These results are also consistent with the very high levels of STAMP in testes. However, despite the attractive mechanistic scenario of STAMP interactions with androgen receptors being required for normal sperm development, we have been unable to find any convincing link. Of the 33 genes for which expression is altered in the Stamptm/tm testes, only six appear to be androgen-regulated (supplemental Table S2). Furthermore, no steroid receptor-mediated pathways were among the top 10 most highly affected pathways identified by preliminary GeneGo analysis of the data at the level of either altered genes or exons (data not shown). Therefore, the effects of eliminating full-length STAMP may be independent of steroid receptors, as has been observed for modifications of cell growth by STAMP (15, 16) and other TTLL family members, including TTLL4 (32) and TTLL7 (33).
Interestingly, expression of all TTLLs is high in the testes (3). TTLL4, 5, and 7 are the only TTLLs that add the first glutamyl group, which is required for polyglutamylation (4). Of these three TTLLs, TTLL5 is selective for α-tubulin, whereas TTLL4 and 7 are reported to act almost exclusively with β-tubulin (3). Microarray analysis detected all TTLLs except 2, 7, 10, and 11 and indicates that STAMP/TTLL5 is the only transcript to be reduced in the testes of TM mice. The level TTLL4, another initiator for polyglutamylation, was unchanged. The qRT-PCR data of Fig. 1H show that the only other initiating TTLL, TTLL7, is also unaffected in the Stamptm/tm mice. Therefore, we conclude that any reduction in polyglutamylation seen in Fig. 4 is due to the reduced amounts of STAMP transcript in TM mouse testes, as revealed by the qRT-PCR with primers for exons 22 and 25 of STAMP (Fig. 1H).
The final question is whether the STAMP transcript lacking exons 23 and 24 in TM mice retains initiating activity for polyglutamylation. This transcript encodes a novel N-terminal fragment of STAMP, which contains the regions required for polyglutamylation (3). The exon array data reveal that the expression levels of all of the Stamp exons probed (other than the deleted exons 23 and 24) are decreased by an average of 2.4-fold (supplemental Table S4). The Uniprot web site lists just one mRNA transcript in addition to the full-length STAMP mRNA. This isoform is missing the C-terminal half of STAMP (amino acids 564–1328) and is expected to also be present in the Stamptm/tm male mice, which are predicted to encode a slightly larger protein containing amino acids 1–718 plus seven extra residues. To the extent that overlapping mRNA sequences would likely be translated at similar efficiencies, the 72% reduction in STAMP transcripts seen by qRT-PCR in Fig. 1H is similar to the 60% reduction in all STAMP exons (other than exons 23 and 24), which nicely parallels both the 76% reduction in sperm of polyglutamylated α-tubulin (Fig. 4, A and B), a known target of STAMP, and the 23–52% reduction of total polyglutamylated tubulins in various regions of the sperm tails (Fig. 4, D and E). We therefore propose that the decrease in polyglutamylation in TM mouse sperm is due to the reduced expression of STAMP isoforms with TTL activity that accompany deletion of exons 23 and 24. Interestingly, the amounts of polyglutamylated α- and β-tubulin in the testes of Stamptm/tm animals are unaltered (Fig. 4C). Because STAMP/TTLL5 is the main initiator of polyglutamylation of α-tubulin (3), this indicates that the effects of STAMP are cell-specific and may be augmented by other factors not present in sperm.
