Background: Mitochondria may utilize different proteins to decode high and low cytosolic Ca2+.
Results: Inhibition of SERCA shifts mitochondrial Ca2+ uptake from being UCP3-dependent to Letm1-dependent.
Conclusion: Depending on the mode of intracellular Ca2+ release, two different mitochondrial Ca2+ uptake pathways are engaged.
Significance: The dissection of two molecularly distinct mitochondrial Ca2+ uptake routes depending on SERCA activity points to the complexity of the mitochondrial Ca2+ uptake machinery.
Keywords: Calcium ATPase, Calcium Intracellular Release, Calcium Signaling, Calcium Transport, Mitochondria
Abstract
The transfer of Ca2+ across the inner mitochondrial membrane is an important physiological process linked to the regulation of metabolism, signal transduction, and cell death. While the definite molecular composition of mitochondrial Ca2+ uptake sites remains unknown, several proteins of the inner mitochondrial membrane, that are likely to accomplish mitochondrial Ca2+ fluxes, have been described: the novel uncoupling proteins 2 and 3, the leucine zipper-EF-hand containing transmembrane protein 1 and the mitochondrial calcium uniporter. It is unclear whether these proteins contribute to one unique mitochondrial Ca2+ uptake pathway or establish distinct routes for mitochondrial Ca2+ sequestration. In this study, we show that a modulation of Ca2+ release from the endoplasmic reticulum by inhibition of the sarco/endoplasmatic reticulum ATPase modifies cytosolic Ca2+ signals and consequently switches mitochondrial Ca2+ uptake from an uncoupling protein 3- and mitochondrial calcium uniporter-dependent, but leucine zipper-EF-hand containing transmembrane protein 1-independent to a leucine zipper-EF-hand containing transmembrane protein 1- and mitochondrial calcium uniporter-mediated, but uncoupling protein 3-independent pathway. Thus, the activity of sarco/endoplasmatic reticulum ATPase is significant for the mode of mitochondrial Ca2+ sequestration and determines which mitochondrial proteins might actually accomplish the transfer of Ca2+ across the inner mitochondrial membrane. Moreover, our findings herein support the existence of distinct mitochondrial Ca2+ uptake routes that might be essential to ensure an efficient ion transfer into mitochondria despite heterogeneous cytosolic Ca2+ rises.
Introduction
The ability of mitochondria to respond to cytosolic Ca2+ elevations is fundamental for cell signaling (1). An accumulation of Ca2+ within mitochondria impacts the rate of oxidative phosphorylation (2) and shapes cytosolic Ca2+ signals (3, 4). Notably, since excessive Ca2+ uptake into mitochondria alters the morphology of the organelle and triggers cell death pathways (5), a proper control of mitochondrial Ca2+ sequestration is essential to maintain mitochondrial and cellular homeostasis (6).
Whereas the phenomenon of mitochondrial Ca2+ uptake is well characterized as to be accomplished by the so-called mitochondrial Ca2+ uniporter, the identification of the actual protein(s) that establish mitochondrial Ca2+ uptake is/are still not entirely completed. Moreover, though early patch-clamp studies revealed one Ca2+ current in mitoplasts (7), recent reports point to the existence of several distinct mitochondrial Ca2+ currents across the inner mitochondrial membrane (IMM)5 (8–10), thus, challenging the concept of one sole, ubiquitous, mitochondrial Ca2+ uniporter. In agreement with these findings several proteins of the IMM have been identified as putative mitochondrial Ca2+ carriers and/or components of mitochondrial Ca2+ uptake sites (6, 11). Among these molecules, a 40 kDa protein initially referred to as CCDC109A and then renamed to “mitochondrial calcium uniporter” (MCU) (12, 13) appears to be a promising candidate for a mitochondrial Ca2+ channel (6). MCU, which was suggested to represent the Ca2+ conducting pore in the IMM, was shown to accomplish mitochondrial Ca2+ uptake independently from the source of Ca2+. In contrast, the leucine zipper-EF-hand containing transmembrane protein 1 (Letm1), which was initially described as a K+/H+ exchanger in the IMM (14), was also shown to function as a mitochondrial Ca2+/H+ antiporter (15) that mainly achieved mitochondrial Ca2+ accumulation as a result of slow cytosolic Ca2+ rises, such as those induced by Ca2+ entry via the store-operated Ca2+ entry (SOCE) pathway in endothelial and HeLa cells (16). In the same cell types, however, the novel uncoupling proteins 2 and 3 (UCP2/3) were found to contribute primarily to the instant transfer of intracellularly released Ca2+ into mitochondria while UCP2/3 did not appear to be engaged in mitochondrial Ca2+ uptake upon activation of SOCE (17–19). Such distinct contribution of Letm1 and UCP2/3 to mitochondrial Ca2+ uptake might explain the versatility of mitochondria to decode the various patterns of the cytosolic Ca2+ signal (20), while the actual molecular function of these proteins remain elusive (6, 21). However, a recent study reports that inhibition of the sarco/endoplasmatic reticulum ATPase (SERCA) abrogates the contribution of UCP3 to mitochondrial Ca2+ uptake in HeLa cells (22). Although these findings confirm our previous report, in which mitochondrial Ca2+ uptake following SERCA inhibition was shown to be independent of UCP3 (23), the individual interpretation of these data are very different. On one hand, based on their measurements of cytosolic ATP, De Marchi et al. concluded that UCP3 is not engaged in mitochondrial Ca2+ uptake, but affects the transfer of Ca2+ into mitochondria by impacting the SERCA activity via the modulation of mitochondrial ATP generation (22). On the other hand, our group provided experimental evidence, that the contribution of UCP2/3 is independent of the organelles ATP production (17). Hence, upon SERCA inhibition mitochondrial Ca2+ uptake is accomplished by a CGP37157-sensitive Ca2+ exchanger (23). However, as the potential contribution of a mitochondrial Ca2+ exchanger to mitochondrial Ca2+ uptake under SERCA inhibition was not evaluated by De Marchi et al., the controversial conclusions remain and await clarification.
Therefore, the present study was designed to solve this controversy. Thus, we employed the same cell model (HeLa cells) and evaluated the contribution of UCP3, Letm1 and MCU to mitochondrial and cytosolic Ca2+signaling using a recently developed, mitochondrially targeted red-shifted Ca2+ sensor (24) and fura-2/am. This technique allowed us to follow simultaneously respective Ca2+ signals in both compartments.
EXPERIMENTAL PROCEDURES
Chemicals and Buffer Solutions
Cell culture materials were obtained from PAA laboratories (Pasching, Austria). Thapsigargin was purchased from Abcam® (London, UK), Histamine and EGTA were from Sigma (Vienna, Austria). Prior to experiments cells were washed and maintained for 20 min in a HEPES buffered solution composed of (in mm): 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 1 HEPES, 2.6 NaHCO3, 0.44 KH2PO4, 0.34 Na2HPO4, 10 d-glucose, 0.1% vitamins, 0.2% essential amino acids, and 1% penicillin/streptomycin; pH adjusted to 7.4 with NaOH. During the experiments cells were continuously perfused with a Ca2+ containing buffer, which consisted of (in mm): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES; pH adjusted to 7.4 with NaOH. In experiments where a Ca2+-free solution was applied to the cells, the CaCl2 was replaced with 1 mm EGTA.
siRNAs and Approval of Their Respective Knock-down Efficiency
The siRNAs against human MCU, UCP2/3 and Letm1 were obtained from Microsynth (Balgach, Switzerland) and their nucleotide sequences (5′-3′) were as follows: si1-hMCU: GCCAGAGACAGACAAUACUtt; si2-hMCU: GGAAAGGGAGCUUAUUGAAtt; si1-hLetm1: UCCACAUUUGAGACUCAGUtt; si2-hLetm1: AUGUUCCAUUUGGCUGCUGtt; si-hUCP2: GCACCGUCAAUGCCUACAAtt; si-hUCP3: GGAACUUUGCCCAACAUCAtt.
For controls, a scrambled siRNA was used: UUCUCCGAACGUGUCACGUtt. Although all siRNAs used in this study have been previously approved to exhibit reliable gene knock-down efficiency (16), their efficiency was again verified by quantitative RT-PCR in HeLa as previously described (25).
