Background: Amot130 regulates cell differentiation and growth signaling.
Results: Amot130 binds and activates overexpressed AIP4 to ubiquitinate Amot130 and YAP resulting in Amot130 stabilization and YAP degradation.
Conclusion: Amot130 and AIP4 cooperatively inhibit YAP and cell growth.
Significance: A mechanism is described whereby Amot130 directs AIP4 to potentially suppress tumor cell growth.
Keywords: Cell Growth, Protein Complexes, Protein Turnover, Ubiquitin Ligase, Ubiquitination, AIP4, Amot, Angiomotin, WW Domain, YAP
Abstract
The adaptor protein Amot130 scaffolds components of the Hippo pathway to promote the inhibition of cell growth. This study describes how Amot130 through binding and activating the ubiquitin ligase AIP4/Itch achieves these effects. AIP4 is found to bind and ubiquitinate Amot130 at residue Lys-481. This both stabilizes Amot130 and promotes its residence at the plasma membrane. Furthermore, Amot130 is shown to scaffold a complex containing overexpressed AIP4 and the transcriptional co-activator Yes-associated protein (YAP). Consequently, Amot130 promotes the ubiquitination of YAP by AIP4 and prevents AIP4 from binding to large tumor suppressor 1. Amot130 is found to reduce YAP stability. Importantly, Amot130 inhibition of YAP dependent transcription is reversed by AIP4 silencing, whereas Amot130 and AIP4 expression interdependently suppress cell growth. Thus, Amot130 repurposes AIP4 from its previously described role in degrading large tumor suppressor 1 to the inhibition of YAP and cell growth.
Introduction
The splice forms of Amot differentially regulate polarity rearrangements (1) associated with cell growth and migration (2). Amot, which is mainly expressed as 130-kDa (Amot130) and 80-kDa (Amot80) splice forms (2), is a member of a family of three architecturally similar adaptor proteins that also includes AmotL1 and AmotL2 (3). Inactivation of murine Amot results in early embryonic lethality from a lack of cell growth and migration of cells in the anterior visceral endoderm (4). Consistently, knockdown of Amot in zebrafish produces defects in endothelial migration (5) as well as reduced rates of cell proliferation in cultured epithelial cells (6, 7) that have been attributed to loss of Amot80 (6). In contrast, Amot130 strongly inhibits the progrowth transcriptional co-activators YAP2 and TAZ (transcriptional co-activator with PDZ-binding motif) (8, 9).
Both YAP and TAZ control organ homeostasis by regulating both cell growth and apoptosis (10–12) through co-activation of the transcriptional enhancer domain protein (13), p73 (11), SMAD (mothers against decapentaplegic protein) (14), and RUNX (Runt-related transcription factor) (15) families of transcription factors. Activation of transcriptional enhancer domain proteins by YAP and TAZ results in the expression of the progrowth cytokines CTGF and Cyr61 (16, 17). These effects are inhibited in response to cell-cell contact through the activation of the LATS1 and LATS2 Hippo kinases (18), which then phosphorylate and inactivate YAP and TAZ. Alternatively, Amot130 can inhibit YAP through poorly defined sequestration mechanisms that are both dependent and independent of LATS1/2 phosphorylation of YAP (9, 19).
This study investigates how Amot130 works coordinately with the Nedd4 (neural precursor cell expressed developmentally down-regulated 4) family ubiquitin ligase AIP4 to repress YAP. Although Amot130 has been shown to associate with AIP4 (20), it is not known whether this complex regulates Hippo signaling or cell growth. This study finds that Amot130 recruits YAP and AIP4 into a common complex. AIP4 upon binding Amot130 has enhanced ubiquitin ligase activity resulting in the catalysis of ubiquitination of itself, Amot130 and YAP. Ubiquitination of Amot130 promotes its stability. Conversely, Amot130 and AIP4 interdependently reduce the stability of YAP and inhibit YAP dependent transcription as well as cell growth.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies used for immunoblot were as follows: Amot (1:1000), 1:5000 (AmotL1), HA (12CA5) (1:1000), Myc (9E10) (1:1000) as described in Ref. 6. FLAG (1:10,000; Sigma, F3165), FLAG (1:1000; Sigma, F7425), Myc (1:1000; Cell Signaling, 2272S), YFP/GFP (1:1000; Invitrogen, A6455), Nedd4 (1:1000; Cell Signaling, C5F5 3607), AIP4 (1:1000; BD Biosciences, 611198), AIP4 (1:200, Santa Cruz Biotechnology, G-11 sc-28367), GAPDH (1:5000; Millipore, MAB374), LATS1 (1:1000; Bethyl Laboratories, A300-477A), YAP1 (1:1000; Abnova, H00010413-MO1), His (Abnova, MAB1274). The antibodies used in this study for immunoprecipitation were as follows: 4 μg of AIP4 (Santa Cruz Biotechnology, G-11 sc-28367), 2 μg of YFP/GFP (Invitrogen, A6455), 2 μg of Myc (9E10), 2 μg of Amot, or 2 μg of FLAG (F3165).
Recombinant DNA
Full-length Amot130, AmotL1, AmotL2, Nedd4 ligases, and YAP2 were subcloned by PCR from Kazusa and IMAGE cDNAs into Creator-based acceptor vectors (21) for mammalian expression with the N-terminal tags 2×Myc, 3×FLAG, monomeric(m) Cherry, or mCitrine (referred to as YFP). Previously described vectors include Myc-AIP4, Myc-AIP4 C830A, Myc-AIP4 ΔC2, Myc-AIP4 4xWW Mut (W313A, W345A, W425A, and Y465A) (22) and HA-ubiquitin (23). The vectors acquired from Addgene were as follows: FLAG-YAP2 (19045), FLAG-LATS1 (18971), and FLAG-YAP2 WW1/2 Mut (19048) (12), HA-Lys-0 ubiquitin (17603) (24). Amot130 P-Y1/2/3 mutants were constructed using the Stratagene QuikChangeTM method. Lentiviral packaging and shRNA scramble control (1864) vectors were acquired from Addgene. AIP4 shRNA (Sigma, catalog no. TRCN0000002087) has been described previously (25). The supplemental Methods more fully describe each clone and cloning method.
Cell Culture
Human embryonic kidney (HEK) 293T cells (ATCC), MCF7 cells (L. Malkas, Indiana University), Madin Darby canine kidney type II cells (K. Mostov, University of California, San Francisco), and MDA-MB-468 cells (L. Mayo, Indiana University) were cultured in DMEM with 10% FBS at 37 °C in 5% CO2 (v/v). Cycloheximide (Sigma, C7698) was used at 50 μg/μl or 200 μg/μl, and MG-132 (Calbiochem, 474790) was used at 25 μm. Silencing via siRNA completed as described previously in Ref. 6 and the supplemental Methods. Transfections used polyethylenimine (Sigma Aldrich) for 293T (6) and Lipofectamine 2000 (Invitrogen) for Madin Darby canine kidney cells (26). Lentiviral production and infection were carried out for MDA-MB-468 and MCF7 cells as described in Ref. 6 and supplemental Methods.
Immunoblot
Cells were lysed in radioimmune precipitation assay buffer (50 mm Tris, pH 8.0, 2 mm EDTA, 10% Triton X-100, 150 mm NaCl, 0.1% SDS) containing 1 mm NaVO4, 2 mm β-glycerol phosphate, and protease inhibitor mixture (Sigma). Lysates were resolved by SDS-PAGE. Images were developed using a LiCor Odyssey and quantified with NIH ImageJ software (27).
Immunoprecipitation
Lysates extracted with phospholipase C buffer (1) were passaged 20× through an 18-gauge syringe, incubated 5 min at 4 °C, and precleared with protein A-Sepharose for 30 min before addition of antibody for 1 h. Protein A or G Sepharose was then added for 1 h.
GST Pulldown Analysis
0.5 μmol of GST-fused protein immobilized on glutathione-Sepharose was incubated with phospholipase C lysates at 500 μl at 2 μg/μl prepared from HEK 293T cells for 2 h at 4 °C with constant mixing. Immobilized protein complexes were washed 3× with phospholipase C buffer, eluted, and analyzed.
Mass Spectrometry Analysis of Affinity-purified Ubiquitinated Proteins
NIK cells were transfected with the His-biotin-ubiquitin vector (28) that expressed a His6-Biotin Ligase Recognition Peptide-ubiquitin fusion protein product allowing for purification of ubiquitinated proteins under denaturing conditions. Protein extraction and purification of ubiquitinated proteins was carried out as described previously (28). Final protein concentrations were estimated by A280. Mass spectrometry and data analysis are described in the supplemental Methods.