Disruption of α-tubulin polyglutamylation in sperm suggests that other organelles dependent upon α-tubulin could also be affected. Indeed, TTLL1−/− mice exhibit disrupted axonemes in tracheal, nasal, and middle ear cilia, resulting in otitis media as well as several phenotypic features of primary ciliary dyskinesia including a reduced rate of cilia-generated flow and rhinosinusitis (34, 35). Disruption of both TTLL3 and TTLL6 by morpholinos in zebrafish has been shown to lead to cilia immotility and structural defects including missing dynein arms and misplaced or rotated microtubule doublets (30). Similarly, when sperm axonemal/flagellar structure formation/maintenance are disrupted, other cilia are often also disrupted including photoreceptors (31, 36, 37) and olfactory and respiratory cilia (37–40). In contrast, we saw no evidence of rhinosinusitis or otitis media in our mice. Furthermore, the absence of full-length STAMP has no effect on the structure of two other α-tubulin-containing structures: the retina and the cochlea (supplemental Fig. S4). Thus, the Stamptm/tm mice appear to have a more restricted set of defects than those associated with the deletion/disruption of other TTLL genes. These results are consistent with those in the testes, where changes in α-tubulin polyglutamylation were noticeable only in sperm.
In many cases, a loss of tubulin polyglutamylation or polyglycylation has resulted in sterility, which has been attributed to disruption of axonemal structures and subsequently flagellar motility. STAMP itself has been previously implicated in sterility in C. elegans via high throughput RNAi screening (29) but was not found to influence sterility in a similar screen by another group (41). In Drosophila, reduction of the initiating glycylase, TTLL3B, using RNAi results in male sterility in 97.6% of the effected population (5). TTLL1, when complexed with other proteins (3), is very similar in polyglutamylation activity to STAMP (6) and is also involved with post-translational modification of tubulin in axonemal microtubules within cilia and flagella (35). In contrast to the Stamptm/tm mice, however, where the heads and tails of the sperm are usually detached with many of the tails being tightly coiled, the TTLL1−/− mice have sperm with detached heads but with very few detached tails in evidence (35). Coupled with evidence that mutation of the C-terminal region of β2-tubulin, which is where polyglutamylation and polyglycylation modifications occur, disrupts only the subset of microtubule functions required for organization of doublets of the sperm tail axoneme (42), these results collectively demonstrate that numerous post-translational modifications of tubulin by members of the TTLL family are integral to proper cilia/flagellar structure and function. However, because microarrays and qRT-PCR reveal that the levels of none of these TTLLs other than STAMP/TTLL5 are altered in the homozygous mutant mice, we conclude that the reduced polyglutamylation caused by lower levels of the truncated STAMP protein is responsible for the disrupted axonemal structures.
A common feature of the axonemes of the Stamptm/tm sperm is the absence of one microtubule doublet. Careful analysis of the electron micrographs of Fig. 3D and others revealed that the missing doublet was almost always doublet 4. This doublet is close to the plane of flagellar beating (18, 21) and could thus affect sperm mobility. The axoneme of the sperm tail consists of nine microtubule doublets surrounding a central pair that extend from the principal to the mid-piece of mature spermatozoa. The α- and β-subunits of microtubules are post-translationally modified primarily in their C-terminal tails and defects affect sperm motility (43). Polyglutamylation initiates with attachment of a glutamate via an isopeptidic bond to the γ-carboxyl of glutamate residue(s) in the tubulin polypeptide. Subsequent extension with additional glutamate residues attached by peptide bonds results in side chains of varying lengths. Specific glutamylases have been isolated (2) and, based on the similarity to their catalytic domains, are grouped as TTLL proteins with specific substrate and reaction preferences (44). Why the axonemes of the Stamptm/tm sperm usually lack just one microtubule doublet, with a nearly normal arrangement of the other eight doublets, is not yet clear. However, it should be noted that doublet 4 has the lowest levels of polyglutamylation (18), which could make doublet 4 more sensitive to the reduced levels of polyglutamylation activity in the sperm of Stamptm/tm mice (Figs. 1, F–H, and 4).