Total RNA was isolated from control and target siRNA treated HeLa cells using a RNA isolation kit (PEQLAB Biotechnologie GmbH, Erlangen, Germany). Reverse transcription was carried out using a cDNA synthesis kit from Applied Biosystems. The efficiency of siRNA was validated by performing Real time PCR using QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) on LightCycler 480 (Roche Diagnostics, Vienna, Austria). RNA polymerase II (RPOL2) was used as housekeeping control. Primers for RPOL2, UCP2, UCP3, MCU, and LETM1 were obtained from Invitrogen (Vienna, Austria) and there sequences (5′-3′) are as follows: RPOL2 for: CATTGACTTGCGTTTCCACC, RPOL2 rev: ACATTTTGTGCAGAGTTGGC, UCP2 for: TCCTGAAAGCCAACCTCATG, UCP2 rev: GGCAGAGTTCATGTATCTCGTC, UCP3 for: AGAAAATACAGCGGGACTATGG, UCP3 rev: CTTGAGGATGTCGTAGGTCAC, MCU for: TTCCTGGCAGAATTTGGGAG, MCU rev: AGAGATAGGCTTGAGTGTGAAC, Letm1 for: TGTTCTTCAAGGCCATCTCC, Letm1 rev: TGTTGCTGTGAAGCTCTTCC. The expression data were analyzed by ΔΔCt method as described previously (25). Knock-down efficiency was in the same range than previously reported for endothelial cells (16).
Cell Culture and Transfection
HeLa cells were cultured as described previously (24). Briefly, cells were grown in Dulbeccos's Modified Eagle Medium (Sigma, Vienna, Austria) containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin and were plated on 30-mm glass coverslips. At 60–80% confluency, cells were transfected with 2 μg (per 30-mm well) of plasmid DNA encoding 4mtD1GO-Cam (24) alone or in combination with 100 μm siRNA using 4 μg/well TransFastTM transfection reagent (Promega, Madison, WI) in 0.5 ml of serum and antibiotic-free transfection medium. Cells were maintained in a humidified incubator (37 °C, 5% CO2, 95% air) for 16–20 h before changing back to complete RPMI 1640 medium. All experiments were performed either 48 h or 72 h after transfection.
Simultaneous Cytosolic and Mitochondrial Ca2+ Measurements
4mtD1GO-Cam (24) transfected HeLa cells were loaded with 2 μm fura-2/AM (TEFLabs, Austin, TX) for 45 min prior to the experiments. Co-imaging of fura-2 and the 4mtD1GO-Cam was achieved with a digital wide field imaging system, the Till iMIC (Till Photonics Graefelfing, Germany) using a 40× objective (alpha Plan Fluar 40×, Zeiss, Göttingen, Germany). For illumination of fura-2 and the 4mtD1GO-Cam an ultra fast switching monochromator, the Polychrome V (Till Photonics) equipped with an excitation filter (E500spuv, Chroma Technology Corp., Rockingham, Vermont) and a dichroic filter (495dcxru, Chroma Technology Corp) was used. fura-2 was excited alternatively at 340 nm and 380 nm and the red-shifted mitochondrial-targeted cameleon was excited at 477 nm, respectively. Emitted light was simultaneously collected at 510 nm (fura-2 and GFP of GO-Cam) and at 560 nm (FRET-channel of GO-Cam) using a single beam splitter design (Dichrotome, Till Photonics) that was equipped with a dual band emission filter (59004m ET Fitc/Tritc Dual Emitter, Chroma Technology Corp.) and a second dichroic filter (560dcxr, Chroma Technology Corp.). Images were recorded with a charged-coupled device (CCD) camera (AVT Stringray F145B, Allied Vision Technologies, Stadtroda, Germany). For the data acquisition and the control of the digital fluorescence microscope the live acquisition software (LA) version 2.0.0.12 (Till Photonics) was used. Post-acquisition image analysis was performed on MetaMorph 7.7.0.0 (Visitron Systems, Puchheim, Germany).
Statistics
Data shown represent the mean ± S.E., where n reflects the number of cells. Statistical analyses were performed with unpaired Student's t test, and p < 0.05 was considered to be significant.
RESULTS
SERCA Inhibition Slows Down the IP3-mediated Transfer of Ca2+ into Mitochondria
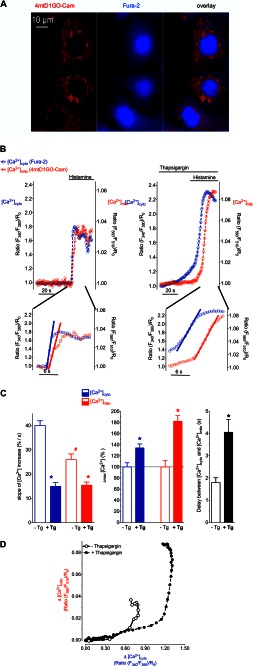
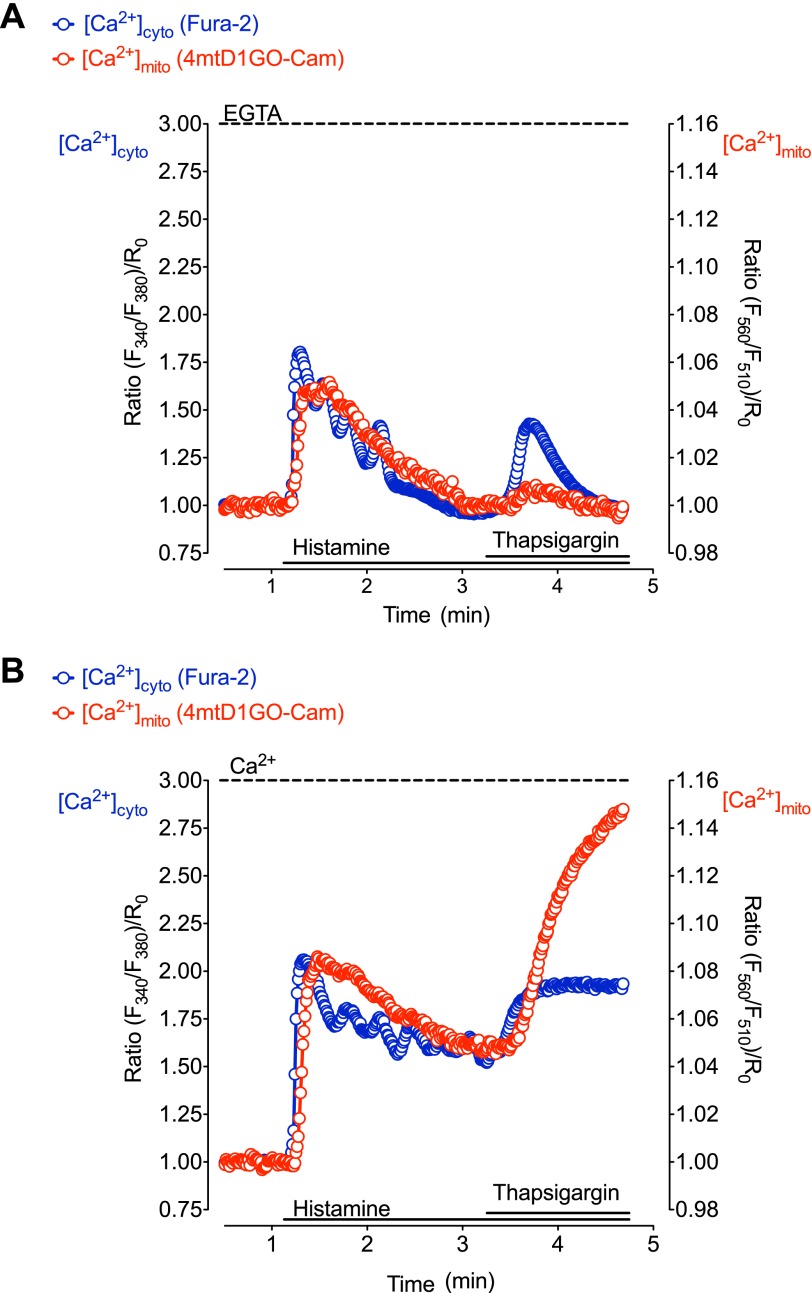
[Ca2+]cyto and [Ca2+]mito were simultaneously measured using Fura-2/AM-loaded cells that transiently expressed 4mtD1GO-Cam, a recently developed red shifted genetically encoded Ca2+ probe targeted to the mitochondrial matrix (Fig. 1A). This approach allowed an accurate temporal correlation of changes in [Ca2+]cyto with [Ca2+]mito. Stimulation with the IP3-generating agonist histamine in the absence of extracellular Ca2+ induced a fast increase of both cytosolic and mitochondrial Ca2+ levels (Fig. 1B, left panel), indicating an efficient transfer of Ca2+ from the endoplasmic reticulum (ER) into mitochondria. The Ca2+ signal in the cytosol occurred slightly faster than the respective Ca2+ elevation within mitochondria of same single individual cells (Fig. 1, B, left panel & C). Pretreating the cells with the SERCA inhibitor thapsigargin 40 s prior to the addition of histamine, enhanced the cytosolic Ca2+ elevation (Fig. 1, B, right panel & C). Notably, in the presence of thapsigargin, [Ca2+]cyto started to increase slowly, indicating Ca2+ leakage from the ER. This weak thaspsigargin-induced cytosolic Ca2+ signal was not accompanied by a significant elevation in [Ca2+]mito (Fig. 1B, right panel). Subsequent addition of histamine evoked a further pronounced rise of both [Ca2+]cyto and [Ca2+]mito. However, these signals increased with a slower kinetics compared with respective Ca2+ elevations in the absence of the SERCA inhibitor (Fig. 1, B & C). In addition, the time gap between the histamine induced rise of [Ca2+]cyto and the respective mitochondrial Ca2+ signal was considerably extended in the presence of thapsigargin (Fig. 1, B & C), indicating that SERCA inhibition decelerates the transfer of Ca2+ into mitochondria upon IP3-mediated Ca2+ release. A correlation between changes of [Ca2+]cyto and respective Ca2+ signals within mitochondria showed that in the presence of thapsigargin almost twice as much cytosolic Ca2+ was elevated, until mitochondrial Ca2+ uptake was activated (Fig. 1D).