Cell Growth Assay
After plating 5000 cells in DMEM with 10% FBS or Opti-MEM reduced serum medium (Invitrogen), cells were harvested at the indicated times and counted with a hemocytometer. Data represent the mean of three separate experiments each performed in triplicate.
RNA Isolation and Real-time PCR
Cells were grown in Opti-MEM (Invitrogen) (10% FBS for Amot) for 24 h and then washed with ice-cold PBS. Total RNA was extracted with Tri Reagent (Sigma). cDNA synthesis using the SuperScript II reverse transcriptase (Invitrogen) method used 2 μg of purified RNA. Real-time PCR with gene-specific primers (supplemental Methods) and SYBR Green SensiMix kit (Bioline) was carried out with a Realplex2 (Eppendorf) system. All values were normalized to GAPDH.
Fluorescence Polarization
P-Y1 and P-Y2 peptides conjugated to FITC were synthesized as described previously (29). Peptides were incubated with GST-tagged fusions of AIP4 or YAP2 fragments for 45 min before equilibrium binding was measured as a function of fluorescence polarization using a Spectramax M5 (Molecular Devices) system. Values from non-binding mutant proteins were used to normalize all data. Non-linear regression analysis and curve fitting were calculated using GraphPad (Prism).
Imaging
Confocal fluorescence images were acquired from live samples using structured light as described in Ref. 26. Fixed images were also acquired using Phalloidin 594 (Santa Cruz Biotechnology, sc-363795) to stain for actin for 20 min at 1:500 in 5% BSA. Images were processed and analyzed with Zeiss Axiovision (version 4.8) and Zeiss Zen.
Statistical Analysis
Immunoblot (pixel intensities), real-time, live cell imaging, and cell count data are presented as the means ± S.D. p values showing differences were calculated by an unpaired two-tailed t test and for showing no differences by a one-tailed t test.
RESULTS
Amot130 Mainly Associates with the First and Second WW domains of AIP4 via Its P-Y2 and P-Y3 Motifs
Consistent with reports that Amot130 binds AIP4 (20), endogenous Amot130 specifically co-immunoprecipitated with AIP4 from lysates of HEK 293T cells (Fig. 1A). The enhanced levels of Amot immunoprecipitated with AIP4 in cells treated with MG-132 suggests that the Amot130·AIP4 complex is regulated by the proteasome. The relatively high level of co-precipitation of Amot130 with AIP4 versus six other Nedd4 family members indicates that AIP4 is a preferred binding partner of Amot130 (supplemental Fig. S1A).
FIGURE 1.

Amot130 binds the ubiquitin ligase AIP4. A, the relative endogenous levels of Amot and AIP4 in an immunoprecipitation with an anti-AIP4 antibody from HEK 293T cells preincubated with (+) 25 μm MG-132 (−) or vehicle were measured by immunoblot. B, a schematic representation of Amot130 and AIP4 protein architecture, including the P-Y1, 2, and 3 motifs in Amot130. C, an immunoblot showing the relative levels of endogenous Amot precipitated from HEK 293T lysates by purified GST-tagged WW domains of YAP2 or AIP4. Below the top panel are the ratios of pixel intensities of bound Amot130 over GST-tagged input. D, the relative levels of Myc-tagged AIP4 that co-immunoprecipitate with YFP-tagged Amot80 or Amot130 from HEK 293T cells were detected by immunoblot (IB). E, an immunoblot showing the relative levels of Myc-tagged AIP4 that co-immunoprecipitate with FLAG-tagged wild-type Amot130 or the P-Y1F (Y109F), P-Y2F (Y242F), and P-Y3F (Y287F) mutants of Amot130.
Because AIP4 has four WW domains and Amot130 encodes three WW binding proline-rich (P-Y) motifs (Fig. 1B), the relative abilities of the WW domains of AIP4 to bind Amot130 were determined. Initially, immunoprecipitation of fragments of AIP4 indicate that only the absence of the WW domains reduces binding to endogenous Amot130 (supplemental Fig. S1B). Furthermore, pulldowns using the purified GST-tagged WW domains of AIP4 and YAP indicate that the WW1 and WW2 domains of AIP4 precipitate endogenous Amot130 from HEK 293T lysates predominantly versus the WW3 or WW4 domains (Fig. 1C). This matches the reported specificity of Nedd4-2 with AmotL1 (30). However, Amot130 and AmotL1 bound mainly to the GST-tagged WW2 and WW3 domains of Nedd4-1 (supplemental Fig. S1C). Thus, Nedd4 family ligases may utilize alternative combinations of WW domains to bind Amot130 or AmotL1. In keeping with Nedd4 members utilizing multiple WW domains to mediate stronger interactions with Amot family members, AmotL1 bound to a greater extant to full-length Nedd4-1 versus its individual WW domains.
Although three P-Y motifs within Amot130 and AmotL1 are reported to mediate their binding to Nedd4-1 (20), their relative importance for binding to AIP4 is undetermined. Consistent with AIP4 binding to the P-Y motifs in Amot130, Amot80 was unable to co-immunoprecipitate AIP4 (Fig. 1D). Furthermore, both the P-Y2 (P-Y2F) and P-Y3 (P-Y3F) mutants of Amot130 and AmotL1 showed substantially reduced immunoprecipitation with AIP4 (Fig. 1E and supplemental Fig. S1D). However, unlike Nedd4–1 (20) but similar to Nedd4–2 (30), AIP4 showed no reduction in binding to P-Y1 (P-Y1F) mutants of Amot130 or AmotL1. Consistent with the pulldown data, the WW2 domains of AIP4 had the highest binding potential for an Amot130 P-Y2 peptide. However, all of the WW domains of AIP4 bound weaker to P-Y1 peptide (2–5-fold higher Kd values) than the WW1 domain of YAP2 (Table 1). Taken together, the WW1 and WW2 domains of AIP4 most likely bind the P-Y2 and P-Y3 motifs of Amot130 and AmotL1.
TABLE 1.
Binding Properties of WW domains of YAP and AIP4
| WW domain | Amot130-based peptide | Bmax | Kd | R2 | Binding potentiala |
|---|---|---|---|---|---|
| AIP4 WW1 | P-Y1b | 75.8 | 16.8 | 0.967 | 4.5 |
| AIP4 WW2 | P-Y1 | 110.0 | 36.6 | 0.956 | 3.0 |
| AIP4 WW3 | P-Y1 | 38.7 | 14.7 | 0.962 | 2.6 |
| AIP4 WW4 | P-Y1 | 40.1 | 28.4 | 0.931 | 1.4 |
| YAP WW1 | P-Y1 | 97.6 | 7.1 | 0.991 | 13.8 |
| YAP WW2 | P-Y1 | NDc | ND | ND | ND |
| YAP WW1 and -2 | P-Y1 | 129.7 | 5.2 | 0.992 | 25.0 |
| AIP4 WW1 | P-Y2d | 91.6 | 17.1 | 0.950 | 5.4 |
| AIP4 WW2 | P-Y2 | 66.1 | 8.5 | 0.971 | 7.8 |
| AIP4 WW3 | P-Y2 | 27.8 | 6.7 | 0.795 | 4.1 |
| AIP4 WW4 | P-Y2 | 29.2 | 7.3 | 0.800 | 4.0 |
| YAP WW1 | P-Y2 | 131.6 | 6.6 | 0.995 | 20.1 |
| YAP WW2 | P-Y2 | 54.5 | 4.4 | 0.928 | 12.5 |
| YAP WW1 and -2 | P-Y2 | 146.5 | 2.9 | 0.996 | 50.1 |
a Bmax over Kd.
b NNEELPTYEEAK.
c ND, not determined, below measurable threshold.
d HRGPPPEYPFKG.
