Abstract
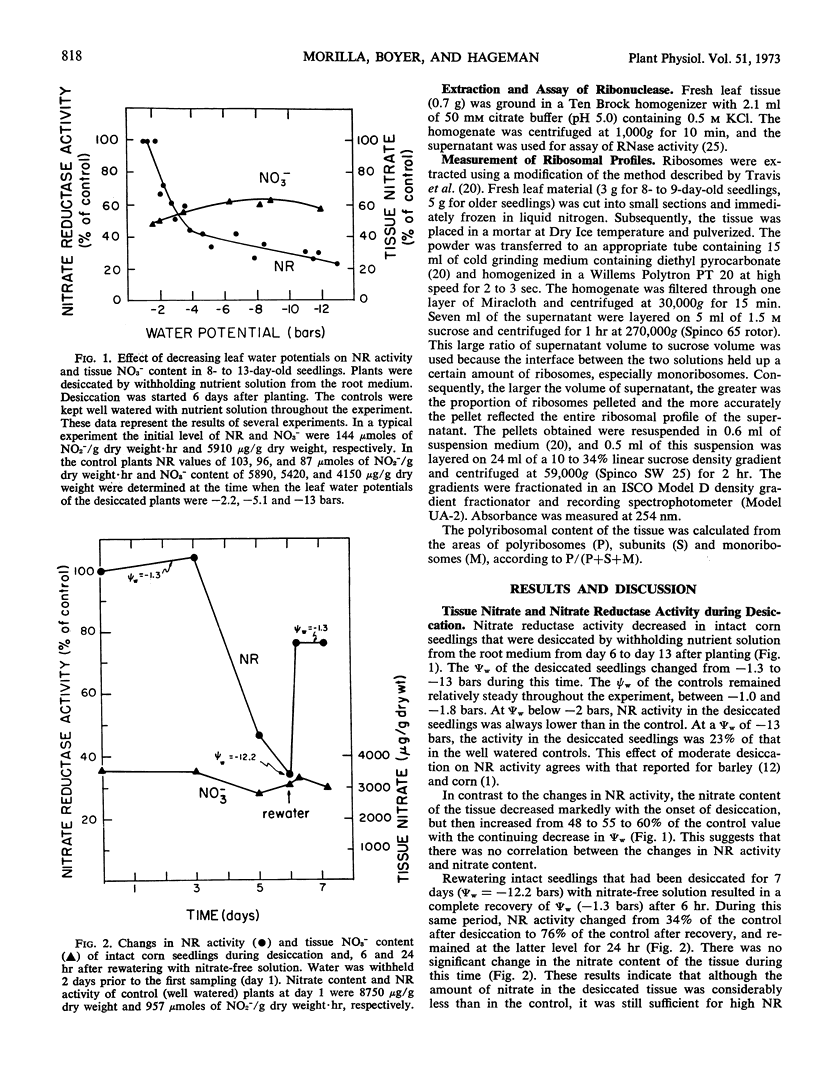
Desiccation of 8- to 13-day-old seedlings, achieved by withholding nutrient solution from the vermiculite root medium, caused a reduction in nitrate reductase activity of the leaf tissue. Activity declined when leaf water potentials decreased below −2 bars and was 25% of the control at a leaf water potential of −13 bars. Experiments were conducted to determine whether the decrease in nitrate reductase activity was due to reduced levels of nitrate in the tissue, direct inactivation of the enzyme by low leaf water potentials, or to changes in rates of synthesis or decay of the enzyme.