FIGURE 1.
SERCA inhibition prior to IP3-mediated Ca2+ release impacts the kinetics of Ca2+ signals and coupling between [Ca2+]cyto and [Ca2+]mito. A, HeLa cells expressing the mitochondrial Ca2+ sensor 4mtD1GO-Cam (red) were loaded with fura-2/AM (blue). Images were taken with a fully automated fluorescence microscope using a camera binning of 4. B, upper panels: representative traces of cytosolic (blue curves) and mitochondrial (red curves) Ca2+ signals in HeLa cells upon stimulation with 100 μm histamine in the absence of Ca2+ (left upper panel). SERCA inhibition was achieved by using 1 μm thapsigargin that was added 40 s prior to cell treatment with histamine (right upper panel). Data are expressed as normalized ratios: (F340/F380)/R0 for [Ca2+]cyto and (F560/F510)/R0 for[Ca2+]mito. R0 was calculated from basal ratio values for each individual cell respectively. Lower panels: zoom-in of upper panels showing which part was used for calculating the slope of Ca2+ increase. Following onset, each curve was fitted with linear regression (bold lines) to assess maximal slope of cytosolic and mitochondrial [Ca2+] elevations. C, statistical evaluation of the Ca2+ signals presented in panel B. Left panel: columns represent the maximal slopes of [Ca2+]cyto (blue columns) and [Ca2+]mito (red columns) increases upon histamine stimulation in the absence (white columns, n = 18) or presence of thapsigargin pretreatment (filled columns, n = 15). Middle panel: columns represent the average of maximum delta ratios in the absence (white columns) or prescence of thapsigargin preincubation (filled columns). Cytosolic and mitochondrial [Ca2+] elevation in response to cell treatment with 100 μm histamine was defined as 100%, respectively (B, left panel). Right panel: lag times in seconds between cytosolic and respective mitochondrial Ca2+ rises in the absence of thapsigargin (white column, n = 18) and upon pretreatment with thapsigargin (black column, n = 15). *, p < 0.05 versus in the absence of thapsigargin (−Tg), #, p < 0.05 versus [Ca2+]cyto in the absence of thapsigargin (−Tg). D, representative temporal correlations between histamine-induced (100 μm) changes of [Ca2+]cyto (x axis) and [Ca2+]mito (y axis) in the absence of thapsigargin (continuous line with open circles) and upon pretreatment with the SERCA inhibitor (dotted line with filled circles).
These protocols that illustrate the distinct kinetics of the compartmental Ca2+ rises and coupling between [Ca2+]cyto and [Ca2+]mito were subsequently used to investigate the contribution of the individual proteins that have been proposed to be involved in mitochondrial Ca2+ uptake (i.e. UCP3, Letm1, and MCU).
SERCA Inhibition Switches Mitochondrial Ca2+ Uptake from a UCP3-dependent into a Letm1-dependent Mode
We speculated that the SERCA-dependent differences in the kinetics of mitochondrial Ca2+ signals probably reflect the involvement of distinct mitochondrial Ca2+ uptake routes. Therefore, we performed experiments, in which the contribution of the mitochondrial proteins UCP2/3, Letm1 and MCU to mitochondrial Ca2+ uptake in various protocols was investigated by diminution of these proteins with a transient transfection of the respective siRNA. The siRNAs against UCP2/3, Letm1, and MCU have already been validated to specifically and significantly reduce mRNA level of the respective proteins (16).
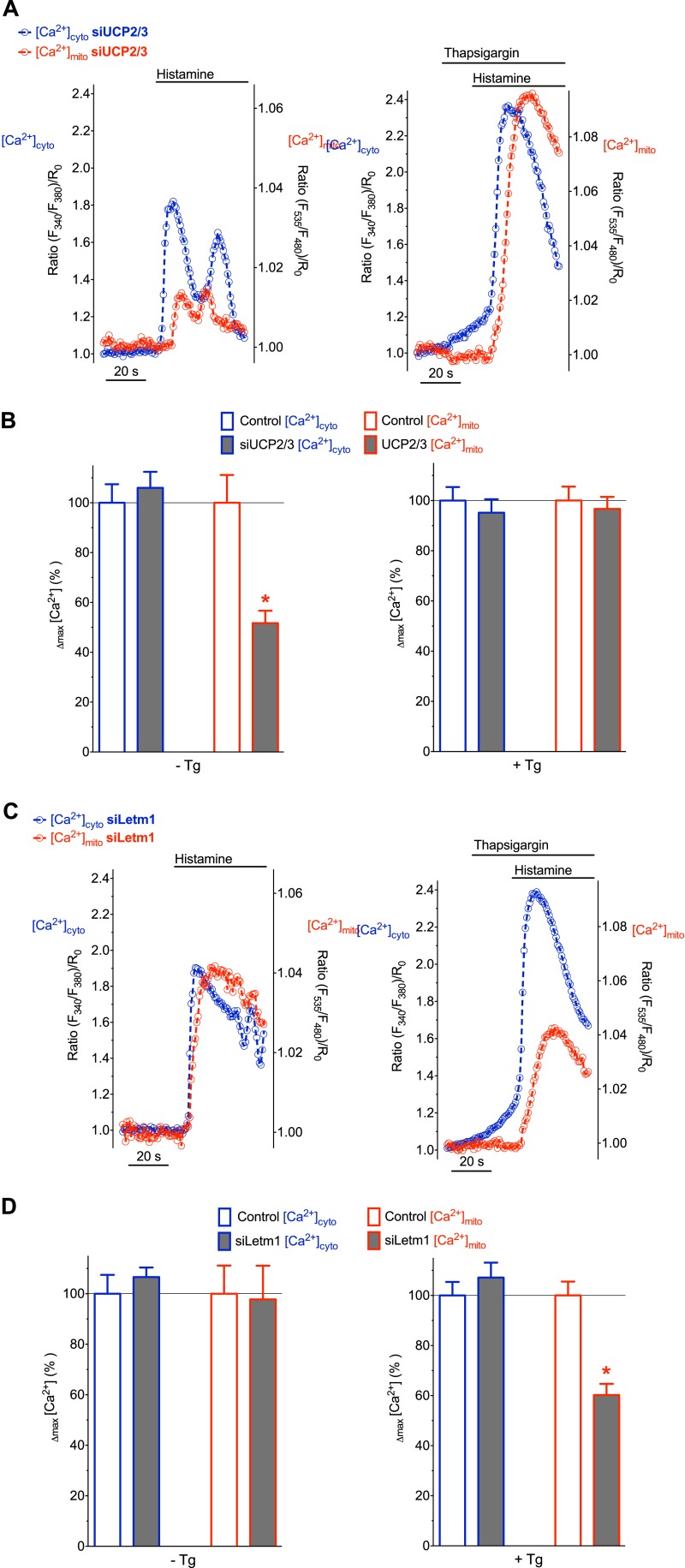
In line with previous studies (17, 18, 22), a transient knock-down of UCP3 significantly reduced the histamine-induced mitochondrial Ca2+ signal, while the respective cytosolic Ca2+ elevation was only minimally affected (Fig. 2, A, left panel & B). As recently demonstrated (22), SERCA inhibition with thapsigargin, which was added shortly before histamine, abolished the effect of UCP3 knock-down on [Ca2+]mito (Fig. 2, A, right panel & B).