Amot130 Is Ubiquitinated by AIP4
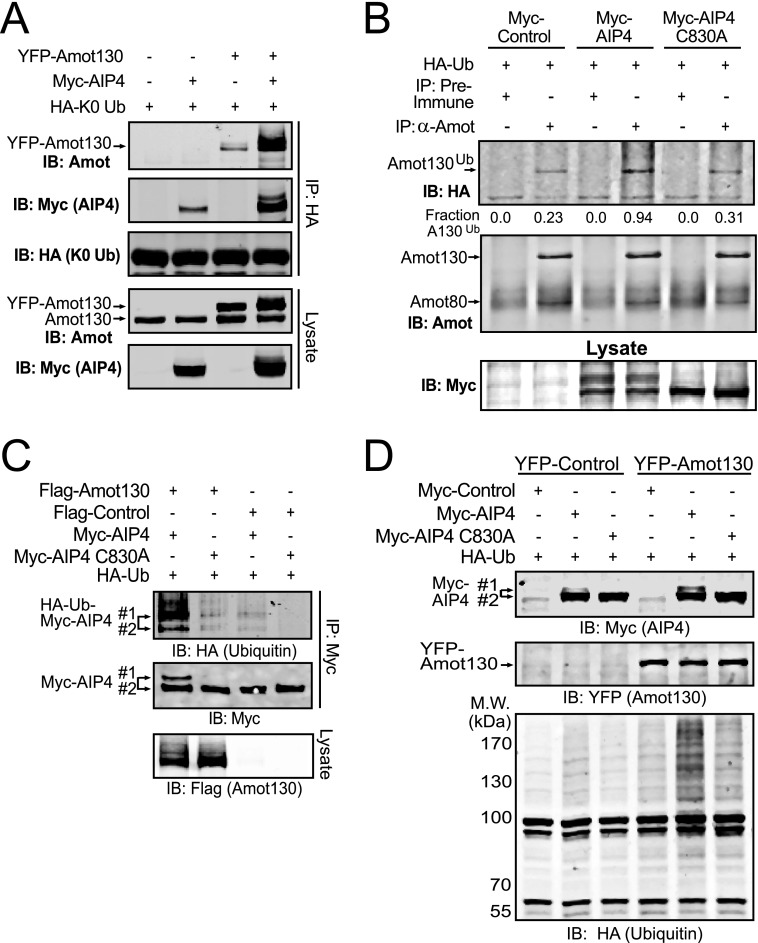
Because AIP4 is a strong binding partner of Amot130, it was investigated as a substrate for this ubiquitin ligase. For this purpose, HA-tagged Lys-0 ubiquitin was used to monitor the capacity of Amot130 to be ubiquitinated. HA-tagged Lys-0 ubiquitin was co-transfected with combinations of Myc-tagged AIP4 and YFP-tagged Amot130 into HEK 293T cells. Ubiquitinated proteins were immunoprecipitated with an anti-HA antibody (Fig. 2A). Immunoblot analysis showed an enhanced ubiquitination of Amot130 when it was co-expressed with AIP4. That Amot130 is a physiologic substrate of AIP4 was further supported by the >3-fold increase in ubiquitination of endogenous immunoprecipitated Amot130 from lysates prepared from HEK 293T cells co-expressing HA-tagged ubiquitin and Myc-tagged AIP4 versus catalytically inactive AIP4 (C830A) or control vector (Fig. 2B). Because Amot130 migrated with a single gel-shifted band that was similar in intensity and position when it incorporated wild-type, mutant K48R, or K63R ubiquitin (supplemental Fig. S2A), Amot130 does not seem to be commonly polyubiquitinated by AIP4. The presence of an analogous Amot130 gel-shifted band in cell lines grown to high density (supplemental Fig. S2B) further suggests that Amot130 is often similarly ubiquitinated as cells are contact inhibited.
FIGURE 2.
Amot130 is ubiquitinated by AIP4. A, the relative levels of ubiquitinated Amot130 and AIP4 following immunoprecipitation of HA-tagged Lys-0 ubiquitin (HA-K0 Ub) in HEK 293T cells co-expressing with and without YFP-tagged Amot130, Myc-tagged AIP4, or control vectors were detected by immunoblot. B, endogenous Amot was immunoprecipitated from HEK 293T cells expressing HA-tagged ubiquitin (HA-Ub) and Myc-tagged AIP4 or the catalytically inactive AIP4 (C830A). The levels of total and ubiquitinated Amot were detected by immunoblot (IB). The ratio of pixel intensities of ubiquitinated Amot130 over total Amot130 is indicated. C, the relative incorporation of HA-ubiquitin into immunoprecipitated Myc-tagged AIP4 or mutant AIP4 (C830A) in HEK 293T cells following co-expression with FLAG-tagged Amot130 was detected by immunoblot. D, HEK 293T cells expressing HA-ubiquitin and Myc-tagged AIP4 or mutant AIP4 (C830A) as well as YFP-tagged Amot130 or control vector were lysed in phospholipase C buffer and soluble HA-ubiquitin-modified proteins were detected by immunoblot. Molecular mass standards (in kDa) are indicated.
The activity of Nedd4 family ligases is often enhanced by binding proteins containing P-Y motifs (31, 32). The impact of Amot130 expression on AIP4 activity was therefore explored. Immunoblot analysis revealed that expression of Amot130 induced a slower migrating gel-shifted form of Myc-tagged AIP4, but not catalytically inactive AIP4 (C830A), which has more incorporated HA-ubiquitin than the lower band (Fig. 2C). Because this auto-ubiquitinated form is reported to indicate active ligase (31–33), Amot130 is likely promoting the ligase activity of AIP4.
Whether AIP4 activation resulted in changes in its ubiquitination of total cellular proteins was also measured. Co-expression of FLAG-tagged Amot130 with Myc-tagged AIP4 but not AIP4 (C830A) resulted in a synergistic increase in the levels of incorporation of ubiquitin into cellular proteins (Fig. 2D). Because this effect occurs in the absence of proteasome inhibitors, these substrates are likely stable following ubiquitination. The absence of this effect in cells expressing AIP4 and the Amot130 P-Y2,3F double mutant further indicates that the direct association of Amot130 with AIP4 induces the activation of its ligase domain (supplemental Fig. S2C).
Amot130 Is Ubiquitinated by AIP4 at Residue Lys-481
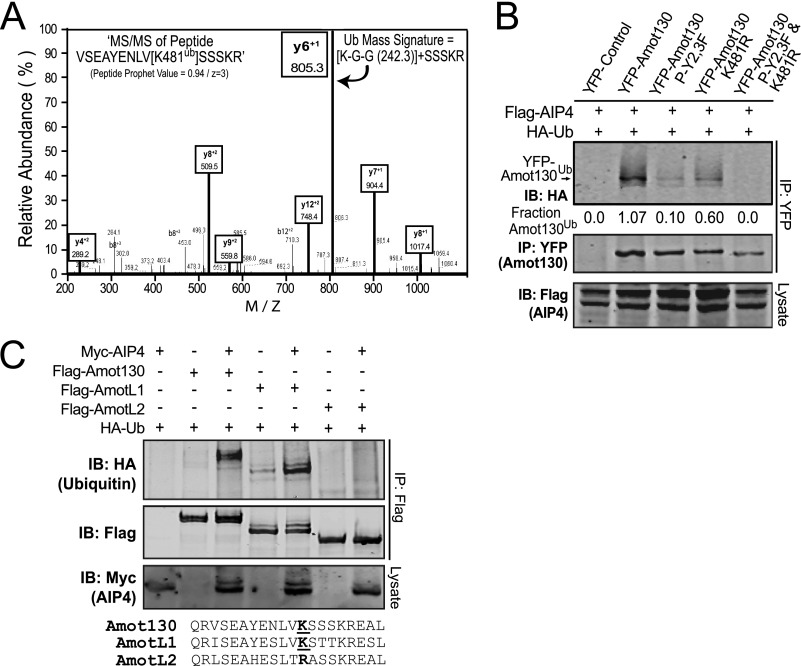
An unbiased approach for mapping ubiquitination sites in primary keratinocytes grown in differentiating (with calcium) conditions was undertaken to map potential ubiquitination sites in Amot. Following stable expression of His6-biotin-ubiquitin, cells were lysed, and ubiquitinated proteins were serially enriched on streptavidin and nickel-nitriloacetic acid columns. Trypsin peptides prepared from eluted proteins were analyzed for ubiquitinated residues by tandem mass spectrometry. In four of five experiments, a single Amot peptide (VSEAYENLVKSSSKR) with a mass signature for ubiquitination at residue Lys-481 was identified (Fig. 3A); a site highly predicted to be ubiquitinated by UbPred (supplemental Fig. S3).
FIGURE 3.
Amot130 is ubiquitinated at lysine 481 by AIP4. A, the y and b ion MS/MS spectra of the VSEAYENLVK*SSSKR trypsinized peptide. Lysine (y6 + 1) is shifted from 692 to 805 m/z; a signature for ubiquitinated residues (Lys + Gly + Gly (K-G-G)). B, ubiquitination of YFP-tagged Amot130 and Amot130 mutants P-Y2,3F (Y242F and Y287F), K481R, and P-Y2,3F/K481R following co-expression with FLAG-tagged AIP4 was examined following immunoprecipitation with an anti-YFP antibody and detected by immunoblot. The ratios of ubiquitinated over total YFP-tagged Amot130 pixel intensities are given. C, FLAG-tagged Amot130, AmotL1, or AmotL2 were co-expressed with HA-tagged ubiquitin and with Myc-tagged AIP4 or control vector. Immunoblot of AIP4 in lysates (anti-Myc) or immunoprecipitated FLAG-tagged total (anti-FLAG) or ubiquitinated (anti-HA) Amot family members were detected by immunoblot (IB). An alignment of the protein sequence encompassing Lys-481 in Amot with AmotL1 and AmotL2 is shown below this blot.
