Background: Neurofibrillary tangles comprising abnormally phosphorylated tau are hallmarks for Alzheimer disease.
Results: Prostate-derived sterile 20-like kinases (PSKs) phosphorylate tau on more than 40 residues. PSKs are activated in tangle-bearing neurons.
Conclusion: PSKs may contribute to Alzheimer pathology and neurodegeneration by mediating tau phosphorylation.
Significance: PSKs may provide novel targets for the treatment of dementia.
Keywords: Alzheimer Disease, MAP Kinases (MAPKs), MAPs, Neurodegenerative Diseases, Signal Transduction, PSK Kinases, Kinase, Neurofibrillary Tangles, Phosphorylation, Tau
Abstract
In Alzheimer disease (AD), the microtubule-associated protein tau is highly phosphorylated and aggregates into characteristic neurofibrillary tangles. Prostate-derived sterile 20-like kinases (PSKs/TAOKs) 1 and 2, members of the sterile 20 family of kinases, have been shown to regulate microtubule stability and organization. Here we show that tau is a good substrate for PSK1 and PSK2 phosphorylation with mass spectrometric analysis of phosphorylated tau revealing more than 40 tau residues as targets of these kinases. Notably, phosphorylated residues include motifs located within the microtubule-binding repeat domain on tau (Ser-262, Ser-324, and Ser-356), sites that are known to regulate tau-microtubule interactions. PSK catalytic activity is enhanced in the entorhinal cortex and hippocampus, areas of the brain that are most susceptible to Alzheimer pathology, in comparison with the cerebellum, which is relatively spared. Activated PSK is associated with neurofibrillary tangles, dystrophic neurites surrounding neuritic plaques, neuropil threads, and granulovacuolar degeneration bodies in AD brain. By contrast, activated PSKs and phosphorylated tau are rarely detectible in immunostained control human brain. Our results demonstrate that tau is a substrate for PSK and suggest that this family of kinases could contribute to the development of AD pathology and dementia.
Introduction
The sterile 20 (STE20)2 group of mammalian protein kinases includes 28 proteins, which have been divided into two subfamilies according to their structure and regulation (1–3). Six p21-activated kinases, which have a C-terminal catalytic domain and an N-terminal Cdc42/Rac-interacting and -binding domain, and 22 germinal center kinase-like kinases, which possess an N-terminal kinase domain but no Cdc42/Rac-interacting and -binding domain (4–6). Prostate-derived STE20-like kinases (PSKs; also referred to as thousand and one amino acid kinases) have been classified as members of the germinal center kinase VIII subfamily of STE20 kinases and include PSK1-α and PSK1-β (splice variants with identical N-terminal kinase domains; TAOK2 isoforms 2 and 1, respectively), PSK2 (TAOK1), and PSK3 (TAOK3) (2, 7–10). Most STE20s activate one or more mitogen-activated protein kinase (MAPK) signaling pathways, and PSK1-α or PSK2 can stimulate c-Jun N-terminal kinase (JNK) and p38 MAPK (7–9, 11, 12).
Previous studies have shown that PSKs can regulate microtubule dynamics and organization (13, 14). Binding of the PSK1-α C terminus (amino acids 745–1235) to microtubules results in stabilized, perinuclear microtubule cables that are nocodazole-resistant and contain increased levels of acetylated α-tubulin (13). In contrast, PSK2 induces microtubule instability via activation of microtubule affinity-regulating kinase (MARK/PAR-1) and phosphorylation of microtubule-associated proteins, including tau, which dissociate from microtubules, resulting in their disassembly (14–16). PSKs are activated catalytically during microtubule-dependent processes such as mitosis and neuritogenesis, and knockdown of PSK expression using small interfering RNAs (siRNAs) inhibits these processes (14, 17).
In the human central nervous system (CNS), the tau gene is alternatively spliced into six isoforms containing up to two inserts at the N terminus (0, 1, or 2N) and three or four microtubule-binding domain repeat sequences toward the C terminus (3R or 4R). The longest human CNS tau isoform comprises 441 amino acids and is referred to as 2N4R (18). Tau is expressed predominantly in neurons and is a major microtubule-associated protein involved in the regulation of microtubule dynamics and organization (19). Repeated motifs in the C terminus of tau are responsible for its binding to and stabilization of microtubules; however, tau phosphorylation causes it to dissociate from microtubules and thereby promotes microtubule instability (20–22). Phosphorylation of tau in the microtubule-binding domain (amino acids 244–368) is important for the regulation of tau-microtubule interactions. Phosphorylation of the repeated KXGS motifs (notably Ser-262 and Ser-356) located within this region inhibits tau-microtubule binding and causes microtubule destabilization (20, 21, 23).