FIGURE 2.
Depending on the SERCA activity either UCP2/3 or Letm1 contribute to mitochondrial Ca2+ uptake. A, representative recordings of [Ca2+]cyto (blue) and [Ca2+]mito (red) in single individual HeLa cells transfected with siRNAs against UCP2/3. Cells were treated with 100 μm histamine alone (left panel) or together with 1 μm thapsigargin (right panel). B, column statistics of histamine-evoked cytosolic (blue-bordered columns) and mitochondrial (red-bordered columns) Ca2+ signals in HeLa cells transfected either with scrambled siRNA (control, white columns) or with siRNA against UCP2/3 (UCP2/3, gray columns). Experiments were performed in the absence (left panel, n = 18 for control and n = 19 for siUCP2/3) or in the presence of 1 μm thapsigargin pretreatment (right panel, n = 15 for control and n = 17 for siUCP2/3). The delta maximum of normalized cytosolic and mitochondrial Ca2+ signals were defined as 100% under control conditions (i.e. cells transfected with scrambled siRNA as shown in Fig. 1C) both in the absence (left panel) and presence (right panel) of thapsigargin, *, p < 0.05 versus Control [Ca2+]mito C, simultaneous, representative recordings of [Ca2+]cyto (blue) and [Ca2+]mito (red) in HeLa cells transfected with siRNA against Letm1. Ca2+ signals were evoked with 100 μm histamine in the absence (left panel) or presence (right panel) of 1 μm thapsigargin. D, column statistics of cytosolic and mitochondrial Ca2+ signals in HeLa cells transfected with siRNA against Letm1 (gray columns) without thapsigargin (left panel, n = 19) and upon pretreatment with thapsigargin (right panel, n = 17). White columns represent control conditions as indicated in panel B. *, p < 0.05 versus Control [Ca2+]mito.
Next we performed analogous experiments with cells, in which Letm1 was silenced (Fig. 2, C & D). Mitochondrial Ca2+ sequestration upon IP3-mediated Ca2+ mobilization in the absence of thapsigargin was not affected in cells that were treated with siRNA against Letm1 (Fig. 2, C, left panel & D). In contrast, if SERCA activity prior to the addition of the agonist was blocked, diminution of Letm1 strongly reduced the histamine-induced mitochondrial Ca2+ signal (Fig. 2, C, right panel & D). Notably, the siRNA-mediated knock-down of UCP2/3, Letm1, and MCU neither affected the mitochondrial membrane potential (supplemental Fig. S1), nor the capacity of mitochondria to extrude Ca2+ (supplemental Fig. S2). These data indicate that SERCA inhibition switches the mode of mitochondrial Ca2+ uptake from a Letm1-independent to a Letm1-dependent one and, hence, explain the lacking contribution of UCP2/3 to mitochondrial Ca2+ uptake under conditions of SERCA inhibition.
MCU Contributes to Mitochondrial Ca2+ Uptake Independently from SERCA Activity and, Hence, the Mode of Ca2+ Mobilization
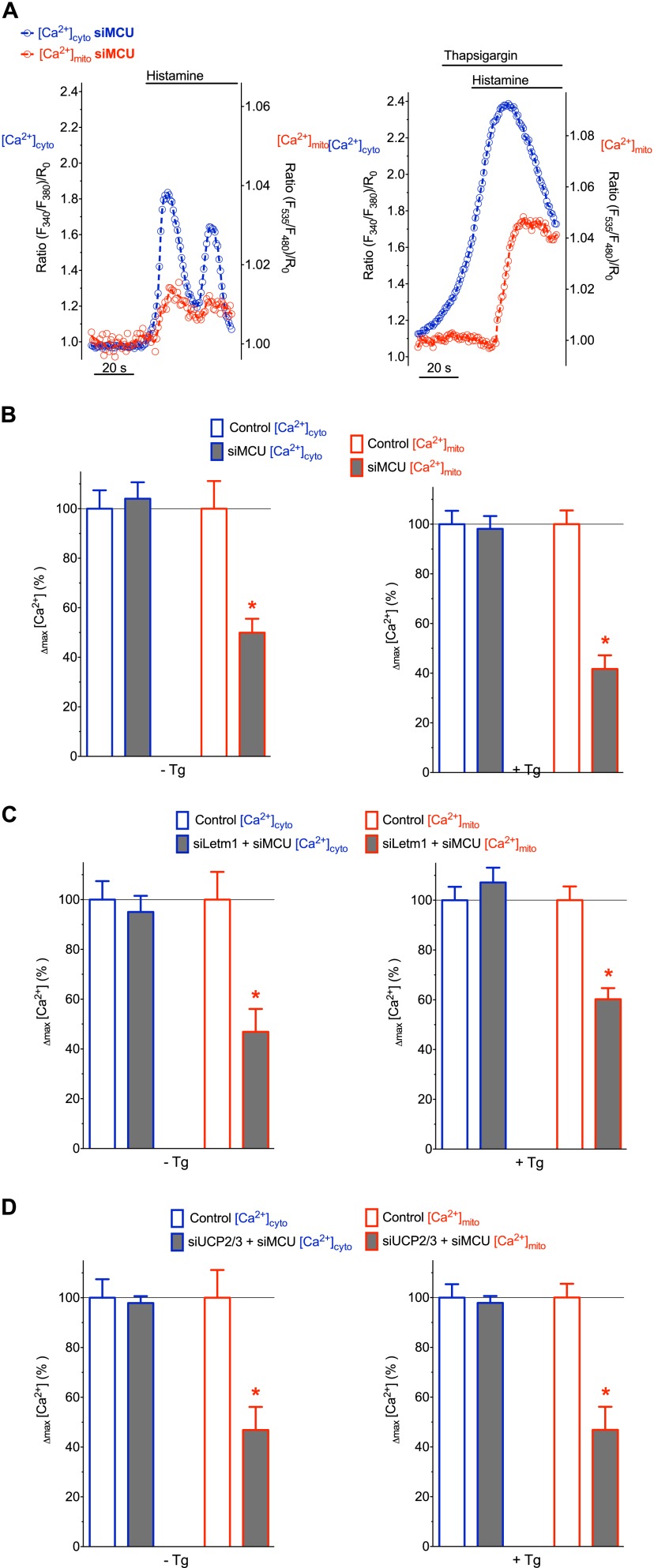
To investigate the participation of MCU in the transfer of intracellularly released Ca2+ into mitochondria, respective experiments were performed with MCU-depleted cells. The knock-down of MCU negligibly altered cytosolic Ca2+ signals in response to cell stimulation (Fig. 3A & Fig. 5B). However, respective mitochondrial Ca2+ signals were strongly diminished in cells depleted of MCU (Fig. 3, A & B). Notably, the inhibitory effect of MCU knock-down on mitochondrial Ca2+ accumulation was independent of the absence or presence of thapsigargin (Fig. 3), indicating that MCU facilitates mitochondrial Ca2+ uptake independently of SERCA activity and, hence, the mode of Ca2+ mobilization.
FIGURE 3.
Transient knock-down of MCU results in a diminished mitochondrial Ca2+ uptake independently from SERCA activity. A, representative curves of [Ca2+]cyto (blue) and [Ca2+]mito (red) in HeLa cells transfected with siRNA against MCU that were treated with 100 μm histamine in the absence of thapsigargin (left panel) and upon pretreatment with the SERCA inhibitor (right panel). B, column statistics of cytosolic (blue border columns) and mitochondrial Ca2+ (red-bordered columns) signals in control HeLa cells (white columns) and cells transfected with siRNA against MCU (gray columns) without thapsigargin (left panel, n = 24) and upon pretreatment with thapsigargin (right panel, n = 14). White columns represent control conditions (defined as 100%) as indicated in Fig. 2. *, p < 0.05 versus Control [Ca2+]mito. C, HeLa cells transfected with siRNA against Letm1 and MCU were stimulated with histamine in the absence (left panel, n = 18) and presence (right panel, n = 14) of thapsigargin in experimental conditions indicated in panel A. D, HeLa cells transfected with siRNA against UCP2/3 and MCU were stimulated with histamine in the absence (left panel, n = 27) and presence (right panel, n = 14) of thapsigargin in experimental conditions indicated in panel A.