The requirement of residue Lys-481 in Amot for its ubiquitination by AIP4 was defined. For this purpose, cells were co-transfected with HA-tagged ubiquitin and Myc-tagged AIP4, as well as YFP-tagged Amot130 or mutants of Amot130 P-Y2,3F, K481R, or P-Y2,3F/K481R. Immunoblot of anti-YFP immunoprecipitates revealed that ubiquitination of P-Y2,3F and K481R was moderately reduced, whereas ubiquitination of the P-Y2,3F/K481R mutant was undetectable (Fig. 3B). Also, consistent with Lys-481 being a primary site of AIP4 ubiquitination, HA-tagged ubiquitin was detected in gel-shifted bands of Amot130 and AmotL1 but not AmotL2, which encodes arginine at residue 364, the site that aligns with Lys-481/Lys-488 in Amot130/AmotL1 (Fig. 3C).
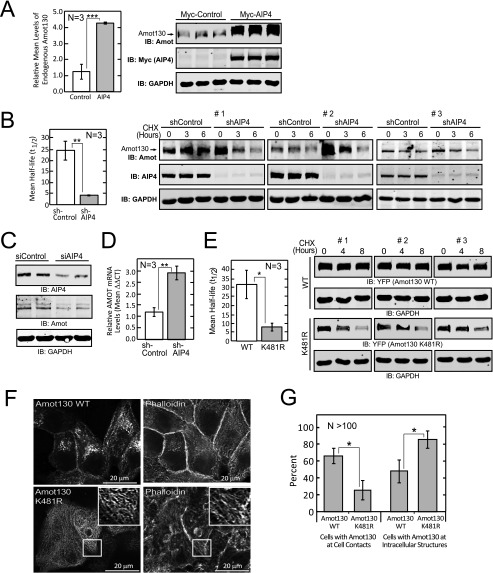
Amot130 Is Stabilized by AIP4
This study finds that the average levels of endogenous Amot130 were elevated >3-fold when Myc-tagged AIP4 was expressed versus control (Fig. 4A). Furthermore, the average increase in the levels of YFP-Amot130 in cells in which AIP4 was expressed remains significantly higher versus in cells expressing control across six independent experiments (supplemental Fig. S4A). However, similar to previous findings (20), the expression of another Nedd4 family member, Nedd4–1, reduced Amot130 steady state levels (supplemental Fig. S4B).
FIGURE 4.
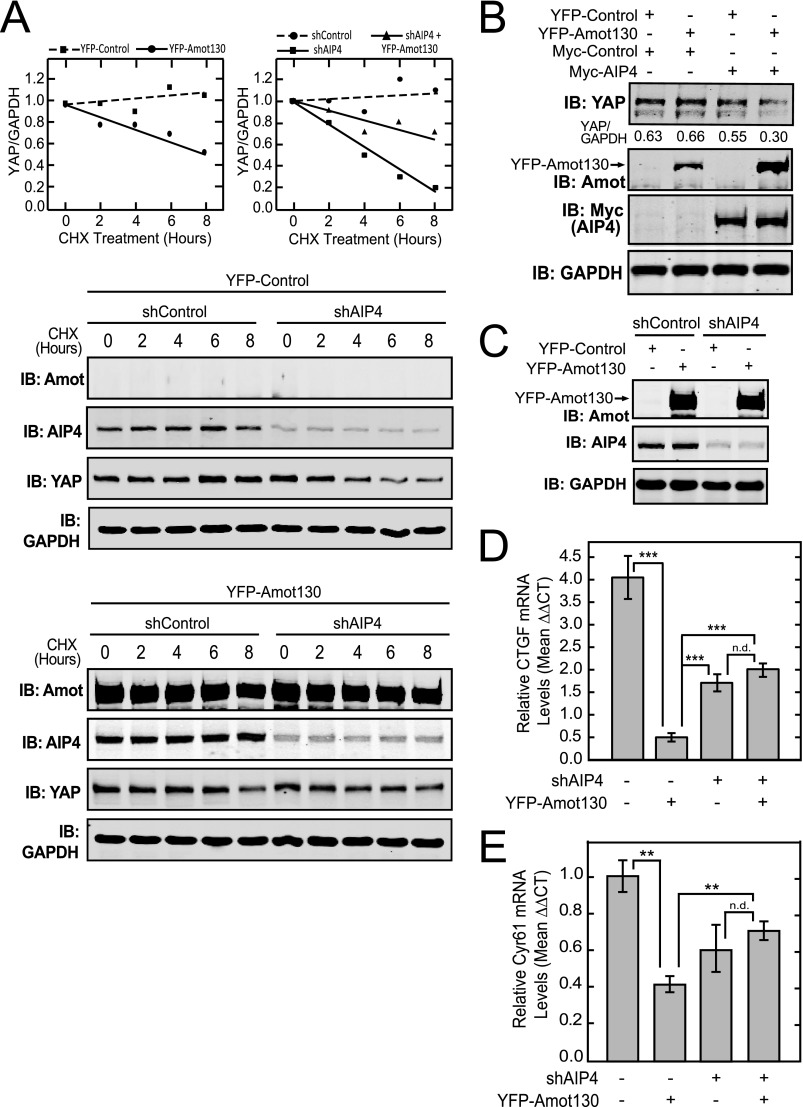
Amot130 ubiquitination by AIP4 results in increased Amot130 stability. A, the levels of endogenous Amot130 were detected by immunoblot analysis of lysates from HEK 293T cells expressing Myc-tagged AIP4. The mean ratio of pixel intensities of endogenous Amot130 over GAPDH from three replicates were graphed (left panel). B, the levels of endogenous Amot130 were detected by immunoblot (IB) from lysates derived from MDA-MB-468 cells expressing AIP4 or control shRNA and treated with vehicle (dimethyl sulfoxide) or 200 μg/μl of cycloheximide (CHX) for indicated times. The ratio of pixel intensities of endogenous Amot130 over GAPDH for both conditions were graphed with linear regression analysis. The mean half-lives were calculated from three independent experiments and graphed (left panel). C, the levels of endogenous Amot130 were detected by immunoblot analysis of lysates from BT474 cells transfected with AIP4 or control siRNA. D, the mRNA transcript levels from cells described in B of Amot were measured from three independent experiments by real-time quantitative PCR. E, the levels of YFP-tagged Amot130 wild-type or K481R mutant were detected by immunoblot analysis of lysates prepared from HEK 293T cells treated with vehicle (dimethyl sulfoxide) or 200 μg/μl of cycloheximide for the indicated times. The ratio of pixel intensities of YFP-Amot130 WT or YFP-Amot130 K481R over GAPDH were graphed with linear regression plots. The mean half-lives were calculated from three independent experiments and graphed (left panel). F, confocal fluorescence images of YFP-tagged Amot130 WT or Amot130 K481R in fixed MCF7 cells stained with phalloidin 594. G, quantification of the percent of live MCF7 cells that show YFP-Amot130 WT and YFP-Amot130 (K481R) localization at intercellular contacts or at intracellular structures. Error bars represent ± S.D. ***, p < 0.0005; **, p < 0.005; *, p < 0.05.
This spurred a more detailed analysis of the modes whereby AIP4 regulates Amot130 protein levels. To determine the effect of silencing AIP4 on Amot130 protein stability, protein synthesis was blocked in MDA-MB-468 cells by treatment with cycloheximide for the indicated times before being lysed. The relative levels of Amot130, AIP4, and GAPDH were then detected by immunoblot analysis. The average half-life derived from three independent experiments of endogenous Amot130 was ∼4 h in cells silenced for AIP4 compared with ∼25 h in control cells (Fig. 4B and supplemental Fig. S4C). However, in an apparent contradiction, it was observed, similar to a previous report (20), that the steady state level of Amot130 was increased in cells silenced for AIP4. The opposite effect was observed, however, in BT474, HEK 293T, and MCF7 cells, which showed decreased levels of Amot130 following silencing of AIP4 (Fig. 4C and supplemental Fig. S4, D and E). Given these conflicting effects, the impact of silencing AIP4 on Amot mRNA transcript levels was measured by real-time quantitative PCR. This revealed that Amot transcript levels in MDA-MB-468 cells were ∼3-fold higher in cells silenced for AIP4 versus control cells (Fig. 4D). Thus, although AIP4 silencing directly reduces Amot130 protein stability, it can also increase Amot mRNA levels. Different balances between these two effects in different cell types may therefore explain the overall impact of silencing AIP4 on Amot130 steady state levels.