The presence of tau gene mutations in familial frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) demonstrated a causal role for tau in neurodegeneration (24, 25). Furthermore, one of the main hallmarks for Alzheimer disease (AD) is the presence of intraneuronal neurofibrillary tangles (NFTs), which are formed from paired helical filaments (PHFs) comprising hyperphosphorylated and insoluble tau aggregates (25). The phosphorylation of tau on multiple residues is an early event in AD that precedes or accompanies pathological aggregation of tau and NFT formation (26). A number of kinases have been reported to phosphorylate tau, at least in vitro, including glycogen synthase kinase-3 (GSK3), MARK, casein kinase 1, AMP-dependent protein kinase, cyclin dependent kinase-5, and stress-activated kinases among others (27–29). However, it is now important to identify the protein kinases that are activated catalytically in tangle-bearing neurons and are responsible for the pathological phosphorylation of tau in AD.
Here we have investigated the potential involvement of PSKs in AD and have demonstrated that tau is phosphorylated on multiple sites by PSK1-α/β and PSK2. Phosphorylated residues are located in the tau microtubule-binding domain and in the regions flanking this domain, and more than half of these modified tau residues are also found in PHF-tau extracted from AD brains (30). PSKs are also activated catalytically in regions of the brain that are susceptible to AD pathology and are present in intraneuronal NFTs, neuropil threads, and granulovacuolar degeneration bodies where tau is also phosphorylated. Each of these structures is characteristic for AD, and our findings suggest potential roles for these proteins in the development of AD and neurodegeneration.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
pRK5-Myc PSK1-α/β(1–349) (catalytically active), pRK5-Myc-PSK1-α/β(1–349) (K57A; kinase-deficient mutant), pRK5-Myc-PSK2(1–416) (catalytically active), and pRK5-Myc-PSK2(1–416) (K57A; kinase-deficient mutant) were made using methods described previously (7–9). pcDNA3.1-human tau was obtained from Prof. B. Anderton (King's College London). Goat PSK1-α/β and mouse Myc or glutathione S-transferase (GST) antibodies were purchased from Santa Cruz Biotechnology. Rabbit PSK1-α antibody was obtained from Proteintech. Mouse anti-PSK2 antibody was obtained from BD Biosciences. Affinity-purified rabbit PSK-Ser(P)-181 antibody was made as described previously (9, 17, 31). Purified PSK-1α/β and PSK2 proteins were obtained from SignalChem, and recombinant human tau (tau-441; 2N4R) was purchased from Sigma-Aldrich. 12E8 tau-Ser(P)-262/356 antibody was a kind gift from Dr P. Seubert (Neotope Biosciences) (32). Rabbit tau-Ser(P)-214, tau-Ser(P)-231, and tau-Ser(P)-422 antibodies were purchased from Chemicon/Millipore, Abcam, and BIOSOURCE, respectively. Mouse β-actin antibody was obtained from Sigma-Aldrich, and mouse GAPDH antibody was obtained from Millipore.
In Vitro Kinase Assays and Mass Spectrometric Analysis of Tau
Purified PSK1-α/β/TAOK2 (amino acids 1–314; 30 ng) or PSK2/TAOK1 (amino acids 1–314; 30 ng) fused to GST and prepared from baculovirus-infected Sf9 insect cells (SignalChem) were placed in 30 μl of kinase buffer (20 mm MgCl2, 2 mm MnCl2, 30 mm Tris-HCl, pH 7.4) containing 20 μm ATP, 2 μCi of [γ-32P]ATP (MP Biomedicals; 3000 Ci/mmol), and 1 μg of recombinant human tau (2N4R) and incubated for 30 min at 30 °C. Kinase assays were terminated in gel sample buffer, proteins were separated by SDS-PAGE, and gels were dried for analysis of phosphorylated tau by autoradiography. Additional preparations performed in the absence of [γ-32P]ATP were separated by SDS-PAGE and transferred to nitrocellulose for immunoblotting using PSK-Ser(P)-181 or 12E8 antibodies and enhanced chemiluminescence.