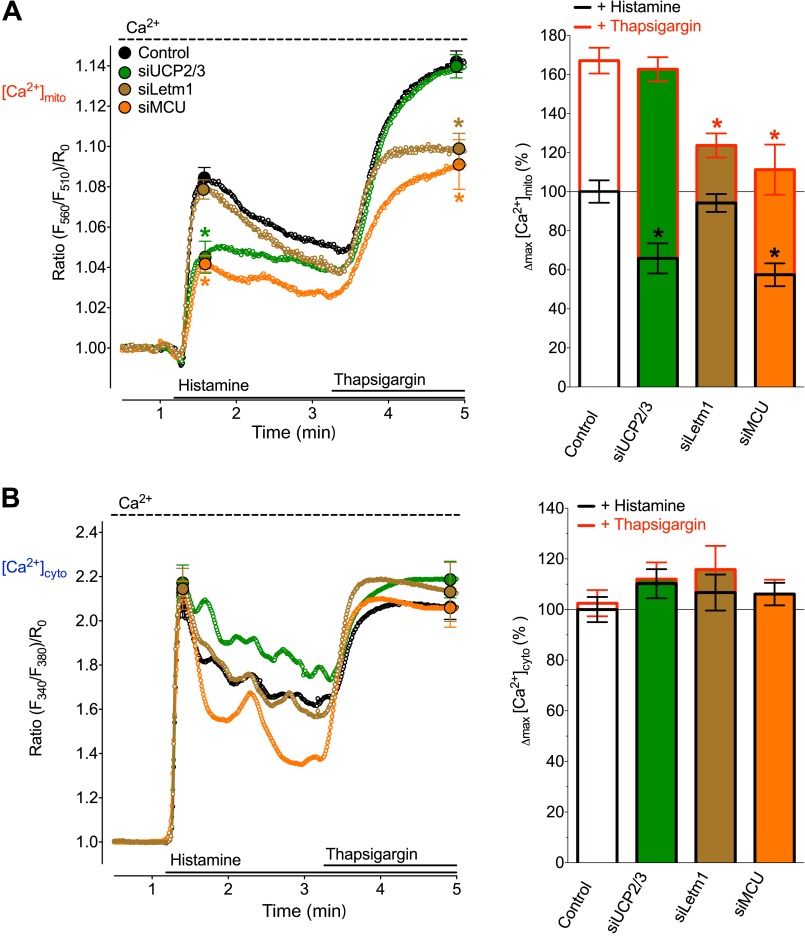
FIGURE 5.
SERCA inhibition during cell stimulation with histamine switches mitochondrial Ca2+ uptake from being UCP2/3 and MCU dependent to an UCP2/3 independent, but Letm1 and MCU-dependent mode. HeLa cells were either transfected with an inoperative Control siRNA (black curves and white columns, n = 18), or siRNAs against UCP2 and UCP3 (green curves and green columns, n = 18), or Letm1 (brown curves and brown columns, n = 20) or MCU (orange curves and orange columns, n = 19). A, average curves (left panel) and statistical data (right panel) of [Ca2+]mito signals measured with 4mtD1GO-Cam upon cell treatment with 100 μm histamine and the subsequent addition of 1 μm thapsigargin in the presence of 2 mm Ca2+. *, p < 0.05 versus respective Controls (B). Respective cytosolic Ca2+ curves (left panel) and statistical analysis of Δmax [Ca2+]cyto values (right panel) from Fura-2 signals of HeLa cells that were treated as indicated in Fig. 1A.
A simultaneous knock-down of either MCU and UCP2/3 or MCU and Letm1 did not further reduce mitochondrial Ca2+ uptake under the various conditions (Fig. 3, C and D & supplemental Fig. S3), thus, pointing to a functional interaction between these putative contributors of mitochondrial Ca2+ uptake.
SERCA Inhibition Following IP3-mediated Ca2+ Release Increases SOCE and Also Abrogates the Contribution of UCP2/3 to MCU-mediated Mitochondrial Ca2+ Uptake, While Letm1 Gets Involved
SERCA inhibition prior to cell stimulation with histamine partially emptied the internal Ca2+ store that results in a decelerated, but higher cytosolic Ca2+ signal and a greater delay of the Ca2+ transfer into the mitochondria (Fig. 1B, right panel) suggesting a shift in the mode/route of mitochondrial Ca2+ uptake.
To test whether or not this change in mitochondrial Ca2+ uptake mode/route also occurs under condition in which the cell is already stimulated by an IP3-generating agonist, we performed different experimental protocols, in which thapsigargin was added after histamine. If cells were continuously exposed to the IP3 generating agonist in the absence of extracellular Ca2+, the subsequent SERCA inhibition transiently elevated [Ca2+]cyto (Fig. 4A). This transient thapsigargin-induced increase of cytsolic Ca2+ levels evoked only small changes of [Ca2+]mito. In contrast, SERCA inhibition following IP3-mediated Ca2+ release in the presence of extracellular Ca2+-induced prominent, longer-lasting elevations of both [Ca2+]cyto and [Ca2+]mito (Fig. 4B), thus, highlighting the involvement of SOCE that promotes mitochondrial Ca2+ accumulation. The latter protocol was further used to test the contribution of UCP3, Letm1, and MCU to mitochondrial Ca2+ sequestration under these conditions of Ca2+ mobilization.
FIGURE 4.
SERCA inhibition during cell stimulation with an IP3-generating agonist enhances [Ca2+]cyto and [Ca2+]mito. Representative curves demonstrating simultaneous measurement of [Ca2+]cyto (blue) and [Ca2+]mito (red) in HeLa cells loaded with fura-2/AM and transiently transfected with 4mtD1GO-Cam. A, cells were treated with 100 μm histamine for 2 min before the addition of 1 μm thapsigargin in the absence of extracellular Ca2+. B, cells were treated with 100 μm histamine for 2 min before the addition of 1 μm thapsigargin in the presence of 2 mm Ca2+ in the extracellular medium.
Consistent with our previous work and the experiments in the absence of extracellular Ca2+ (Fig. 2, B and D) cells depleted of UCP3 showed greatly reduced mitochondrial Ca2+ accumulation in response to histamine (Fig. 5A). The subsequent addition of thapsigargin evoked a substantial rise of [Ca2+]mito that was not affected by the diminution of UCP3.
In contrast, knock-down of Letm1 negligibly influenced mitochondrial Ca2+ uptake that was elicited by histamine, whereas [Ca2+]mito was significantly reduced in response to a subsequent SERCA inhibition (Fig. 5A). These findings demonstrated that in protocols in which SERCA was blocked after the initiation of IP3-mediated Ca2+ release, mitochondrial Ca2+ uptake also switched from an UCP3-reliant to a Letm1-dependent mode.
Consistent with previous experiments, cells that were treated with siRNA against MCU showed attenuated mitochondrial Ca2+ signals in response to histamine and to the subsequent addition of thapsigargin (Fig. 5A). Notably, the initial cytosolic Ca2+ peak and the thapsigargin induced rise remained unaffected under all conditions (Fig. 5B).