Because silencing and overexpression of AIP4 has pleiotropic effects, the direct impact of ubiquitination of Amot130 on its stability was investigated. For this purpose, the stability of the Amot130 K481R mutant, which has substantially reduced ubiquitination by AIP4, was compared with that of wild-type Amot130. Consistent with ubiquitination having a significant stabilizing effect on Amot, the half-life of wild-type YFP-tagged Amot130 was ∼30 h in cycloheximide-treated HEK 293T cells, whereas YFP-tagged Amot130 (K481R) was ∼8 h (Fig. 4E and supplemental Fig. S4F). Similarly, the stability of the Amot130 K481R mutant was ∼3-fold less stable than Amot130 in MCF7 cells (supplemental Fig. S4G). Taken together, ubiquitination of Amot130 by AIP4 appears to significantly increase Amot130 stability in cells.
One possibility for the observed reduced stability of Amot130 (K481R) is that it may have an altered subcellular distribution. Consistently, in >60% of live MCF7 cells, YFP-tagged Amot130 localized at intercellular contacts and at intracellular compartments that are likely endosomes (26). However, Amot130 (K481R) localized at cell-cell contacts in a significantly lower fraction of cells (∼20%) and instead was mainly observed along actin fibers that were not close to cell-cell contacts (Fig. 4, F and G) (2). Similarly, in live Madin Darby canine kidney cells, YFP-tagged Amot130 and the mutant Amot130 (P-Y1F) distributed mainly to cell-cell contacts, whereas the YFP-tagged Amot130 P-Y2,3F and K481R mutants distributed predominantly at intracellular fibers (supplemental Fig. S4H). These data suggest that Amot130, when not ubiquitinated by AIP4, exhibits increased actin association and reduced membrane localization at endosomes and at the plasma membrane.
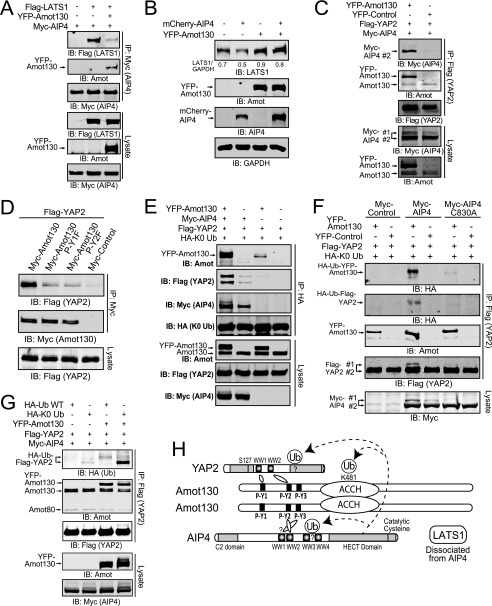
Amot130 Redirects AIP4 from LATS1 to the Ubiquitination of YAP
Because AIP4 binds to P-Y motifs in both Amot130 and LATS1 via its WW domains, their competition for binding to AIP4 was explored. Myc-tagged AIP4 was immunoprecipitated from cells that also expressed a control vector and FLAG-tagged LATS1 or YFP-tagged Amot130 and FLAG-tagged LATS1. Immunoblot analysis revealed that YFP-tagged Amot130 expression resulted in dramatically less LATS1 being precipitated by AIP4 and concomitant binding to Amot130 (Fig. 5A). Consistent with AIP4 mediating the degradation of LATS1 (25), the steady state levels of endogenous LATS1 were reduced upon expression of AIP4 (Fig. 5B). However, expression of Amot130 increased the levels of endogenous LATS1 even when AIP4 was co-expressed. Amot130 is therefore proposed to uncouple AIP4 from binding and inducing the degradation of LATS1.
FIGURE 5.
Amot130 induces AIP4-dependent ubiquitination of YAP. A, the levels of Myc-tagged AIP4, FLAG-tagged LATS1, and YFP-tagged Amot130 were detected by immunoblot (IB) from lysates and immunoprecipitations (anti-Myc) prepared from HEK 293T cells expressing these proteins in the indicated combinations. B, the levels of endogenous LATS1 were measured by immunoblot in MDA-MB-468 cells expressing mCherry-tagged AIP4 and YFP-tagged Amot130 alone or in combination. The ratio of pixel intensities of LATS1 over GAPDH is presented. C, the levels of Myc-tagged AIP4, YFP-tagged Amot130, and FLAG-tagged YAP2 were detected by immunoblot from lysates and immunoprecipitations (anti-FLAG) prepared from HEK 293T cells expressing these three proteins in the indicated combinations. D, the levels of FLAG-tagged YAP2 and Myc-tagged Amot130 wild-type, P-Y1F (Y109F), or P-Y2F (Y242F) mutants or control vector were detected by immunoblot from lysates and immunoprecipitations (anti-Myc) prepared from HEK 293T cells expressing these proteins in the indicated combinations. E, the levels of ubiquitinated FLAG-tagged YAP2 were detected by immunoblot following immunoprecipitation (anti-HA) from HEK 293T cells expressing HA-tagged Lys-0 ubiquitin (HA-K0 Ub) in combination with YFP-tagged Amot130 or control vector as well as Myc-tagged AIP4 and FLAG-tagged YAP2. F, the relative incorporation of HA-Lys-0 ubiquitin into immunoprecipitated FLAG-tagged YAP2 in HEK 293T cells co-expressing YFP-tagged Amot130, Myc-tagged AIP4, mutant AIP4 (C830A), or control vectors was detected by immunoblot. G, the levels of total and ubiquitinated FLAG-tagged YAP2 were detected by immunoblot following immunoprecipitation from HEK 293T cells expressing HA-tagged wild-type or Lys-0 ubiquitin in combination with YFP-tagged Amot130 or control vector as well as Myc-tagged AIP4 and FLAG-tagged YAP2. H, model of the binding and ubiquitination relationships of the Amot130·AIP4·YAP2 complex. A question mark denotes unknown sites of ubiquitination or binding.
Given that Amot130 binds YAP, it was investigated whether AIP4 was a part of this complex. To do this, we tested whether YAP required Amot130 to immunoprecipitate AIP4. The results showed that Myc-tagged AIP4 and YFP-tagged Amot130 strongly co-precipitated with FLAG-tagged YAP2, whereas only trace levels of Myc-tagged AIP4 (along with endogenous Amot130) bound FLAG-tagged YAP2 in the absence of YFP-tagged Amot130 (Fig. 5C). Thus, YAP2 and AIP4 are likely scaffolded by Amot130 into a single protein complex.
Because AIP4 and YAP2 potentially bind to overlapping sites in Amot130, the mechanisms of interaction of YAP2 with Amot130 were further examined. Direct equilibrium binding of GST-tagged YAP2-WW1 and YAP2-WW1 and -2 domains with the Amot130 P-Y1 peptide occurs with Kd values of 7.1 and 5.2 μm, respectively. This is >2-fold lower (stronger) than any WW domains of AIP4. No binding was detected by GST-tagged YAP2-WW2. GST-tagged YAP2-WW1, -WW2, and -WW1 and -2 domains bound the P-Y2 peptide with Kd values of 6.6, 4.4, and 2.9 μm respectively (Table 1). P-Y3 was not examined as it does not bind YAP2 (30). Consistent with YAP2 requiring both P-Y1 and P-Y2 for binding full-length protein, it showed almost no detectable association with Amot130 P-Y1F or P-Y2F mutants (Fig. 5D). Overall, these data are most consistent with YAP2 binding in a fixed orientation, where its WW2 domain binds preferably to P-Y2 and only its WW1 domain is capable of binding P-Y1.
Based on the evidence that Amot130 brings YAP2 and AIP4 into a common complex, the effects of Amot130 on the ubiquitination of YAP2 by AIP4 were measured. Ubiquitinated FLAG-tagged YAP2 was immunoprecipitated from cells expressing HA-tagged Lys-0 ubiquitin with an anti-HA antibody. Although ubiquitinated YAP2 was undetectable in samples not expressing Amot130 or AIP4, expression of Amot130 or AIP4 alone modestly increased the levels of ubiquitinated YAP2. However, co-expression of AIP4 and Amot130 resulted in a strong synergistic increase in the levels of ubiquitinated YAP2, indicating the importance of Amot130 in adapting YAP2 as a substrate for AIP4 (Fig. 5E). This effect requires catalytically active AIP4 as co-expression of Amot130, and the catalytically inactive mutant of AIP4 (C830A) did not induce the ubiquitination of YAP2 (Fig. 5F). The inability of Amot80 to induce ubiquitination of YAP2 also suggests that scaffolding of AIP4 and YAP2 by Amot130 is required for this event (supplemental Fig. S5).