Cell Culture
COS-1 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics (10% CO2 at 37 °C). For transfection, 5 × 105 cells/4 ml of medium were seeded onto 60-mm Petri dishes. After 16 h, the indicated plasmids were transfected into COS-1 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For electroporation, cells were washed with 5 ml of cold electroporation buffer (120 mm KCl, 10 mm K2PO4·KH2PO4, pH 7.6, 25 mm Hepes, 2 mm MgCl2, 0.5% Ficoll). The buffer was removed, and cells were resuspended in 250 μl of cold electroporation buffer and electroporated at 250 V and 960 microfarads with 5 μg of total DNA. The cells (106) were then plated onto 10-cm-diameter dishes and incubated for 24 h prior to lysis.
Mass Spectrometry
For mass spectrometric analysis of phosphorylated tau residues, in vitro kinase assays were performed as described above using 150 ng of purified PSK1-α/β/TAOK2 or PSK2/TAOK1, 3 μg of recombinant human tau (2N4R), and 3 mm ATP in a total volume of 60 μl and incubated for 6 h at 30 °C. Samples were resolved by SDS-PAGE; fixed in 40% MeOH, 7% glacial acetic acid (30 min); and stained with Coomassie Brilliant Blue (MeOH:H2O, 1:4) (>2 h). Samples were destained once in 25% MeOH, 7% glacial acetic acid (10 min) and then twice in 25% MeOH, 2% glacial acetic acid (30 min each) and rinsed and stored in H2O. Tau bands were then excised from the gel, and in-gel reduction, alkylation, and trypsin digestion were carried out as described previously (33). Peptides were extracted from the gel pieces using acetonitrile and aqueous washes and lyophilized. Samples were resuspended in ammonium bicarbonate (50 mm) and analyzed by LC-MS/MS. Peptides were resolved by reverse phase chromatography on a 75-μm C18 PepMap column using a three-step linear gradient of acetonitrile in 0.1% formic acid (33). The eluate was ionized by nanoelectrospray ionization (Orbitrap Velos Pro, Thermo Fisher Scientific), running the instrument in automated data-dependent switching mode. Precursor ions were selected for sequencing by collision-induced fragmentation using a Top20 collision-induced dissociation method. Mass spectral data were processed into peak lists using Proteome Discoverer v1.3, and peak lists were searched against the UniProt database using Mascot software v2.2. Peptide assignments were accepted if they contained at least two unique peptide assignments and were established at 100% identification probability by the ProteinProphet algorithm. Phosphorylation sites were identified by neutral loss of phosphoric acid (98 Da) and manual validation to determine the precise site of phosphorylation.
Analysis of Human Brain Tissues
Post-mortem human brain from clinically and pathologically confirmed AD or control human brain (Table 1) was obtained from the MRC London Neurodegenerative Diseases Brain Bank (Institute of Psychiatry, King's College London).
TABLE 1.
Characteristics of the subjects whose brain tissues were used in this study
PMD refers to post-mortem delay in hours before samples were processed and stored. M, male; F, female; Path Dx, pathologic diagnosis.