Numerous other genetic defects that do not involve polyglutamylation have also been reported to affect the head-to-tail connections of sperm and cause axonemal and flagellum anomalies in mice (43, 45–48) and in the flagellated protozoan parasite Trypanosoma brucei (49). Twenty-one knock-out mouse models have been shown to lead to male infertility as a result of specific abnormalities of sperm flagellum leading to motility disorders (50). Importantly, none of these genetic defects are indicated in the microarray analysis of genes that are altered in the testes of Stamptm/tm mice (supplemental Table S2 and S3). A new knock-out mouse with a defective Odf1 gene shares several phenotypic traits with the Stamptm/tm mice, i.e., male infertility and sperm with missing heads, coiled tails, and defective motility (51). Odf1, also known as HSPB10, is a member of the family of small heat shock proteins. Interestingly, the sperm of the Odf1 KO mice not only have tails without heads but also do not have any detached heads, unlike the sperm of Stamptm/tm mice. Although the biochemical defect for this is not known, it is speculated that the heads of the Odf1 KO mice are less firmly attached and lost as a result of the shear forces during passage of the sperm from the testis to epididymis. The presence of detached heads among the sperm of Stamptm/tm mice suggests that a different underlying cause is operative in these mice. Other differences between the Odf1 KO (51) and Stamptm/tm mice include the presence of abnormal axonemal structures (Fig. 3D) and negligible changes in Odf1 mRNA (<1.5-fold) in the Stamptm/tm mice (supplemental Table S3).
In summary, STAMP appears to be a new gene that, upon disruption in mice, produces male infertility caused by phenotypic features that are different from the numerous other genes that also result in male infertility. The precise molecular defect has yet to be identified, but a consistent mechanistic hypothesis can be formulated. The level of polyglutamylation of α-tubulin in sperm, but not whole testes or several other tissues, is reduced by disrupting the Stamp gene in Stamptm/tm mice to cause markedly reduced levels of a prematurely terminated protein retaining the required structural features to initiate polyglutamylation. Reduced α-tubulin polyglutamylation could result in abnormal axonemal structures with reduced progressivity and sharply reduced fertility. The expression of 33 genes in mouse testes are altered in the Stamptm/tm mice, but none of these have been previously implicated in male fertility. Thus, STAMP represents an intriguing new protein in the study of male fertility.
Supplementary Material
Acknowledgments
We thank Rong Zhu (Steroid Hormones Section, NIDDK, National Institutes of Health) for excellent assistance in the care and maintenance of the mouse colonies, Huiyan Lu (NIDDK, National Institutes of Health) for conducting the in vitro fertilization experiments and mouse surgery, Lei Bao (UCSD) for help with bioinformatics analyses of the Stamp gene, Heiner Westphal (NICHD, National Institutes of Health) for providing EIIa-Cre transgenic mice, and Carsten Janke (CNRS, Montpellier, France) for the generous gift of monoclonal polyglutamylation antibody.
This work was supported, in whole or in part, by the NIDDK, National Institutes of Health Intramural Research Program.

This article contains supplemental Movie S1, Tables S1–S4, and Figs. S1–S4.
- TTLL
- tubulin tyrosine ligase-like
- qRT-PCR
- quantitative RT-PCR
- TM
- targeted mutant.
REFERENCES
- 1. Trichet V., Ruault M., Roizès G., De Sario A. (2000) Characterization of the human tubulin tyrosine ligase-like 1 gene (TTLL1) mapping to 22q13.1. Gene 257, 109–117 [DOI] [PubMed] [Google Scholar]
- 2. Janke C., Rogowski K., Wloga D., Regnard C., Kajava A. V., Strub J. M., Temurak N., van Dijk J., Boucher D., van Dorsselaer A., Suryavanshi S., Gaertig J., Eddé B. (2005) Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758–1762 [DOI] [PubMed] [Google Scholar]