The differential effects of incorporation of HA-tagged wild-type ubiquitin versus HA-tagged Lys-0 ubiquitin induced by Amot130 and AIP4 into YAP2 were compared (Fig. 5G). Expression of Amot130 with AIP4 resulted in a gel-shifted weak smear of wild-type ubiquitinated YAP2, which is consistent with polyubiquitination and therefore potential targeting for degradation, whereas Lys-0-ubiquitinated YAP2, induced by expression of Amot130 and AIP4, migrated as a faster/smaller and much stronger single band. The absence of this lower band with wild-type ubiquitin supports that AIP4 catalyzed ubiquitination of YAP2 is mainly coupled to polyubiquitination. A model of the proposed binding and ubiquitination relationships based on the preceding data between Amot130, AIP4, and YAP2 is depicted in Fig. 5H.
Amot130 Works Interdependently with AIP4 to Inhibit YAP
Initially, the effects of exogenous expression of Amot130 on the endogenous stability of YAP protein in the presence or absence of silencing of AIP4 expression were defined. For this, MDA-MB-468 cells stably expressing YFP-tagged Amot130 or control vector in combination with shRNA control or shRNA targeting AIP4 were lysed at the indicated times following treatments with cycloheximide (Fig. 6A). Unlike control cells which showed no reduction of endogenous YAP by 8 h, cells expressing YFP-tagged Amot130 showed a 40% loss of YAP. (Fig. 6A, top left). Furthermore, as reported previously (25), silencing of AIP4 reduced the levels of YAP by >80% during this time. Consistent with Amot130 uncoupling YAP from regulation of LATS1/2 (8, 9), the expression of Amot130 partially reversed the effects of silencing AIP4 (Fig. 6A, top right). However, the reported destabilization of LATS1 by AIP4 likely explains the inability to see a reversal of the inhibition of YAP by Amot130 expression upon the silencing of AIP4. This event is also complicated by the potential for Amot130 to regulate other Nedd4 family members (20). However, because co-expression of Amot130 and AIP4 result in a synergistic reduction in the steady state levels of YAP, there appears to be some role for Amot130 in directing AIP4 to induce the reduction of YAP protein levels (Fig. 6B).
FIGURE 6.
Amot130 and AIP4 interdependently inhibit YAP. A, the levels of YFP-tagged Amot130 as well as endogenous AIP4, YAP, and GAPDH were detected in lysates from MDA-MB-468 cells stably expressing control or AIP4 shRNA in combination with YFP-tagged Amot130 or control vector and treated for the indicated times with vehicle (dimethyl sulfoxide) or 200 μg/μl of cycloheximide (CHX). The ratio of pixel intensities with linear regression analysis was plotted from the immunoblots, where the YAP/GAPDH levels in cells expressing YFP-control (■) or YFP-Amot130 (●) are depicted (left graph) and the YAP/GAPDH levels in cells expressing shControl (●), shAIP4 (■), or YFP-tagged (▴) Amot130 and shAIP4 are shown (right graph). B, the levels of endogenous YAP were detected by immunoblot (IB) analysis of lysates from HEK 293T cells expressing combinations of YFP-tagged Amot130 and Myc-tagged AIP4. The ratio of pixel intensities of endogenous YAP over GAPDH bands are below the blot. C, MDA-MB-468 cells stably expressing YFP-tagged Amot130 or control vector in combination with control or AIP4 shRNA. The mRNA transcript levels from cells described in C of CTGF (D) or Cyr61 (E) were measured by real-time quantitative PCR. Error bars represent ± S.D. ***, p < 0.00001; **, p < 0.01; n.d., no statistical difference.
Given the complex effects of AIP4 and Amot130 on YAP stability, functional analysis was undertaken to determine the role of AIP4 in mediating Amot130 induced inhibition of YAP dependent transcription. The transcription of CTGF/CCN2 and Cyr61/CCN1 are regulated by transcriptional enhancer domain protein transcription factors that in turn are under the control of YAP (16, 17). Therefore, we measured the effects of AIP4 silencing in combination with Amot130 expression (Fig. 6C) on the levels of endogenous CTGF (Fig. 6D) and Cyr61 (Fig. 6E) transcripts in MDA-MB-468 cells. This revealed that cells stably expressing Amot130 had a >85% reduction in CTGF transcript, whereas CTGF transcript levels in AIP4 stably silenced cells were reduced by roughly 50%. However, CTGF levels were reduced by <45% in cells simultaneously silenced for AIP4 expression and expressing YFP-tagged Amot130. Similar effects were seen with Cyr61 transcript levels. Thus, AIP4 appears to be at least partially required for Amot130 to suppress the transcript levels of CTGF and Cyr61.
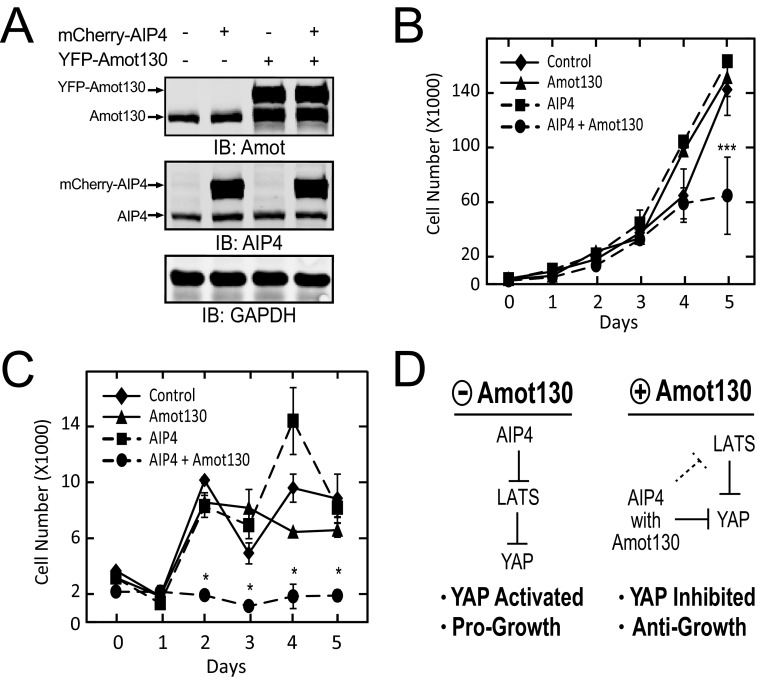
Because Amot and YAP are implicated in the regulation of cell proliferation, the impact of co-expression of Amot130 and AIP4 on cell accrual was measured. MDA-MB-468 cells expressing either a control vector, Amot130, AIP4, or Amot130 and AIP4 (Fig. 7A) were plated in the presence or absence of serum and then counted at the indicated days. Cells grown in full serum expressing Amot130 or AIP4 alone grew similarly to control cells. However, cells expressing both Amot130 and AIP4 accumulated at a significantly reduced level by day 5 (Fig. 7B). Similarly, significantly fewer serum-starved cells expressing both Amot130 and AIP4 were present at days 2 through 5 versus cells expressing Amot130, AIP4, or the control vector alone (Fig. 7C). Taken together, this data supports a role for Amot130 and AIP4 in cooperatively mediating anti-growth functions, at least in part, through the inhibition of YAP (Fig. 7D).
FIGURE 7.
Co-expression of Amot130 and AIP4 inhibits cell accumulation. A, immunoblot (IB) analysis of the protein levels of YFP-tagged Amot130 and mCherry-tagged AIP4 from lysates prepared from MDA-MB-468 cells stably expressing with and without these proteins. These cells expressing either control (♦), mCherry-AIP4 (■), YFP-Amot130 (▴), or mCherry-AIP4 and YFP-Amot130 (●) were plated in triplicate and counted after the indicated days of growth in DMEM with 10% (B) or 0% (C) serum. Data represent the average of three independent experiments. D, model depicting the proposed role of Amot130 in rewiring AIP4 from inducing the degradation of LATS1 to promoting the inhibition of YAP and cell growth. Error bars represent ± S.D. ***, p < 0.005; *, p < 0.05.
DISCUSSION
AIP4 is reported to both promote and inhibit cell growth. However, far less is known regarding the means by which AIP4 is switched between these opposing roles. Here, the binding of Amot130 to AIP4 is shown to prevent AIP4 association with LATS1 and to increase AIP4 recruitment into a complex with YAP. The consequence of this contextual change is a functional reversal of AIP4, where it now mediates Amot130 induced inhibition of YAP2 controlled transcription and cell accumulation.