| Case no. | Path Dx | Braak | Age | Sex | PMD |
|---|---|---|---|---|---|
| h | |||||
| 1 | AD | VI | 70 | M | 60 |
| 2 | AD | VI | 62 | F | 40 |
| 3 | AD | VI | 68 | M | 25 |
| 4 | AD | VI | 86 | F | 55 |
| 5 | AD | VI | 80 | F | 47 |
| 6 | AD | VI | 72 | M | 5 |
| 7 | AD | IV | 82 | M | 80 |
| 8 | AD | VI | 97 | F | 28 |
| 9 | AD | VI | 87 | F | 48 |
| 10 | AD | VI | 90 | F | 23 |
| 11 | AD | IV | 89 | F | 15 |
| 12 | AD | IV | 85 | F | 24 |
| 13 | Control | I-II | 89 | F | 41 |
| 14 | Control | I | 66 | M | 35 |
| 15 | Control | I | 81 | M | 18 |
| 16 | Control | I | 81 | F | 17 |
| 17 | Control | II | 79 | M | 37 |
| 18 | Control | II | 82 | F | 13 |
| 19 | Control | I | 81 | F | 17 |
| 20 | Control | III | 86 | M | 7 |
| 21 | Control | IV | 81 | M | 47 |
For immunoblotting, tissue samples were taken from different areas of the same brain slice for each individual, and regions included entorhinal cortex, anterior hippocampus, superior temporal gyrus of the temporal cortex, medial frontal gyrus of the frontal cortex, angular gyrus of the occipital cortex, and cerebellum taken from the first ventral lobe lateral to the vermis in a midvermal section (34). Brain tissue was pulverized into powder in a liquid nitrogen-cooled pestle and mortar. Samples were homogenized (6 volumes/g of tissue) in a glass homogenizer in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% IGEPAL, 0.1% SDS, 0.5% deoxycholate) containing 10 mm NaF, 1 mm β-glycerophosphate, protease inhibitor mixture (Complete, Roche Applied Science), and phosphatase inhibitor mixture (PhosSTOP, Roche Applied Science). The homogenates were incubated on ice for 30 min, centrifuged at 100,000 × g, and stored as aliquots at −70 °C. 50 μg of total protein were separated using Novex 8% precast gels (Invitrogen), transferred to nitrocellulose, and immunoblotted using methods described previously (7, 34). Additional frozen post-mortem human hippocampus from pathologically confirmed cases of AD were homogenized (200 mg/ml) in 2× sample buffer (120 mm Tris, pH 6.8, 4% (w/v) sodium dodecyl sulfate, 20% (v/v) glycerol, 10% (v/v) β-mercaptoethanol) using a mechanical homogenizer. Homogenates were sonicated (VibraCellsTM probe sonicator, Sonics & Materials, Inc.) for 10 s at output setting 4, heated to 95 °C for 5 min, and briefly centrifuged prior to SDS-PAGE and immunoblotting.
For immunohistochemistry, 7-μm tissue sections (×10) were cut from formalin-fixed paraffin-embedded blocks of AD or control human brain tissue. Sections were deparaffinized and pretreated with methanol containing 2.5% H2O2 for 30 min, and antigen retrieval was enhanced by microwaving in 10 mm sodium citrate buffer, pH 6.0. Endogenous peroxidase activity was inhibited by incubating samples in 3% hydrogen peroxide for 30 min. Sections were then blocked for 30 min in either PBS, 10% BSA (PSK-Ser(P)-181 antibody) or TBS, 10% normal rabbit serum (12E8 antibody) before incubating with PSK-Ser(P)-181 (1:25 in PBS, 2% BSA) or 12E8 tau-Ser(P)-262/356 (1:100 in TBS, 1% rabbit serum) antibody overnight at 4 °C. Sections were then incubated with biotinylated secondary antibodies swine anti-rabbit IgG (Dako) or rabbit anti-mouse IgG (Dako) for 45 min. Sections were developed using the VECTASTAIN Elite ABC kit (Vector Laboratories) and 0.5 mg/ml 3,3′-diaminobenzidine chromogen (Sigma-Aldrich). All samples were counterstained with hematoxylin.
Double Immunofluorescence Staining
For double labeling, sections were treated as above in citrate buffer and blocked in 10% goat serum. Primary antibodies were then applied (1:25 PSK-Ser(P)-181 and 1:50 12E8) and incubated overnight at 4 °C. Secondary Alexa Fluor antibodies (goat anti-rabbit 568 and goat anti-mouse 488; Invitrogen) were applied. Autofluorescence was quenched by Sudan black, and coverslips were mounted using VECTASHIELD (Vector Laboratories). Sections were visualized using a fluorescence microscope (Zeiss Axiovert-S100), and images were captured with a Zeiss LSM 510 confocal laser-scanning microscope.