- 3. van Dijk J., Rogowski K., Miro J., Lacroix B., Eddé B., Janke C. (2007) A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437–448 [DOI] [PubMed] [Google Scholar]
- 4. Janke C., Bulinski J. C. (2011) Post-translational regulation of the microtubule cytoskeleton. Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 12, 773–786 [DOI] [PubMed] [Google Scholar]
- 5. Rogowski K., Juge F., van Dijk J., Wloga D., Strub J. M., Levilliers N., Thomas D., Bré M. H., Van Dorsselaer A., Gaertig J., Janke C. (2009) Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137, 1076–1087 [DOI] [PubMed] [Google Scholar]
- 6. Janke C., Rogowski K., van Dijk J. (2008) Polyglutamylation. A fine-regulator of protein function? “Protein Modifications. Beyond the Usual Suspects” review series. EMBO Rep. 9, 636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He Y., Simons S. S., Jr. (2007) STAMP, a novel predicted factor assisting TIF2 actions in glucocorticoid receptor-mediated induction and repression. Mol. Cell. Biol. 27, 1467–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun Y., Tao Y. G., Kagan B. L., He Y., Simons S. S., Jr. (2008) Modulation of transcription parameters in glucocorticoid receptor-mediated repression. Mol. Cell. Endocrinol. 295, 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szapary D., Song L.-N., He Y., Simons S. S., Jr. (2008) Differential modulation of glucocorticoid and progesterone receptor transactivation. Mol. Cell. Endocrinol. 283, 114–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKenna N. J., Lanz R. B., O'Malley B. W. (1999) Nuclear receptor coregulators. Cellular and molecular biology. Endocr. Rev. 20, 321–344 [DOI] [PubMed] [Google Scholar]
- 11. Needham M., Raines S., McPheat J., Stacey C., Ellston J., Hoare S., Parker M. (2000) Differential interaction of steroid hormone receptors with LXXLL motifs in SRC-1a depends on residues flanking the motif. J. Steroid Biochem. Mol. Biol. 72, 35–46 [DOI] [PubMed] [Google Scholar]
- 12. Ichijo T., Voutetakis A., Cotrim A. P., Bhattachryya N., Fujii M., Chrousos G. P., Kino T. (2005) The Smad6-histone deacetylase 3 complex silences the transcriptional activity of the glucocorticoid receptor. Potential clinical implications. J. Biol. Chem. 280, 42067–42077 [DOI] [PubMed] [Google Scholar]
- 13. Reeb C. A., Gerlach C., Heinssmann M., Prade I., Ceraline J., Roediger J., Roell D., Baniahmad A. (2011) A designed cell-permeable aptamer-based corepressor peptide is highly specific for the androgen receptor and inhibits prostate cancer cell growth in a vector-free mode. Endocrinology 152, 2174–2183 [DOI] [PubMed] [Google Scholar]
- 14. Awasthi S., Simons S. S. (2012) Separate regions of glucocorticoid receptor, coactivator TIF2, and comodulator STAMP modify different parameters of glucocorticoid-mediated gene induction. Mol. Cell. Endocrinol. 355, 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Y., Blackford J. A., Jr., Kohn E. C., Simons S. S., Jr. (2010) STAMP alters the growth of transformed and ovarian cancer cells. BMC Cancer 10, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang H., Cheung L. W., Li J., Ju Z., Yu S., Stemke-Hale K., Dogruluk T., Lu Y., Liu X., Gu C., Guo W., Scherer S. E., Carter H., Westin S. N., Dyer M. D., Verhaak R. G., Zhang F., Karchin R., Liu C. G., Lu K. H., Broaddus R. R., Scott K. L., Hennessy B. T., Mills G. B. (2012) Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 22, 2120–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newman G. R. (1999) LR White embedding for immunoelectron microscopy. Histochem. J. 31, 79. [DOI] [PubMed] [Google Scholar]
- 18. Fouquet J. P., Prigent Y., Kann M. L. (1996) Comparative immunogold analysis of tubulin isoforms in the mouse sperm flagellum. Unique distribution of glutamylated tubulin. Mol. Reprod. Dev. 43, 358–365 [DOI] [PubMed] [Google Scholar]
- 19. Montagutelli X. (2000) Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 11, S101–S105 [PubMed] [Google Scholar]