Ubiquitination is a key determinate of protein function and stability. The Nedd4 family of HECT (homologous to the E6-AP carboxyl terminus) domain ubiquitin ligases (34) have diverse roles in cell signaling, including the ubiquitination and regulation of proteins that control cell growth (35). For instance, the Nedd4 family member AIP4, which localizes to endosomes and the trans-Golgi network (36, 37), promotes cell accrual by inhibiting apoptosis through polyubiquitinating and thereby signaling the degradation of the transcription factor p73 (38) and the proapoptotic factor tBid (39). AIP4 also promotes cell proliferation by similarly inducing the degradation of LATS1 (25, 40). This degradation of LATS1 results in YAP and TAZ being less restricted from activating cellular growth. Conversely, AIP4 induces both poly- and monoubiquitination of Notch1 (41) and blocks hematopoietic stem cell proliferation by promoting its degradation (42). This study highlights how binding of AIP4 to the adaptor protein Amot130 can mediate the changeover of AIP4 from a pro-growth to an anti-growth role.
AIP4 is proposed to achieve a higher affinity interaction with Amot130 through binding both the P-Y2 and P-Y3 motifs through its first and second WW domains. A similar precedence is seen with Nedd4 ligases (43, 44) as well as YAP2 (45). However, this study indicates that YAP2 achieves this by binding the P-Y1 and P-Y2 motifs of Amot130 and not through the P-Y3 motif (9, 30). Consistently, YAP2 strongly binds AmotL2 (9), which lacks a P-Y3 motif. This also suggests that YAP2 and AIP4 through competition at the P-Y2 site inhibit binding of the other. However, because both proteins associate in a single complex with Amot130, single site binding may be sufficient or this could be achieved via Amot130 homodimerization or heterodimerization with AmotL1 (46).
A significant consequence of Amot130 binding to AIP4 is the induction of its ligase activity for self and substrate ubiquitination. Nedd4 ligases exist naturally in an auto-inhibited conformation (33, 47). However, when their WW domains bind to P-Y motifs in another protein, the inhibitory HECT domain conformation is relieved (31–33), and ligase activity is elevated leading to self and substrate ubiquitination (31, 32). Amot130, similar to other adaptors (31, 32), further defines the substrate availability of AIP4 by both preventing its association with LATS1 as well as coupling its activation with providing itself and YAP as substrates. Because AIP4 targets LATS1 for degradation via the 26 S proteasome (25), this may in part explain how Amot130 prevents AIP4 from inhibiting Hippo kinase signaling to maintain its normal inhibition of cell growth. AIP4, also through stabilizing Amot130 likely contributes to the established role of Amot130 in inhibiting cell growth (8, 9).
The stabilization of Amot130 by AIP4 may be a result of ubiquitinated Amot130 being targeted to intercellular contacts. Although the finding that Amot130 is stabilized by AIP4 differs from the conclusion of a previous study (20), the findings here are supported by multiple approaches. First, the effects of silencing AIP4 both reduce Amot130 stability, and it also increases Amot mRNA transcript levels. The cell type-specific effects of silencing AIP4 on Amot130 steady state levels are therefore likely due to a dominance of either effect. Furthermore, although silencing AIP4 is a fairly nonspecific approach, the reduced stability of mutants of Amot130 that are less ubiquitinated by AIP4 is a more direct indicator that ubiquitination generally enhances Amot130 stability. Furthermore, in cells at confluence where Amot130 is reported to be more stable (48), it is found here that Amot130 is more likely to be ubiquitinated. For instance, the ubiquitinated peptide of Amot was identified in differentiated keratinocytes four of five times but was never detected in undifferentiated keratinocytes. Furthermore, the gel-shifted band of Amot130, which is associated with ubiquitination, is strongly evident in cells at high density but not in cells at low density (data not shown). This change in stability is also consistent with the finding that wild-type Amot130 but not the ubiquitination reduced mutant is localized to a much greater extant to intercellular contacts, where it may promote cell polarity as well as inhibition of YAP.
Canonical inhibition of YAP involves its phosphorylation by LATS1/2, which induces its cytosolic sequestration and/or marks it for recognition and targeting for ubiquitination by the E3 ligase SCFβ-TRCP (49). Alternatively, inhibition of YAP by Amot130 is reported to both require and be independent on the phosphorylation of YAP by LATS1/2 (8, 9). This study describes a mechanism whereby this latter LATS1-independent event may involve Amot130/AIP4. This might be similar to BCL10 (B-cell CLL/lymphoma 10 protein), which is targeted separately for ubiquitination and degradation by both AIP4 (50) and SCFβ-TRCP (51). The ability of Amot130 to partially reverse the instability of YAP induced by AIP4 silencing further suggests that Amot130 removes YAP from regulation by LATS1/2. However, because AIP4 silencing only partially reverses the inhibition of YAP dependent transcription by Amot130, there are likely pools of YAP that are still available to be inhibited by increased LATS1 activity.
Overall, Amot130 likely inhibits YAP through a combination of mechanisms involving sequestration from the nucleus and by inducing its degradation. This may include both direct coupling with LATS1/2 (52) and, as found here, independent effects with AIP4. Future studies examining how these mechanisms are coordinated by microenvironmental cues will likely lead to a better understanding of growth control in cancer and normal development (10).
Supplementary Material
Acknowledgments
The HA-tagged Lys-0 ubiquitin was obtained through Addgene from T. Dawson (The Johns Hopkins University). The FLAG-tagged YAP2 and LATS1 constructs were obtained through Addgene from M. Sudol (Weis Center for Research). We also thank J. Russ (Indiana University Purdue University Indianapolis) for proofreading.
This work was supported by National Institutes of Health/NCI Grant R01CA151765 and Department of Defense Grant W81XWH (to C. D. W).

This article contains supplemental “Methods,” Tables 1–4, Figs. S1–S5, and additional references.
- YAP1
- Yes-associated protein 1
- CTGF
- connective tissue growth factor
- LATS1
- large tumor suppressor homolog 1
- AIP4
- atrophin-1 interacting protein 4
- P-Y
- proline and tyrosine conserved motif
- WW
- domain containing 2 conserved tryptophans
- SCFβ-TRCP
- Skp1/Cdc53/Cullin/F-box receptor/β-transducin repeat-containing protein.