RESULTS
Tau is Phosphorylated on Multiple Sites by PSK1-α/β and PSK2
In initial experiments, we set out to determine whether purified PSK1-α/β(1–314) or PSK2(1–314) could phosphorylate recombinant human tau (2N4R) in vitro. Both PSKs were shown to be active under these conditions by immunoblotting samples with a phospho-PSK antibody (PSK-Ser(P)-181), which detects catalytically activated forms of PSK1-α/β and PSK2 that are phosphorylated on serine 181 (Fig. 1A) (9, 17, 35). We found that PSK1-α/β and PSK2 both phosphorylated human tau directly in these in vitro kinase assays, demonstrating that tau is a substrate for both of these kinases (Fig. 1B).
FIGURE 1.

PSK1-α/β and PSK2 phosphorylate tau. A, purified GST-PSK1-α/β(1–314) or GST-PSK2(1–314) or control samples without recombinant proteins were subjected to in vitro kinase assays and immunoblotted with the PSK-Ser(P)-181 antibody (top row) or GST antibody (bottom row). Proteins were detected by enhanced chemiluminescence. B, purified GST-PSK1-α/β(1–314) or GST-PSK2(1–314) and/or human tau (2N4R) were added as indicated to in vitro kinase assays, and phosphorylated tau (top row) was detected using SDS-PAGE and autoradiography. Tau (middle row) and GST-PSK1-α/β(1–314) or GST-PSK2(1–314) (bottom row) were detected by immunoblotting and enhanced chemiluminescence.
PHF-tau extracted from AD brain is phosphorylated on multiple sites, and to date, ∼45 phosphorylated serine or threonine residues have been detected in Alzheimer tau using mass spectrometry and phosphospecific tau antibodies (30). To identify tau residues that are phosphorylated by PSK1-α/β or PSK2, phosphorylated tau protein bands obtained in in vitro kinase assays were extracted from gels, digested, and then analyzed using mass spectrometry (LC-MS/MS). We obtained sequence coverage of 92 and 94% of 2N4R human tau for PSK1-α/β and PSK2, respectively. The sequence information included all but three serine residues of a possible 80 phosphorylatable serine or threonine residues (Figs. 2 and 3). We assigned 41 phosphorylation sites for human tau treated with PSK1-α/β and 44 phosphorylation sites for PSK2 (Figs. 2 and 3). In contrast, no phosphorylation events were detected in the control tau sample incubated in the absence of PSKs (data not shown).
FIGURE 2.
Mass spectrometric identification and location of human tau (2N4R) residues phosphorylated by PSK1-α/β. A, following LC-MS/MS analysis and UniProt database searches, Mascot assigned 41sites on tau phosphorylated by PSK1-α/β that were validated manually by analyzing phosphopeptides and fragmentation spectra. Tau residues are numbered according to 2N4R nomenclature, and phosphorylated serine (pS) and threonine (pT) residues and their locations are shown. B, the amino acid sequence of the longest tau isoform (2N4R) is shown. Residues in lowercase were not detected by LC-MS/MS analysis, and the overall sequence coverage was 92% for PSK1-α/β. The location of each phosphorylated tau residue (Ser/Thr) is illustrated in bold. The microtubule-binding domain (amino acids 244–368) is underlined.
FIGURE 3.
Mass spectrometric identification and location of human tau (2N4R) residues phosphorylated by PSK2. A, following LC-MS/MS analysis and UniProt database searches, Mascot assigned 44 sites on tau phosphorylated by PSK2 that were validated manually by analyzing phosphopeptides and fragmentation spectra. Tau residues are numbered according to 2N4R nomenclature, and phosphorylated serine (pS) and threonine (pT) residues and their locations are shown. B, the amino acid sequence of the longest tau isoform (2N4R) is shown. Residues in lowercase were not detected by LC-MS/MS analysis, and the overall sequence coverage was 94% for PSK2. The location of each phosphorylated tau residue (Ser/Thr) is illustrated in bold. The microtubule-binding domain (amino acids 244–368) is underlined.
PSK1-α/β and PSK2 Phosphorylate Tau on KXGS Motifs
Analysis of the distribution of phosphorylated tau sites modified by PSKs shows that PSK1-α/β and PSK2 can phosphorylate 9 and 12 residues, respectively, within the tau microtubule-binding domain (Figs. 2B and 3B). Phosphorylated tau residues include three of the four KXGS motifs located within this domain, including Ser-262, Ser-324, and Ser-356, each of which is targeted by both kinases. We were unable to obtain sequence coverage for either kinase over the remaining KXGS motif at residue Ser-293 (Figs. 2B and 3B). Phosphorylation of Ser-262 and to a lesser extent Ser-356 on tau is notable because these modifications are known to strongly inhibit tau-microtubule binding and microtubule stability, and their phosphorylation is also an early event in AD development (20, 21, 23, 26).