- 20. Yan W. (2009) Male infertility caused by spermiogenic defects. Lessons from gene knockouts. Mol. Cell. Endocrinol. 306, 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inaba K. (2011) Sperm flagella. Comparative and phylogenetic perspectives of protein components. Mol. Hum. Reprod. 17, 524–538 [DOI] [PubMed] [Google Scholar]
- 22. Matzuk M. M., Lamb D. J. (2008) The biology of infertility. Research advances and clinical challenges. Nat. Med. 14, 1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Osman B. A., Kawashima A., Tamba M., Satoh E., Kato Y., Iki A., Konishi K., Matsuda M., Okamura N. (2011) Localization of a novel RNA-binding protein, SKIV2L2, to the nucleus in the round spermatids of mice. J. Reprod. Dev. 57, 457–467 [DOI] [PubMed] [Google Scholar]
- 24. Pinto F. M., Ravina C. G., Fernández-Sánchez M., Gallardo-Castro M., Cejudo-Román A., Candenas L. (2009) Molecular and functional characterization of voltage-gated sodium channels in human sperm. Reprod. Biol. Endocrinol. 7, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei N., Heckert L. L. (2002) Sp1 and Egr1 regulate transcription of the Dmrt1 gene in Sertoli cells. Biol. Reprod. 66, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horowitz E., Zhang Z., Jones B. H., Moss S. B., Ho C., Wood J. R., Wang X., Sammel M. D., Strauss J. F. (2005) Patterns of expression of sperm flagellar genes. Early expression of genes encoding axonemal proteins during the spermatogenic cycle and shared features of promoters of genes encoding central apparatus proteins. Mol. Hum. Reprod. 11, 307–317 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z., Jones B. H., Tang W., Moss S. B., Wei Z., Ho C., Pollack M., Horowitz E., Bennett J., Baker M. E., Strauss J. F. (2005) Dissecting the axoneme interactome. The mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol. Cell. Proteomics 4, 914–923 [DOI] [PubMed] [Google Scholar]
- 28. Gagnon C., White D., Cosson J., Huitorel P., Eddé B., Desbruyères E., Paturle-Lafanechère L., Multigner L., Job D., Cibert C. (1996) The polyglutamylated lateral chain of α-tubulin plays a key role in flagellar motility. J. Cell Sci. 109, 1545–1553 [DOI] [PubMed] [Google Scholar]
- 29. Maeda I., Kohara Y., Yamamoto M., Sugimoto A. (2001) Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 11, 171–176 [DOI] [PubMed] [Google Scholar]
- 30. Pathak N., Austin C. A., Drummond I. A. (2011) Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J. Biol. Chem. 286, 11685–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mykytyn K., Mullins R. F., Andrews M., Chiang A. P., Swiderski R. E., Yang B., Braun T., Casavant T., Stone E. M., Sheffield V. C. (2004) Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc. Natl. Acad. Sci. U.S.A. 101, 8664–8669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kashiwaya K., Nakagawa H., Hosokawa M., Mochizuki Y., Ueda K., Piao L., Chung S., Hamamoto R., Eguchi H., Ohigashi H., Ishikawa O., Janke C., Shinomura Y., Nakamura Y. (2010) Involvement of the tubulin tyrosine ligase-like family member 4 polyglutamylase in PELP1 polyglutamylation and chromatin remodeling in pancreatic cancer cells. Cancer Res. 70, 4024–4033 [DOI] [PubMed] [Google Scholar]
- 33. Ikegami K., Mukai M., Tsuchida J., Heier R. L., Macgregor G. R., Setou M. (2006) TTLL7 is a mammalian β-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 281, 30707–30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikegami K., Sato S., Nakamura K., Ostrowski L. E., Setou M. (2010) Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc. Natl. Acad. Sci. U.S.A. 107, 10490–10495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vogel P., Hansen G., Fontenot G., Read R. (2010) Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet. Pathol. 47, 703–712 [DOI] [PubMed] [Google Scholar]