REFERENCES
- 1. Wells C. D., Fawcett J. P., Traweger A., Yamanaka Y., Goudreault M., Elder K., Kulkarni S., Gish G., Virag C., Lim C., Colwill K., Starostine A., Metalnikov P., Pawson T. (2006) A Rich1/Amot Complex Regulates the Cdc42 GTPase and Apical-Polarity Proteins in Epithelial Cells. Cell 125, 535–548 [DOI] [PubMed] [Google Scholar]
- 2. Ernkvist M., Aase K., Ukomadu C., Wohlschlegel J., Blackman R., Veitonmäki N., Bratt A., Dutta A., Holmgren L. (2006) p130-Angiomotin associates to actin and controls endothelial cell shape. FEBS J. 273, 2000–2011 [DOI] [PubMed] [Google Scholar]
- 3. Bratt A., Wilson W. J., Troyanovsky B., Aase K., Kessler R., Van Meir E. G., Holmgren L., Meir E. G. (2002) Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene 298, 69–77 [DOI] [PubMed] [Google Scholar]
- 4. Shimono A., Behringer R. R. (2003) Angiomotin regulates visceral endoderm movements during mouse embryogenesis. Curr. Biol. 13, 613–617 [DOI] [PubMed] [Google Scholar]
- 5. Aase K., Ernkvist M., Ebarasi L., Jakobsson L., Majumdar A., Yi C., Birot O., Ming Y., Kvanta A., Edholm D., Aspenström P., Kissil J., Claesson-Welsh L., Shimono A., Holmgren L. (2007) Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 21, 2055–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranahan W. P., Han Z., Smith-Kinnaman W., Nabinger S. C., Heller B., Herbert B. S., Chan R., Wells C. D. (2011) The adaptor protein AMOT promotes the proliferation of mammary epithelial cells via the prolonged activation of the extracellular signal-regulated kinases. Cancer Res. 71, 2203–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yi C., Troutman S., Fera D., Stemmer-Rachamimov A., Avila J. L., Christian N., Persson N. L., Shimono A., Speicher D. W., Marmorstein R., Holmgren L., Kissil J. L. (2011) A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19, 527–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan S. W., Lim C. J., Chong Y. F., Pobbati A. V., Huang C., Hong W. (2011) Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 286, 7018–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao B., Li L., Lu Q., Wang L. H., Liu C. Y., Lei Q., Guan K. L. (2011) Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao B., Li L., Lei Q., Guan K. L. (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strano S., Munarriz E., Rossi M., Castagnoli L., Shaul Y., Sacchi A., Oren M., Sudol M., Cesareni G., Blandino G. (2001) Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J. Biol. Chem. 276, 15164–15173 [DOI] [PubMed] [Google Scholar]
- 12. Oka T., Mazack V., Sudol M. (2008) Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J. Biol. Chem. 283, 27534–27546 [DOI] [PubMed] [Google Scholar]
- 13. Vassilev A., Kaneko K. J., Shu H., Zhao Y., DePamphilis M. L. (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., Rossant J., Wrana J. L. (2010) The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 [DOI] [PubMed] [Google Scholar]
- 15. Vitolo M. I., Anglin I. E., Mahoney W. M., Jr., Renoud K. J., Gartenhaus R. B., Bachman K. E., Passaniti A. (2007) The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biol. Ther. 6, 856–863 [DOI] [PubMed] [Google Scholar]
- 16. Lai D., Ho K. C., Hao Y., Yang X. (2011) Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 71, 2728–2738 [DOI] [PubMed] [Google Scholar]
- 17. Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C. Y., Chinnaiyan A. M., Lai Z. C., Guan K. L. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao Y., Chun A., Cheung K., Rashidi B., Yang X. (2008) Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283, 5496–5509 [DOI] [PubMed] [Google Scholar]
- 19. Wang W., Huang J., Chen J. (2011) Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 286, 4364–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang C., An J., Zhang P., Xu C., Gao K., Wu D., Wang D., Yu H., Liu J. O., Yu L. (2012) The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitin-dependent degradation. Biochem. J. 444, 279–289 [DOI] [PubMed] [Google Scholar]
- 21. Colwill K., Wells C.D., Elder K., Goudreault M., Hersi K., Kulkarni S., Hardy W. R., Pawson T., Morin G. B. (2006) Modification of the Creator recombination system for proteomics applications - improved expression by addition of splice sites. BMC Biotechnol. 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ingham R. J., Colwill K., Howard C., Dettwiler S., Lim C. S., Yu J., Hersi K., Raaijmakers J., Gish G., Mbamalu G., Taylor L., Yeung B., Vassilovski G., Amin M., Chen F., Matskova L., Winberg G., Ernberg I., Linding R., O'donnell P., Starostine A., Keller W., Metalnikov P., Stark C., Pawson T. (2005) WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell. Biol. 25, 7092–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo C., Tang T. S., Bienko M., Parker J. L., Bielen A. B., Sonoda E., Takeda S., Ulrich H. D., Dikic I., Friedberg E. C. (2006) Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 26, 8892–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim K. L., Chew K. C., Tan J. M., Wang C., Chung K. K., Zhang Y., Tanaka Y., Smith W., Engelender S., Ross C. A., Dawson V. L., Dawson T. M. (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci. 25, 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salah Z., Melino G., Aqeilan R. I. (2011) Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 71, 2010–2020 [DOI] [PubMed] [Google Scholar]
- 26. Heller B., Adu-Gyamfi E., Smith-Kinnaman W., Babbey C., Vora M., Xue Y., Bittman R., Stahelin R. V., Wells C. D. (2010) Amot recognizes a juxtanuclear endocytic recycling compartment via a novel lipid binding domain. J. Biol. Chem. 285, 12308–12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasband W. S. (2011) ImageJ Version 1.44o U. S. National Institutes of Health, Bethesda, MD [Google Scholar]
- 28. Tagwerker C., Flick K., Cui M., Guerrero C., Dou Y., Auer B., Baldi P., Huang L., Kaiser P. (2006) A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivo cross-linking. Mol. Cell. Proteomics 5, 737–748 [DOI] [PubMed] [Google Scholar]
- 29. Lee J. H., Zhang Q., Jo S., Chai S. C., Oh M., Im W., Lu H., Lim H. S. (2011) Novel pyrrolopyrimidine-based α-helix mimetics: cell-permeable inhibitors of protein−protein interactions. J. Am. Chem. Soc. 133, 676–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skouloudaki K., Walz G. (2012) YAP1 recruits c-Abl to protect angiomotin-like 1 from Nedd4-mediated degradation. PLoS One 7, e35735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mund T., Pelham H. R. (2009) Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep. 10, 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hooper C., Puttamadappa S. S., Loring Z., Shekhtman A., Bakowska J. C. (2010) Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol. 8, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallagher E., Gao M., Liu Y. C., Karin M. (2006) Activation of the E3 ubiquitin ligase Itch through a phosphorylation-induced conformational change. Proc. Natl. Acad. Sci. U.S.A. 103, 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rotin D., Kumar S. (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 [DOI] [PubMed] [Google Scholar]
- 35. Chen C., Matesic L. (2007) The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev. 26, 587–604 [DOI] [PubMed] [Google Scholar]
- 36. Marchese A., Raiborg C., Santini F., Keen J. H., Stenmark H., Benovic J. L. (2003) The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev. Cell 5, 709–722 [DOI] [PubMed] [Google Scholar]
- 37. Angers A., Ramjaun A. R., McPherson P. S. (2004) The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. J. Biol. Chem. 279, 11471–11479 [DOI] [PubMed] [Google Scholar]
- 38. Rossi M., De Laurenzi V., Munarriz E., Green D. R., Liu Y. C., Vousden K. H., Cesareni G., Melino G. (2005) The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 24, 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azakir B. A., Desrochers G., Angers A. (2010) The ubiquitin ligase Itch mediates the antiapoptotic activity of epidermal growth factor by promoting the ubiquitylation and degradation of the truncated C-terminal portion of Bid. FEBS J. 277, 1319–1330 [DOI] [PubMed] [Google Scholar]
- 40. Ho K. C., Zhou Z., She Y. M., Chun A., Cyr T. D., Yang X. (2011) Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc. Natl. Acad. Sci. U.S.A. 108, 4870–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qiu L., Joazeiro C., Fang N., Wang H. Y., Elly C., Altman Y., Fang D., Hunter T., Liu Y. C. (2000) Recognition and ubiquitination of Notch by Itch, a hect-type E3 ubiquitin ligase. J. Biol. Chem. 275, 35734–35737 [DOI] [PubMed] [Google Scholar]
- 42. Rathinam C., Matesic L. E., Flavell R. A. (2011) The E3 ligase Itch is a negative regulator of the homeostasis and function of hematopoietic stem cells. Nat. Immunol. 12, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lott J. S., Coddington-Lawson S. J., Teesdale-Spittle P. H., McDonald F. J. (2002) A single WW domain is the predominant mediator of the interaction between the human ubiquitin-protein ligase Nedd4 and the human epithelial sodium channel. Biochem. J. 361, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chong P. A., Lin H., Wrana J. L., Forman-Kay J. D. (2010) Coupling of tandem Smad ubiquitination regulatory factor (Smurf) WW domains modulates target specificity. Proc. Natl. Acad. Sci. U.S.A. 107, 18404–18409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webb C., Upadhyay A., Giuntini F., Eggleston I., Furutani-Seiki M., Ishima R., Bagby S. (2011) Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry 50, 3300–3309 [DOI] [PubMed] [Google Scholar]
- 46. Gagné V., Moreau J., Plourde M., Lapointe M., Lord M., Gagnon E., Fernandes M. J. (2009) Human angiomotin-like 1 associates with an angiomotin protein complex through its coiled-coil domain and induces the remodeling of the actin cytoskeleton. Cell Motil. Cytoskeleton 66, 754–768 [DOI] [PubMed] [Google Scholar]
- 47. Wiesner S., Ogunjimi A. A., Wang H. R., Rotin D., Sicheri F., Wrana J. L., Forman-Kay J. D. (2007) Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain. Cell 130, 651–662 [DOI] [PubMed] [Google Scholar]
- 48. Bratt A., Birot O., Sinha I., Veitonmäki N., Aase K., Ernkvist M., Holmgren L. (2005) Angiomotin regulates endothelial cell-cell junctions and cell motility. J. Biol. Chem. 280, 34859–34869 [DOI] [PubMed] [Google Scholar]
- 49. Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β)-TRCP. Genes Dev. 24, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scharschmidt E., Wegener E., Heissmeyer V., Rao A., Krappmann D. (2004) Degradation of Bcl10 induced by T-cell activation negatively regulates NF-κB signaling. Mol. Cell. Biol. 24, 3860–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lobry C., Lopez T., Israël A., Weil R. (2007) Negative feedback loop in T cell activation through IκB kinase-induced phosphorylation and degradation of Bcl10. Proc. Natl. Acad. Sci. U.S.A. 104, 908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paramasivam M., Sarkeshik A., Yates J. R., 3rd, Fernandes M. J., McCollum D. (2011) Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol. Biol. Cell 22, 3725–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.