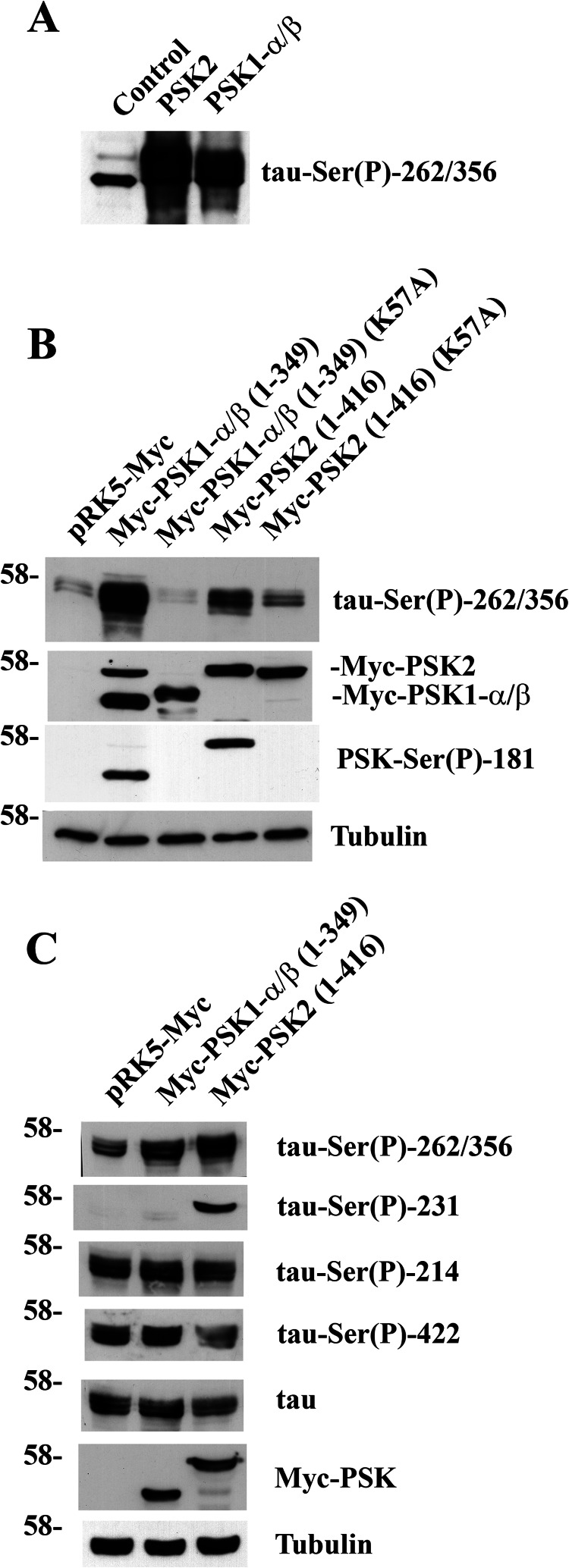
Because both kinases phosphorylate tau on Ser-262/Ser-356, we used the well characterized 12E8 phosphospecific tau antibody, which recognizes tau-Ser(P)-262/356 (32). Following incubation of recombinant tau with PSK1-α/β or PSK2, immunoblots probed with 12E8 revealed an enhanced detection of tau, demonstrating that these kinases both phosphorylate tau on the Ser-262/Ser-356 phosphoepitope in vitro (Fig. 4A). The ability of PSKs to phosphorylate tau in cells was also examined with 12E8, and we found that exogenously expressed PSK1-α/β or PSK2 each increased tau-Ser(P)-262/Ser(P)-356 when coexpressed with tau in COS-1 cells (Fig. 4B). In contrast, kinase-deficient PSK1-α/β (K57A) and PSK2 (K57A) did not enhance the phosphorylation of tau on Ser-262/Ser-356 in COS-1 cells (Fig. 4B). In further experiments examining the effects of exogenously expressed PSK1-α/β or PSK2 on additional tau sites known to regulate tau-microtubule interactions, we found that PSK2 stimulated phosphorylation of tau on Ser-231, but PSK1-α/β did not (Fig. 4C). In contrast, we were unable to detect enhanced phosphorylation of tau on Ser-214 or Ser-422 following expression of exogenous PSK1-α/β or PSK2 when compared with control cells because transfected tau was phosphorylated already on these residues most likely due to the activity of endogenous kinases present in these cells (Fig. 4C).