DISCUSSION
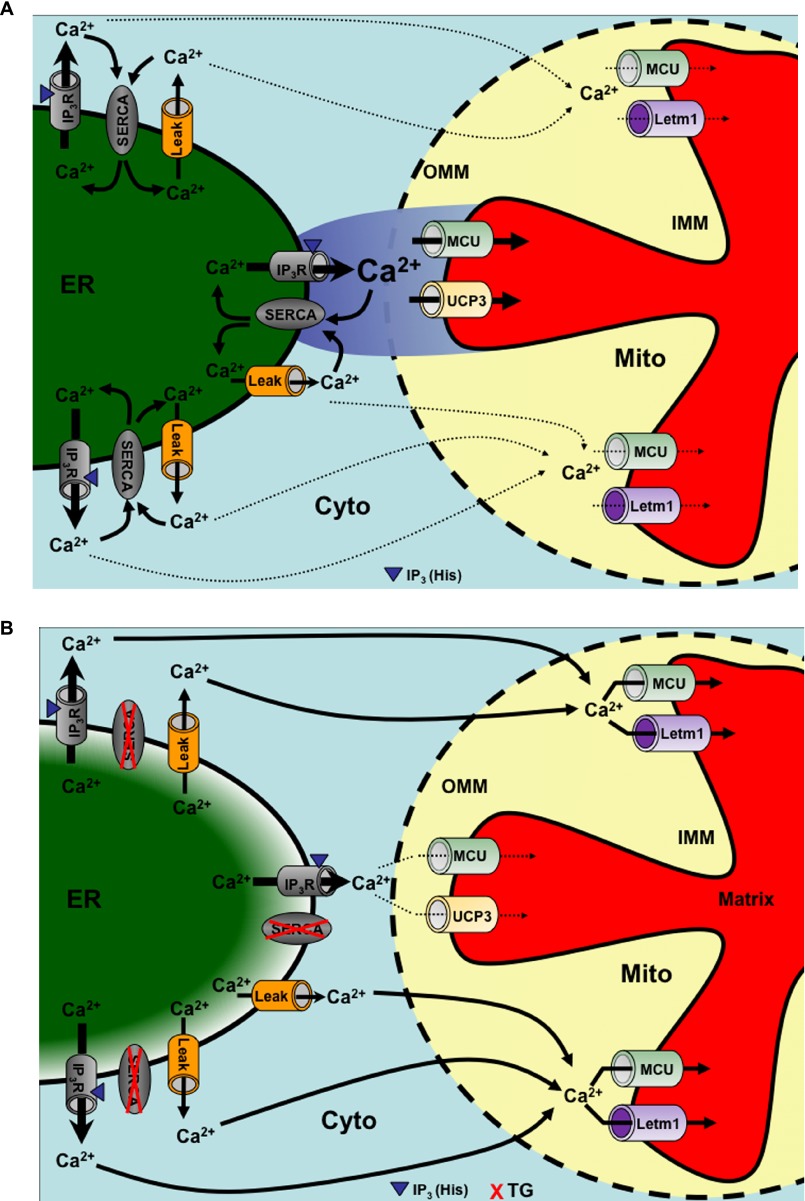
Our present data reveal that IP3-mediated, rapid Ca2+ rises, that are associated with the generation of high Ca2+ micro-domains at the surface of mitochondria (26) were transferred into these organelles via an UCP3- and MCU-dependent but Letm1-independent pathway (Fig. 6A). However, if the mode of Ca2+ mobilization was decelerated by SERCA inhibition, which increased ER Ca2+ leakage and, thus, probably attenuated the inter-organelle Ca2+ micro-domains, mitochondria slowly accumulated Ca2+ via an UCP3-independent, but Letm1- and MCU-reliant route (Fig. 6B).
FIGURE 6.
Schematic illustration of a hypothetical switch of mitochondrial Ca2+ uptake by SERCA inhibition upon IP3-mediated Ca2+ release. A, SERCA activity counteracting ER Ca2+ leakage and recycling Ca2+ into the ER supports the generation of high Ca2+ micro-domains upon IP3-mediated ER Ca2+ release. Under these conditions Ca2+ hot spots at close sites of mitochondrial Ca2+ uptake are sensed by UCP2/3 and MCU, which accomplish the transfer of Ca2+ across the IMM. B, impaired SERCA activity yields partial ER Ca2+ depletion counteracting the generation of high Ca2+ micro-domains at mitochondrial contact sites. Under these conditions the global slow cytosolic Ca2+ elevation is partially transferred into mitochondria by Letm1 and MCU. Arrows indicate Ca2+ fluxes.
Basically, our current findings indicate that a modification of the IP3-mediated Ca2+ release by SERCA inhibition significantly alters the molecular characteristics of mitochondrial Ca2+ uptake. At first glance, these findings are not surprising, considering the central role of ER Ca2+ pumps in the control of the cellular Ca2+ homeostasis (27, 28). Virtually, in all cells, SERCA activity is necessary to maintain high Ca2+ levels within the ER by counteracting Ca2+ leakage and to restore Ca2+ upon events of ER Ca2+ release (29, 30). In line with these reports, our measurements show that a combination of the SERCA inhibitor with the IP3-generating agonist boosts the total increases in global cytosolic and mitochondrial Ca2+ signals, thus, further supporting the hypothesis that during IP3-mediated ER depletion, SERCA activity counteracts cytosolic Ca2+ rises under control conditions (31).
One possible explanation for this obvious switch in the mode of mitochondrial Ca2+ uptake is an attenuation of high Ca2+ micro-domains in the inter-organelle gap between the ER and mitochondria upon inhibition of SERCA. High Ca2+ micro-domains at ER-mitochondria contact sites were supposed to be fundamental for the activation of the low Ca2+ sensitive mitochondrial Ca2+ uniport upon IP3-mediated ER Ca2+ release (32–34). In sophisticated studies the existence of such high Ca2+ micro-domains, also referred to as Ca2+ hot spots, on sites of mitochondrial Ca2+ uptake has been recently demonstrated (26, 35). Although we did not measure local Ca2+ hot spots, based on the clear effects of the SERCA activity on global cytosolic Ca2+ signals we reported herein, it is likely that an acute deactivation of ER Ca2+ pumps prior to or during the activation of the IP3-mediated pathway also considerably impedes the formation of such local Ca2+ domains. In agreement with this assumption, our data demonstrate that a loss of SERCA activity in HeLa cells instantly increases a leak of Ca2+ from the ER resulting in a slow and moderate cytosolic Ca2+ elevation that is accompanied with a tiny but measurable increase in [Ca2+]mito. Due to the slightly lower Ca2+ affinity of the cameleon probe used for measuring [Ca2+]mito (1.53 μm, (24)) than that of fura-2 (224 nm, (36)), the mitochondrial Ca2+ elevation upon thapsigargin might be underestimated. However, these data clearly show that despite the massive and pronounced ER depletion and its associated slow, but considerable cytosolic Ca2+ elevation, mitochondrial Ca2+ elevation remains small. Accordingly, the ER Ca2+ leakage might be locally facilitated by neighboring mitochondria that sequester and buffer the leaked Ca2+ from the inter-organelle gap and, thus, maintain the great Ca2+ gradient. Consequently, such phenomenon might result in an accelerated local ER Ca2+ depletion in ER regions that are in the vicinity of mitochondria. Such scenario would explain the decelerated cytosolic Ca2+ rise in response to IP3-mediated Ca2+ mobilization upon the short preincubation with a SERCA inhibitor. Under such conditions, the IP3-triggered formation of inter-organelle Ca2+ hot spots is hampered, thus, a mitochondrial Ca2+ carrier such as UCP3 that, might require high Ca2+ domains to be activated due to its low Ca2+ sensitivity (19) is inactive.
In addition, the increased Ca2+ leak from the ER upon SERCA inhibition might per se impact the transfer of Ca2+ into mitochondria. Such a scenario is feasible, as it was shown that an increased ER Ca2+ leakage in cells expressing an inactive truncated version of SERCA1 inhibited mitochondrial movements and increased ER-mitochondria contact sites, which consequently led to mitochondrial Ca2+ overload (37). However, our data showed that the thapsigargin induced Ca2+ leak in HeLa cells is only slowly and moderately transferred into mitochondria. Nevertheless, the increased Ca2+ leak upon SERCA inhibition might indeed affect the local organization and architecture of ER-mitochondria contact sites and, thus, the mode of mitochondrial Ca2+ uptake (38–41). Notably, using genetically encoded linker proteins that allowed a definite tethering of the ER to mitochondria indicated that the distance of the gap between both organelles is determent for the ability of mitochondria to sense Ca2+ hot spots upon ER Ca2+ release (42).
Irrespective of high local Ca2+ signals at inter-organelle junctions between mitochondria and the ER, mitochondria have been shown to accomplish also the uptake of smooth, moderate cytosolic Ca2+ elevations (18, 32–34). The coexistence of both rapid mitochondrial uptake of high Ca2+ micro-domains and mitochondrial Ca2+ sequestration of global slow rising Ca2+ signals is consistent with our data presented in this study. Moreover, our findings highlight a clear delay between cytosolic and respective mitochondrial Ca2+ signals, particularly if the IP3-mediated cytosolic Ca2+ elevation was decelerated by SERCA inhibition. Accordingly, we assumed that the slow and delayed mitochondrial Ca2+ accumulation in the presence of the SERCA inhibitor exhibits a specific mode of Ca2+ transfer across the IMM, which is distinct from mitochondrial Ca2+ uptake during fast IP3-mediated ER Ca2+ release. This “slow” presumably highly sensitive type of mitochondrial Ca2+ accumulation in the presence of thapsigargin might be comparable with mitochondrial uptake of Ca2+ entering the cell via the SOCE pathway (19). Notably, it was shown that mitochondria in HeLa cells are not exposed to Ca2+ hot spots in response to SOCE (26). These findings are also consistent with our previous study using endothelial cells, which showed a diffusion dependent and, hence, slow mode of mitochondrial Ca2+ sequestration if Ca2+ was mobilized via the SOCE pathway (18). In this endothelial cell model we unveiled that the slow mode of mitochondrial Ca2+ sequestration upon SOCE especially requires Letm1, while UCP2/3 contributed exclusively to fast mitochondrial uptake of Ca2+ that was mobilized via the IP3 pathway (16). These findings are in line with the observed switch of mitochondrial Ca2+ uptake from an UCP3-dependent to a Letm1-dependent mode upon SERCA inhibition in HeLa cells, we reported herein.