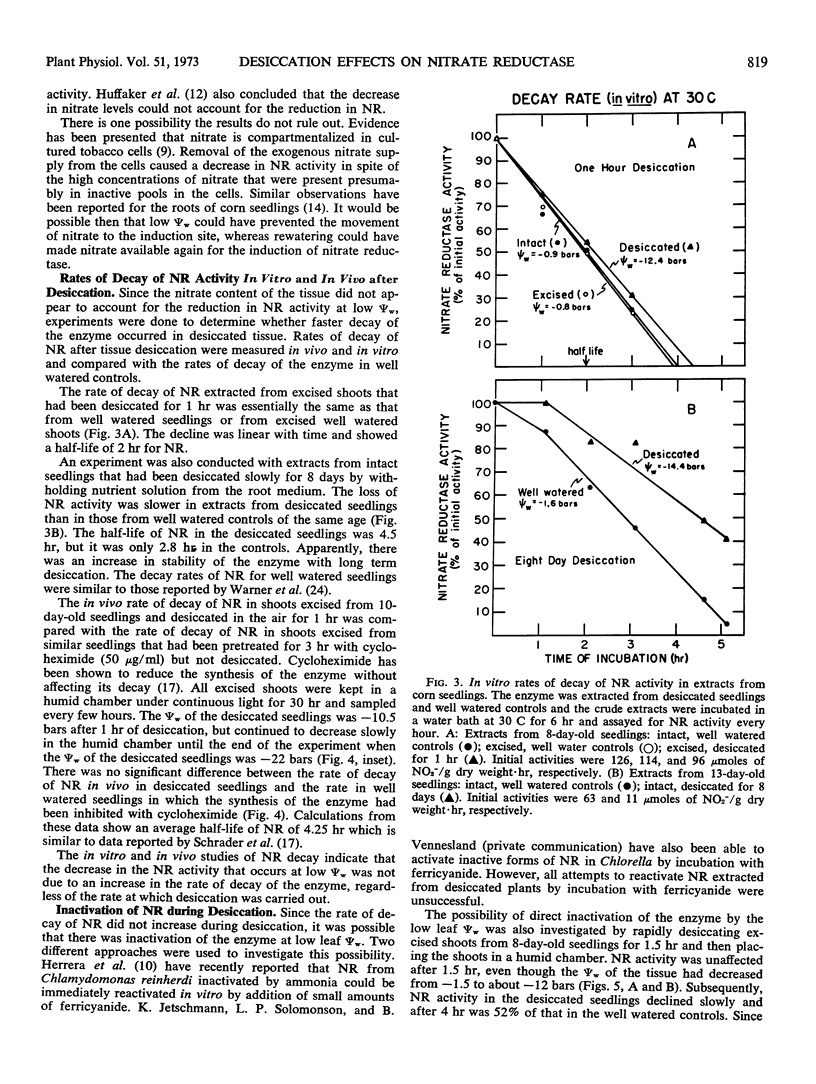
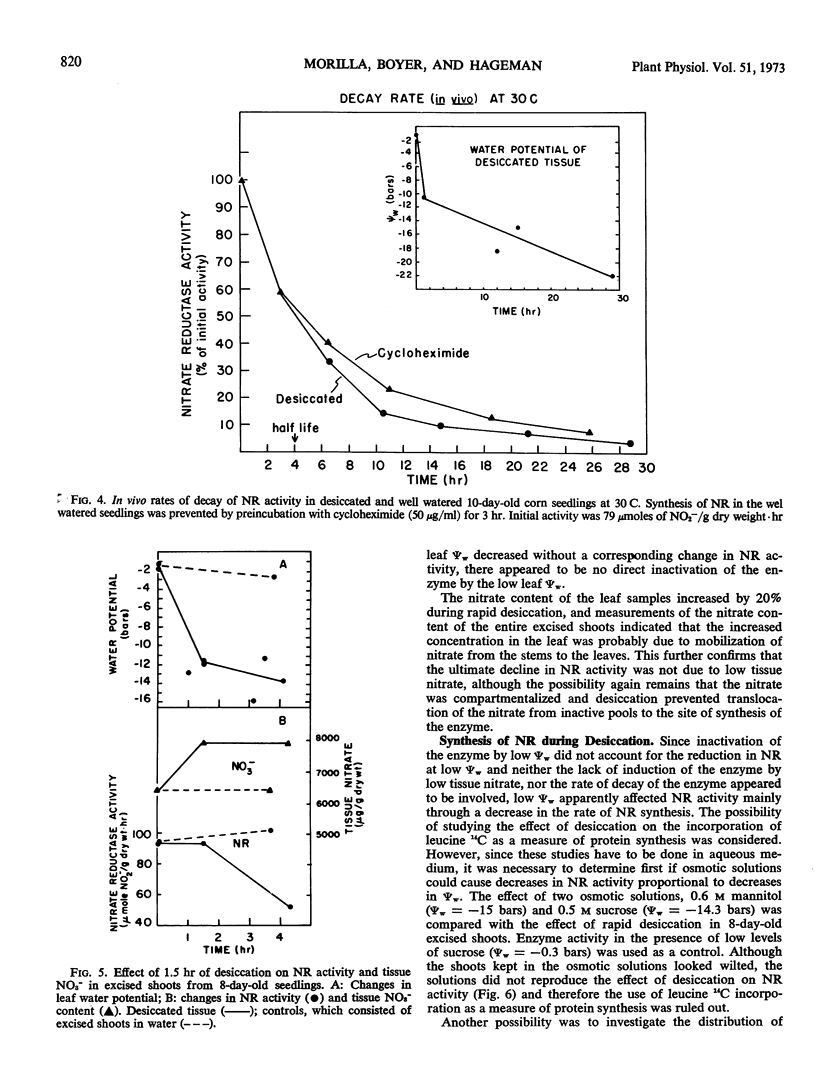
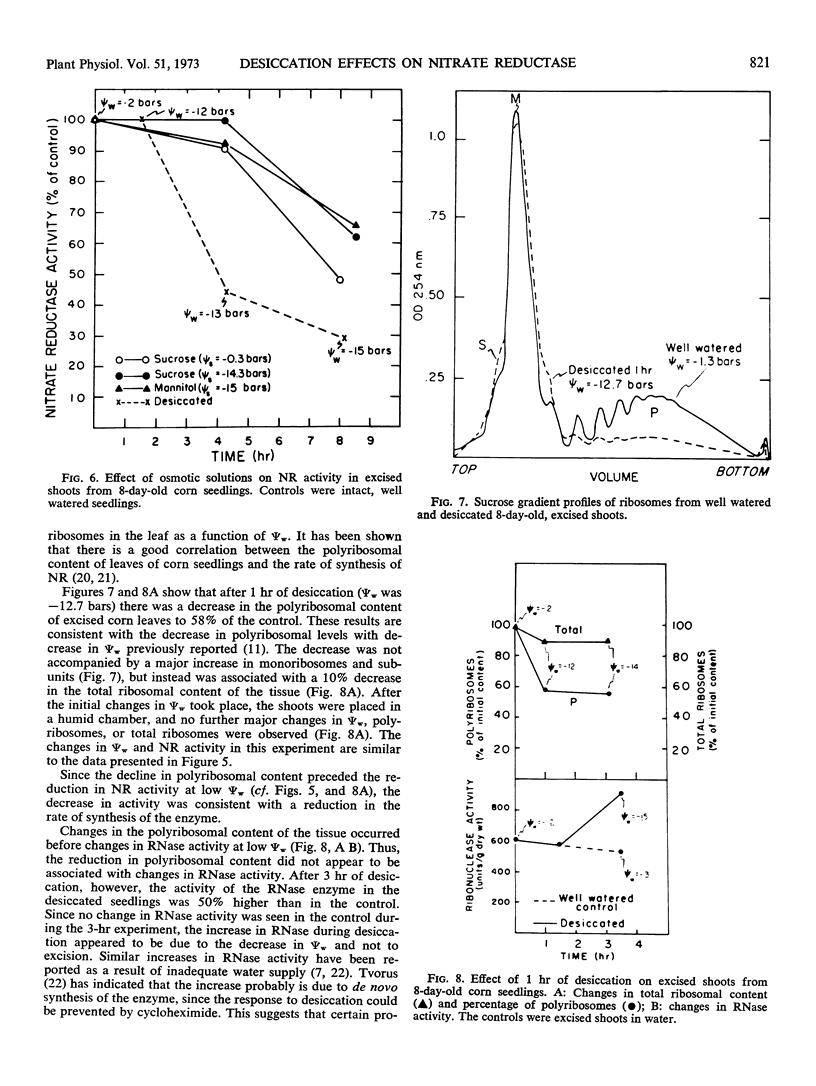
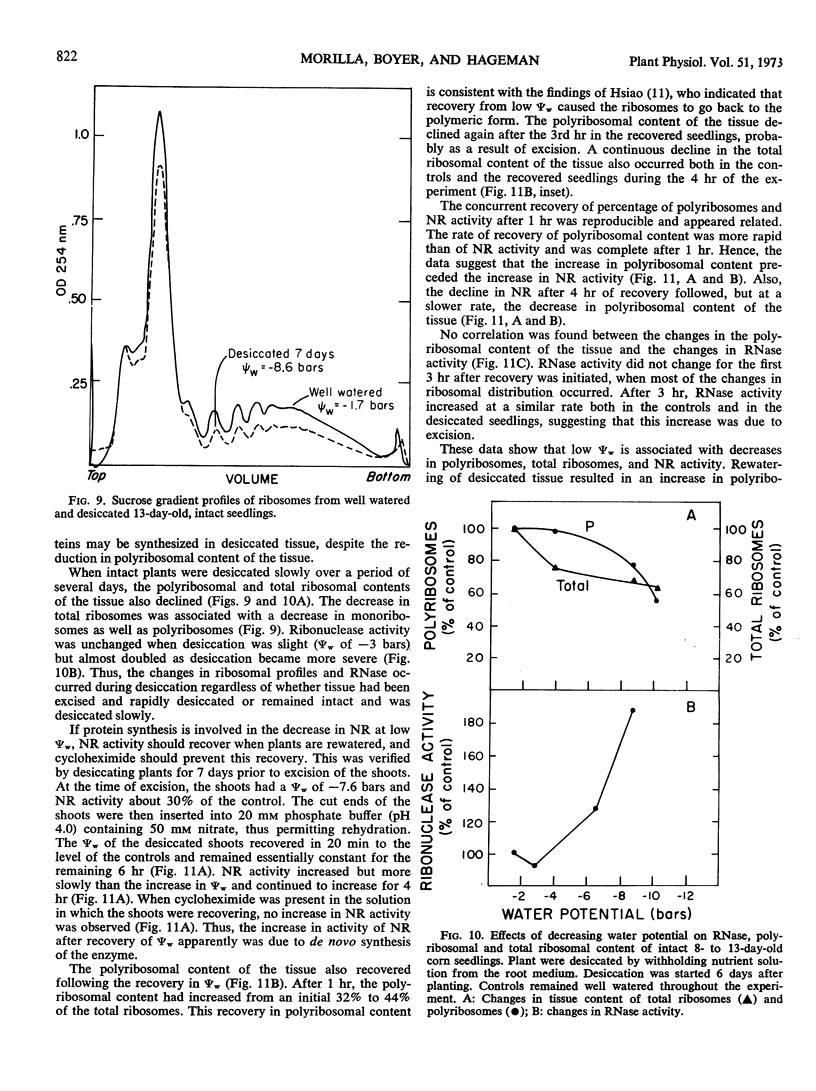
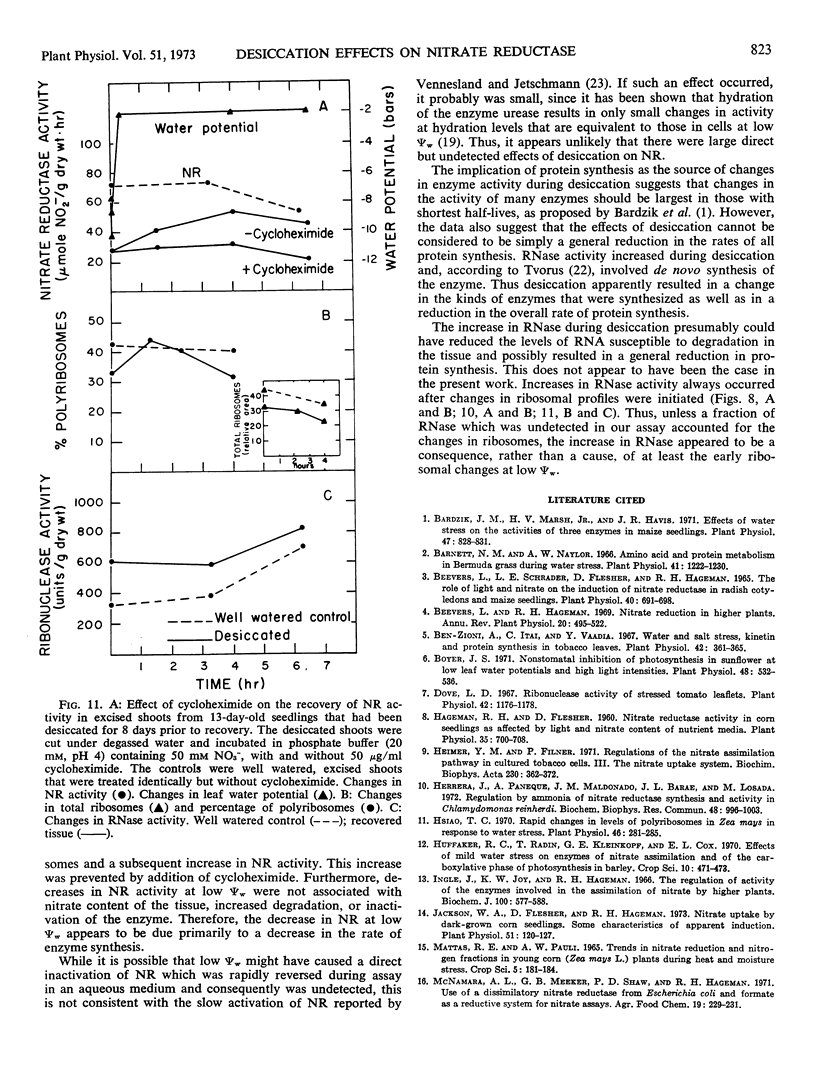
Although tissue nitrate content decreased with the onset of desiccation, it did not continue to decline with tissue desiccation and loss of enzyme activity. Nitrate reductase activity recovered when the plants were rewatered with nitrate-free medium, suggesting that the nitrate in the plant was adequate for high nitrate reductase activity. The rate of decay of nitrate reductase activity from desiccated tissue was essentially identical to that of the control, in vivo or in vitro, regardless of the rapidity of desiccation of the tissue. Direct inactivation of the enzyme by the low water potentials was not detected. Polyribosomal content of the tissue declined with the decrease in water potential, prior to the decline in nitrate reductase activity. Changes in ribosomal profiles occurred during desiccation, regardless of whether the tissue had been excised or not and whether desiccation was rapid or slow. Reduction in polyribosomal content did not appear to be associated with changes in ribonuclease activity. Nitrate reductase activity and the polyribosomal content of the tissue recovered upon rewatering, following the recovery in water potential. The increase in polyribosomal content preceded the increase in nitrate reductase activity. Recovery of enzyme activity was prevented by cycloheximide.