FIGURE 4.
PSK1-α/β and PSK2 phosphorylate tau on Ser-262 and/or Ser-356 in vitro and in cells. A, purified GST-PSK1-α/β(1–314) or GST-PSK2(1–314) was added or no additions were made to recombinant human tau (2N4R) as indicated, and in vitro kinase assays were carried out. Samples were immunoblotted with 12E8 antibody to detect tau-Ser(P)-262/356. B, COS-1 cells were cotransfected with pCDNA3.1-human tau and either pRK5-Myc, pRK5-Myc-PSK1-α/β(1–349), pRK5-Myc-PSK1-α/β(1–349) (K57A; kinase-deficient), pRK5-Myc-PSK2(1–416), or pRK5-Myc-PSK2(1–416) (K57A; kinase-deficient) as indicated. Cell lysates were prepared after 24 h and immunoblotted with 12E8 tau-Ser(P)-262/356 antibody (top row), anti-Myc antibody (second row), PSK-Ser(P)-181 antibody (third row), or α-tubulin antibody (bottom row). C, COS-1 cells were coelectroporated with pCDNA3.1-human tau and either pRK5-Myc, pRK5-Myc-PSK1-α/β(1–349), or pRK5-Myc-PSK2(1–416) as indicated. Cell lysates were prepared after 24 h and immunoblotted with 12E8 tau-Ser(P)-262/356 antibody (top row); antibodies to detect tau-Ser(P)-231, tau-Ser(P)-214, tau-Ser(P)-422, or total tau (second through fifth rows, respectively); anti-Myc antibody (sixth row); or α-tubulin antibody (bottom row). The position of the 58-kDa protein marker is shown in B and C.
Thus, PSK1-α/β and PSK2 both phosphorylate tau on multiple sites in vitro. Furthermore, both PSKs phosphorylate tau on KXGS motifs (notably Ser-262/Ser-356) located within the microtubule-binding domain both in vitro and when overexpressed in cells.
PSKs Are Activated Catalytically in AD Brain
Previous studies have shown that NFTs spread throughout the human brain in an ordered fashion during AD progression (36). Consequently, the catalytic activity of PSKs was investigated in regions of the human brain susceptible to AD (entorhinal cortex and hippocampus followed by temporal, frontal, and occipital cortices) or resistant to AD pathology (cerebellum) (34, 36). Extracts of post-mortem brain tissue from different regions of AD brains were resolved by SDS-PAGE and probed with PSK-Ser(P)-181 antibody. This antibody has been shown previously to detect only catalytically active and autophosphorylated PSK1-α/β and PSK2 but not their inactive counterparts (9, 17). In AD brain, we identified PSK catalytic activity in all six of the brain regions analyzed. PSK activity was most intense in the hippocampus and weakest in the cerebellum where it was barely detectible (Fig. 5A). Entorhinal cortex was examined in three AD and three control brains, and catalytically active PSK was detected in all samples analyzed (Fig. 5B). Extracts prepared from the hippocampus of four AD cases also contained activated PSK-Ser(P)-181, which ran with an apparent molecular mass of 175 kDa (Fig. 5C). Additional probing of stripped blots suggests that the upper band of the characteristic PSK1-α doublet reported previously comigrates with catalytically active PSK, whereas the PSK2 antibody detects a band that runs ahead of PSK-Ser(P)-181 (Fig. 5C) (9). This approach using immunoblotting to analyze brain extracts shows that PSKs are activated catalytically in AD but is unable to establish whether their activity is enhanced in diseased tissues when compared with control tissues.
FIGURE 5.