- 36. Hunter D. G., Fishman G. A., Mehta R. S., Kretzer F. L. (1986) Abnormal sperm and photoreceptor axonemes in Usher's syndrome. Arch. Ophthalmol. 104, 385–389 [DOI] [PubMed] [Google Scholar]
- 37. Fath M. A., Mullins R. F., Searby C., Nishimura D. Y., Wei J., Rahmouni K., Davis R. E., Tayeh M. K., Andrews M., Yang B., Sigmund C. D., Stone E. M., Sheffield V. C. (2005) Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum. Mol. Genet. 14, 1109–1118 [DOI] [PubMed] [Google Scholar]
- 38. Neesen J., Kirschner R., Ochs M., Schmiedl A., Habermann B., Mueller C., Holstein A. F., Nuesslein T., Adham I., Engel W. (2001) Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 10, 1117–1128 [DOI] [PubMed] [Google Scholar]
- 39. Lorenzetti D., Bishop C. E., Justice M. J. (2004) Deletion of the Parkin coregulated gene causes male sterility in the quaking(viable) mouse mutant. Proc. Natl. Acad. Sci. U.S.A. 101, 8402–8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nayernia K., Vauti F., Meinhardt A., Cadenas C., Schweyer S., Meyer B. I., Schwandt I., Chowdhury K., Engel W., Arnold H. H. (2003) Inactivation of a testis-specific Lis1 transcript in mice prevents spermatid differentiation and causes male infertility. J. Biol. Chem. 278, 48377–48385 [DOI] [PubMed] [Google Scholar]
- 41. Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D. P., Zipperlen P., Ahringer J. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 [DOI] [PubMed] [Google Scholar]
- 42. Fackenthal J. D., Hutchens J. A., Turner F. R., Raff E. C. (1995) Structural analysis of mutations in the Drosophila β2-tubulin isoform reveals regions in the β-tubulin molecular required for general and for tissue-specific microtubule functions. Genetics 139, 267–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell P. K., Waymire K. G., Heier R. L., Sharer C., Day D. E., Reimann H., Jaje J. M., Friedrich G. A., Burmeister M., Bartness T. J., Russell L. D., Young L. J., Zimmer M., Jenne D. E., MacGregor G. R. (2002) Mutation of a novel gene results in abnormal development of spermatid flagella, loss of intermale aggression and reduced body fat in mice. Genetics 162, 307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaertig J., Wloga D. (2008) Ciliary tubulin and its post-translational modifications. Curr. Top. Dev. Biol. 85, 83–113 [DOI] [PubMed] [Google Scholar]
- 45. Vollrath B., Pudney J., Asa S., Leder P., Fitzgerald K. (2001) Isolation of a murine homologue of the Drosophila neuralized gene, a gene required for axonemal integrity in spermatozoa and terminal maturation of the mammary gland. Mol. Cell. Biol. 21, 7481–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sampson M. J., Decker W. K., Beaudet A. L., Ruitenbeek W., Armstrong D., Hicks M. J., Craigen W. J. (2001) Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 276, 39206–39212 [DOI] [PubMed] [Google Scholar]
- 47. Tokuhiro K., Isotani A., Yokota S., Yano Y., Oshio S., Hirose M., Wada M., Fujita K., Ogawa Y., Okabe M., Nishimune Y., Tanaka H. (2009) OAZ-t/OAZ3 is essential for rigid connection of sperm tails to heads in mouse. PLoS Genet. 5, e1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Uhrin P., Dewerchin M., Hilpert M., Chrenek P., Schöfer C., Zechmeister-Machhart M., Krönke G., Vales A., Carmeliet P., Binder B. R., Geiger M. (2000) Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J. Clin. Invest. 106, 1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dawe H. R., Farr H., Portman N., Shaw M. K., Gull K. (2005) The Parkin co-regulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J. Cell Sci. 118, 5421–5430 [DOI] [PubMed] [Google Scholar]
- 50. Escalier D. (2006) Knockout mouse models of sperm flagellum anomalies. Hum. Reprod. Update 12, 449–461 [DOI] [PubMed] [Google Scholar]
- 51. Yang K., Meinhardt A., Zhang B., Grzmil P., Adham I. M., Hoyer-Fender S. (2012) The small heat shock protein ODF1/HSPB10 is essential for tight linkage of sperm head to tail and male fertility in mice. Mol. Cell. Biol. 32, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.