Letm1 as well as UCP2/3 were described to accomplish the transfer of Ca2+ into mitochondria, while their functioning and contribution to mitochondrial Ca2+ uptake is debated (6, 11, 21, 43, 44). Despite strong functional data, the concerns against the idea that Letm1 and UCP2/3 indeed accomplish a transfer of Ca2+ across the IMM, are primarily based on the postulation of a unique, ubiquitous Ca2+ uniporter being a low sensitive Ca2+ channel protein that is activated exclusively by high Ca2+ signals. A recently identified protein of the IMM, referred to as MCU, was shown to fulfill some of the criteria that have been expected for a protein that accomplishes mitochondrial Ca2+ uniport (12, 13). In agreement with these landmark publications, siRNA-mediated knock-down of MCU attenuated mitochondrial Ca2+ signals independently from the mode of Ca2+ mobilization in the present study. These observations indicate that MCU is activated over a large range of Ca2+ concentration and, hence, contributes to mitochondrial uptake of high and low cellular Ca2+ signals, which is, however, in disagreement with the low Ca2+ sensitivity of the mitochondrial Ca2+ uniport phenomenon (45, 46).
Notably, in several reports elimination of the mitochondrial Ca2+ buffering increases cytosolic Ca2+ peak and accelerates its decline (13, 47, 48). However, our findings are in agreement with other reports using the same cell type (HeLa) (17, 49, 50) where inhibition of mitochondrial Ca2+ uptake failed or only slightly affected cytosolic Ca2+ elevation upon stimulation, while the kinetics of decline remained unchanged. Accordingly, these data may indicate that in the cell type used herein, mitochondria have a rather low Ca2+ buffer capacity and do not accumulate large proportions of released Ca2+, thus, resulting in the lack of strong changes in cytosolic Ca2+ signaling by MCU knock-down.
The contribution of the MCU, to mitochondrial Ca2+ uptake was shown to be tightly regulated by an associated protein, referred to as mitochondrial calcium uptake 1 (MICU1) (51) that, in contrast to MCU, has Ca2+ binding domains. Initially, it was reported that MICU1 facilitates MCU-dependent mitochondrial Ca2+ uptake in HeLa (51) and clonal pancreatic beta-cells (25) but not endothelial cells (16). However, in a very recent study a contrary function of MICU1 in HeLa and endothelial cells acting as a gatekeeper and, thus, preventing MCU-dependent mitochondrial Ca2+ loads was unveiled (52), thus, indicating that further studies are necessary to understand the definite role of MICU1 in the control of mitochondrial Ca2+ uptake. Nevertheless, the intricate regulation of the MCU activity by MICU1 and other associated proteins such as the recently identified mitochondrial calcium uniporter regulator 1 (MCUR1) (53) might explain why the MCU catalyzes mitochondrial Ca2+ uptake of both high and low Ca2+ signals. From this point of view our data might also indicate that depending on the SERCA activity either UCP2/3 or Letm1 contribute to mitochondrial Ca2+ uptake by modulating the activity of MICU1, MCUR1, and/or the MCU. This assumption is further supported by our observation that a double knock-down of either MCU and UCP2/3 or MCU and Letm1 did not further impact mitochondrial Ca2+ accumulation. The lack of any further reduction of mitochondrial Ca2+ uptake in MCU depleted cells by an additional knock-down of either UCP2/3 or Letm1 also suggest that these proteins might function as upstream regulators of the MCU. However, whether or not the remaining uptake under such conditions indicates a so far unknown additional mitochondrial Ca2+ carrier or is due to incomplete diminution of the proteins by the siRNA remains unclear.
The findings that SERCA inhibition abrogates the contribution of UCP3 to mitochondrial Ca2+ uptake in HeLa cells was recently interpreted as an indication that UCP3 do not accomplish mitochondrial Ca2+ uniport or directly modulate a mitochondrial Ca2+ channel (22). The authors suggested that UCP3 reduces SERCA activity by limiting mitochondrial ATP generation, which increases the amount of Ca2+ at sites of mitochondrial Ca2+ uptake. However such interpretation is in contradiction to the reported lack of uncoupling activity of UCP2/3 (54), findings that overexpression of UCP2/3 boosts mitochondrial ATP generation upon Ca2+ mobilization and UCP2/3 contributed to mitochondrial Ca2+ uptake also under conditions in which mitochondrial ATP production was prevented (17).This is further supported by the observation that silencing of UCP2/3 failed to hyperpolarize mitochondrial membrane potential (supplemental Fig. S1) However, the present findings confirm the assumption of Demaurex's group and demonstrate that SERCA activity affects mitochondrial Ca2+ uptake.
Overall the present study demonstrates that the inhibition of SERCA affects the kinetics of IP3-triggered intracellular Ca2+ release, and, subsequently, shifts the mode of mitochondrial Ca2+ uptake from an UCP3- and MCU-dependent and Letm1-independent toward a Letm1- and MCU-dependent but UCP3-independent route (Fig. 6). These observations indicate that SERCA activity is a crucial determinant for the mode of mitochondrial Ca2+ uptake and appoints which proteins of the IMM actually contribute to the transfer of Ca2+ into mitochondria.
Supplementary Material
Acknowledgments
We thank Sandra Blass, Therese Macher, Florian Enzinger, and Dr. Rene Rost for excellent technical assistance.
This work was supported by the Austrian Science Funds (FWF, P20181-B05, P21857-B18, and P22553-B18).

This article contains supplemental Figs. S1—S3.
- IMM
- inner mitochondrial membrane
- [Ca2+]cyto
- cytosolic free Ca2+ concentration
- Letm1
- leucine zipper EF hand-containing transmembrane protein 1
- MCUR1
- mitochondrial calcium uniporter regulator 1
- MICU1
- mitochondrial Ca2+ uptake 1
- [Ca2+]mito
- mitochondrial Ca2+ concentration
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- SOCE
- store-operated Ca2+ entry
- UCP2/3
- uncoupling protein 2 and 3.