Based on these results, it appears that nitrate reductase activity was affected primarily by a decrease in the rate of enzyme synthesis at low leaf water potentials.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardzik J. M., Marsh H. V., Havis J. R. Effects of water stress on the activities of three enzymes in maize seedlings. Plant Physiol. 1971 Jun;47(6):828–831. doi: 10.1104/pp.47.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett N. M., Naylor A. W. Amino Acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966 Sep;41(7):1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Schrader L. E., Flesher D., Hageman R. H. The Role of Light and Nitrate in the Induction of Nitrate Reductase in Radish Cotyledons and Maize Seedlings. Plant Physiol. 1965 Jul;40(4):691–698. doi: 10.1104/pp.40.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zioni A., Itai C., Vaadia Y. Water and salt stresses, kinetin and protein synthesis in tobacco leaves. Plant Physiol. 1967 Mar;42(3):361–365. doi: 10.1104/pp.42.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Nonstomatal inhibition of photosynthesis in sunflower at low leaf water potentials and high light intensities. Plant Physiol. 1971 Nov;48(5):532–536. doi: 10.1104/pp.48.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove L. D. Ribonuclease activity of stressed tomato leaflets. Plant Physiol. 1967 Sep;42(9):1176–1178. doi: 10.1104/pp.42.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Filner P. Regulation of the nitrate assimilation pathway in cultured tobacco cells. 3. The nitrate uptake system. Biochim Biophys Acta. 1971 Feb 23;230(2):362–372. doi: 10.1016/0304-4165(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Herrera J., Paneque A., Maldonado J. M., Barea J. L., Losada M. Regulation by ammonia of nitrate reductase synthesis and activity in Chlamydomonas reinhardi. Biochem Biophys Res Commun. 1972 Aug 21;48(4):996–1003. doi: 10.1016/0006-291x(72)90707-3. [DOI] [PubMed] [Google Scholar]
- Hsiao T. C. Rapid Changes in Levels of Polyribosomes in Zea mays in Response to Water Stress. Plant Physiol. 1970 Aug;46(2):281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J. The regulation of activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem J. 1966 Sep;100(3):577–588. doi: 10.1042/bj1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W. A., Flesher D., Hageman R. H. Nitrate Uptake by Dark-grown Corn Seedlings: Some Characteristics of Apparent Induction. Plant Physiol. 1973 Jan;51(1):120–127. doi: 10.1104/pp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skujins J. J., McLaren A. D. Enzyme reaction rates at limited water activities. Science. 1967 Dec 22;158(3808):1569–1570. doi: 10.1126/science.158.3808.1569. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Huffaker R. C. Light-induced Development of Polyribosomes and the Induction of Nitrate Reductase in Corn Leaves. Plant Physiol. 1970 Dec;46(6):800–805. doi: 10.1104/pp.46.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Key J. L. Correlation between Polyribosome Level and the Ability to Induce Nitrate Reductase in Dark-grown Corn Seedlings. Plant Physiol. 1971 Nov;48(5):617–620. doi: 10.1104/pp.48.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennesland B., Jetschmann C. The nitrate reductase of Chlorella pyrenoidosa. Biochim Biophys Acta. 1971 Mar 10;227(3):554–564. doi: 10.1016/0005-2744(71)90006-4. [DOI] [PubMed] [Google Scholar]
- WILSON C. M. Chromatographic separation of ribonucleases in corn. Biochim Biophys Acta. 1963 Feb 26;68:177–184. doi: 10.1016/0006-3002(63)90133-1. [DOI] [PubMed] [Google Scholar]
- Warner R. L., Hageman R. H., Dudley J. W., Lambert R. J. Inheritance of nitrate reductase activity in Zea mays L. Proc Natl Acad Sci U S A. 1969 Mar;62(3):785–792. doi: 10.1073/pnas.62.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