REFERENCES
- 1. Duchen M. R. (2000) Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium 28, 339–348 [DOI] [PubMed] [Google Scholar]
- 2. Jouaville L. S., Pinton P., Bastianutto C., Rutter G. A., Rizzuto R. (1999) Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U.S.A. 96, 13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szabadkai G., Simoni A. M., Bianchi K., De Stefani D., Leo S., Wieckowski M. R., Rizzuto R. (2006) Mitochondrial dynamics and Ca2+ signaling. Biochim. Biophys. Acta 1763, 442–449 [DOI] [PubMed] [Google Scholar]
- 4. Graier W. F., Frieden M., Malli R. (2007) Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch. 455, 375–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rimessi A., Giorgi C., Pinton P., Rizzuto R. (2008) The versatility of mitochondrial calcium signals: From stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta 1777, 808–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pizzo P., Drago I., Filadi R., Pozzan T. (2012) Mitochondrial Ca2+ homeostasis: mechanism, role, and tissue specificities. Pflugers Arch. 464, 3–17 [DOI] [PubMed] [Google Scholar]
- 7. Kirichok Y., Krapivinsky G., Clapham D. E. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 [DOI] [PubMed] [Google Scholar]
- 8. Ryan T., Sharma P., Ignatchenko A., MacLennan D. H., Kislinger T., Gramolini A. O. (2011) Identification of Novel Ryanodine Receptor 1 (RyR1) Protein Interaction with calcium homeostasis endoplasmic reticulum protein (CHERP). J. Biol. Chem. 286, 17060–17068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jean-Quartier C., Bondarenko A. I., Alam M. R., Trenker M., Waldeck-Weiermair M., Malli R., Graier W. F. (2012) Studying mitochondrial Ca2+ uptake - A revisit. Mol. Cell. Endocrinol. 353, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bondarenko A. I., Jean-Quartier C., Malli R., Graier W. F. (2013) Characterization of distinct single-channel properties of Ca2+ inward currents in mitochondria. Pflugers Arch. 2013. (in press) DOI: 10.1007/s00424-013-1224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryu S. Y., Beutner G., Dirksen R. T., Kinnally K. W., Sheu S. S. (2010) Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 584, 1948–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nowikovsky K., Froschauer E. M., Zsurka G., Samaj J., Reipert S., Kolisek M., Wiesenberger G., Schweyen R. J. (2004) The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 279, 30307–30315 [DOI] [PubMed] [Google Scholar]
- 15. Jiang D., Zhao L., Clapham D. E. (2009) Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldeck-Weiermair M., Jean-Quartier C., Rost R., Khan M. J., Vishnu N., Bondarenko A. I., Imamura H., Malli R., Graier W. F. (2011) The leucine zipper EF hand-containing transmembrane protein 1 (LETM1) and uncoupling proteins-2 and -3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J. Biol. Chem. 286, 28444–28455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W. F. (2007) Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 9, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waldeck-Weiermair M., Malli R., Naghdi S., Trenker M., Kahn M. J., Graier W. F. (2010) The contribution of UCP2 and UCP3 to mitochondrial Ca2+ uptake is differentially determined by the source of supplied Ca2+. Cell Calcium 47, 433–440 [DOI] [PubMed] [Google Scholar]
- 19. Waldeck-Weiermair M., Duan X., Naghdi S., Khan M. J., Trenker M., Malli R., Graier W. F. (2010) Uncoupling protein 3 adjusts mitochondrial Ca2+ uptake to high and low Ca2+ signals. Cell Calcium 48, 288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szanda G., Koncz P., Várnai P., Spät A. (2006) Mitochondrial Ca2+ uptake with and without the formation of high-Ca2+ microdomains. Cell Calcium 40, 527–537 [DOI] [PubMed] [Google Scholar]
- 21. Malli R., Graier W. F. (2010) Mitochondrial Ca2+ channels: Great unknowns with important functions. FEBS Lett. 584, 1942–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Marchi U., Castelbou C., Demaurex N. (2011) Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production. J. Biol. Chem. 286, 32533–32541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trenker M., Fertschai I., Malli R., Graier W. F. (2008) UCP2/3-likely to be fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 10, 1237–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waldeck-Weiermair M., Alam M. R., Khan M. J., Deak A. T., Vishnu N., Karsten F., Imamura H., Graier W. F., Malli R. (2012) Spatiotemporal Correlations between Cytosolic and Mitochondrial Ca2+ Signals Using a Novel Red-Shifted Mitochondrial Targeted Cameleon. PLoS ONE 7, e45917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alam M. R., Groschner L. N., Parichatikanond W., Kuo L., Bondarenko A. I., Rost R., Waldeck-Weiermair M., Malli R., Graier W. F. (2012) Mitochondrial Ca2+ Uptake 1 (MICU1) and Mitochondrial Ca2+ Uniporter (MCU) Contribute to Metabolism-Secretion Coupling in Clonal Pancreatic β-Cells. J. Biol. Chem. 287, 34445–34454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giacomello M., Drago I., Bortolozzi M., Scorzeto M., Gianelle A., Pizzo P., Pozzan T. (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 38, 280–290 [DOI] [PubMed] [Google Scholar]
- 27. Carafoli E., Brini M. (2000) Calcium pumps: structural basis for and mechanism of calcium transmembrane transport. Curr. Opin Chem. Biol. 4, 152–161 [DOI] [PubMed] [Google Scholar]
- 28. Brini M., Bano D., Manni S., Rizzuto R., Carafoli E. (2000) Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca2+ signalling. EMBO J. 19, 4926–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Camello C., Lomax R., Petersen O. H., Tepikin A. V. (2002) Calcium leak from intracellular stores-the enigma of calcium signalling. Cell Calcium 32, 355–361 [DOI] [PubMed] [Google Scholar]
- 30. Bakowski D., Parekh A. B. (2001) Sarcoplasmic/endoplasmic-reticulum-Ca2+-ATPase-mediated Ca2+ reuptake, and not Ins(1,4,5)P3 receptor inactivation, prevents the activation of macroscopic Ca2+ release-activated Ca2+ current in the presence of physiological Ca2+ buffer in rat basophilic leukaemia-1 cells. Biochem. J. 353, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Csordás G., Hajnóczky G. (2001) Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium 29, 249–262 [DOI] [PubMed] [Google Scholar]
- 32. Spät A., Fülöp L., Koncz P., Szanda G. (2009) When is high-Ca2+ microdomain required for mitochondrial Ca2+ uptake? Acta Physiol. 195, 139–147 [DOI] [PubMed] [Google Scholar]
- 33. Spät A., Szanda G., Csordás G., Hajnóczky G. (2008) High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium 44, 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spät A. (2006) Calcium microdomains and the fine control of cell function: an introduction. Cell Calcium 40, 403–404 [DOI] [PubMed] [Google Scholar]
- 35. Csordás G., Várnai P., Golenár T., Roy S., Purkins G., Schneider T. G., Balla T., Hajnóczky G. (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 37. Chami M., Oulès B., Szabadkai G., Tacine R., Rizzuto R., Paterlini-Bréchot P. (2008) Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol. Cell 32, 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kornmann B., Walter P. (2010) ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J. Cell Sci. 123, 1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merkwirth C., Langer T. (2008) Mitofusin 2 builds a bridge between ER and mitochondria. Cell 135, 1165–1167 [DOI] [PubMed] [Google Scholar]
- 40. de Brito O. M., Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 [DOI] [PubMed] [Google Scholar]
- 41. Csordás G., Thomas A. P., Hajnóczky G. (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnóczky G. (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Santo-Domingo J., Demaurex N. (2010) Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta 1797, 907–912 [DOI] [PubMed] [Google Scholar]
- 44. Pan S., Ryu S. Y., Sheu S.-S. (2011) Distinctive characteristics and functions of multiple mitochondrial Ca2+ influx mechanisms. Sci. China Life Sci. 54, 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernardi P. (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155 [DOI] [PubMed] [Google Scholar]
- 46. Azzolin L., Basso E., Argenton F., Bernardi P. (2010) Mitochondrial Ca2+ transport and permeability transition in zebrafish (Danio rerio). Biochim. Biophys. Acta 1797, 1775–1779 [DOI] [PubMed] [Google Scholar]
- 47. Raffaello A., De Stefani D., Rizzuto R. (2012) The mitochondrial Ca2+ uniporter. Cell Calcium 52, 16–21 [DOI] [PubMed] [Google Scholar]
- 48. Poburko D., Potter K., van Breemen E., Fameli N., Liao C.-H., Basset O., Ruegg U. T., van Breemen C. (2006) Mitochondria buffer NCX-mediated Ca2+-entry and limit its diffusion into vascular smooth muscle cells. Cell Calcium 40, 359–371 [DOI] [PubMed] [Google Scholar]
- 49. Rizzuto R., Bastianutto C., Brini M., Murgia M., Pozzan T. (1994) Mitochondrial Ca2+ homeostasis in intact cells. J. Cell Biol. 126, 1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marchi S., Lupini L., Patergnani S., Rimessi A., Missiroli S., Bonora M., Bononi A., Corrà F., Giorgi C., De Marchi E., Poletti F., Gafà R., Lanza G., Negrini M., Rizzuto R., Pinton P. (2013) Downregulation of the Mitochondrial Calcium Uniporter by Cancer-Related miR-25. Current Biology, 23, 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perocchi F., Gohil V. M., Girgis H. S., Bao X. R., McCombs J. E., Palmer A. E., Mootha V. K. (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mallilankaraman K., Doonan P., Cárdenas C., Chandramoorthy H. C., Müller M., Miller R., Hoffman N. E., Gandhirajan R. K., Molgó J., Birnbaum M. J., Rothberg B. S., Mak D.-O., Foskett J. K., Madesh M. (2012) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151, 630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mallilankaraman K., Cárdenas C., Doonan P. J., Chandramoorthy H. C., Irrinki K. M., Golenár T., Csordás G., Madireddi P., Yang J., Müller M., Miller R., Kolesar J. E., Molgó J., Kaufman B., Hajnóczky G., Foskett J. K., Madesh M. (2012) MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 14, 1336–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nedergaard J., Cannon B. (2003) The 'novel' “uncoupling” proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp. Physiol. 88, 65–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.