PSKs are activated catalytically in AD brain extracts. A, extracts were prepared from six different regions of AD brains, and catalytically active and phosphorylated PSKs were detected by immunoblotting samples with the PSK-Ser(P)-181 antibody. Equal loading of extracts was confirmed by immunoblotting samples with a β-actin antibody, and two representative cases marked i and ii are shown. Brain regions are as follows: C, cerebellum; EC, entorhinal cortex; OC, occipital cortex; STG, superior temporal gyrus; FC, frontal cortex; and H, hippocampus. B, extracts were prepared from the entorhinal cortex of control or AD brains, and catalytically active PSKs were detected by immunoblotting samples with the PSK-Ser(P)-181 antibody. Equal loading of extracts was confirmed by immunoblotting samples with a GAPDH antibody. C, extracts were prepared from the hippocampus of AD brains (n = 4) and immunoblotted with antibodies to detect PSK-Ser(P)-181 (top row), PSK1-α/β (second row), PSK2 (third row), or α-tubulin (bottom row). The positions of the 175- and 58-kDa protein markers are shown.
Catalytically Active PSKs and Phosphorylated Tau Colocalize with NFTs in AD Brain
To examine the localization of activated PSK in AD and control brains, immunohistochemical analysis was performed using the PSK-Ser(P)-181 and 12E8 antibodies. Serial sections prepared from the hippocampal region of AD and control brains were immunostained with each antibody. No detectible immunoreactivity was observed in the CA1 segment of the hippocampus in control brain probed with the PSK-Ser(P)-181 antibody (Fig. 6A, panel a). However, in AD brain (Braak stage VI), strong positive immunoreactivity with the PSK-Ser(P)-181 antibody was observed in the CA1 region, the subiculum, and the tempero-occipital gyrus (Fig. 6B, panels a, c, and e). In these diseased brains, catalytically active and phosphorylated PSKs were detected in the nucleus and cytoplasm of neurons and were also clearly present on NFTs and in dystrophic neurites surrounding the neuritic plaques. Additional positive immunoreactivity with the PSK-Ser(P)-181 antibody was observed in neuropil threads and in granulovacuolar degeneration bodies (Fig. 6B, panels a, c, and e), another major pathological structure associated with AD. AD brain sections immunostained with 12E8 antibody showed strong positive immunoreactivity with tangle-bearing neurons, dystrophic neurites surrounding neuritic plaques, and neuropil threads and to a lesser extent with granulovacuolar degeneration bodies (Fig. 6B, panels b, d, and f). Neither the PSK-Ser(P)-181 antibody nor the 12E8 antibody demonstrated significant immunoreactivity in control brain (Fig. 6A, panels a and b), but both antibodies labeled rare NFTs and neuropil threads that occur in control brain tissues due to normal aging (Fig. 6A, panels c and d) and are also present in mild tauopathies (e.g. hyperphosphorylated tau Braak stage II) (Fig. 6A, panels e and f).
FIGURE 6.
Catalytically active PSKs and phosphorylated tau are associated with tangle-bearing neurons in AD brain. Formalin-fixed control or advanced AD (Braak stage VI) brains were immunostained with the PSK-Ser(P)-181 antibody to detect catalytically active and phosphorylated PSKs (panels a, c, and e; left side) or 12E8 antibody to detect tau-Ser(P)-262/356 (panels b, d, and f; right side). A, control brains. Negative staining with the PSK-Ser(P)-181 and 12E8 antibodies is seen in the CA1 segment of the hippocampus (panels a and b), a neuron in the subiculum shows mild immunoreactivity with the PSK-Ser(P)-181 antibody within granulovacuolar degeneration bodies (panel c), and an NFT and rare neuropil thread are stained with the 12E8 antibody in the subiculum (panel d). Mild tauopathy (hyperphosphorylated tau Braak stage II) shows immunopositivity in NFTs treated with the PSK-Ser(P)-181 antibody (panel e) and immunostaining with the 12E8 antibody (panel f) that is more evident in neuropil threads. B, AD brains. Strong positive immunoreactivity is observed in NFTs treated with the PSK-Ser(P)-181 (panels a, c, and e) and 12E8 (panels b, d, and f) antibodies in hyperphosphorylated tau Braak stage VI AD brain tissues. Some of the dystrophic neurites of the neuritic plaques (*) and neuropil threads were also labeled with the PSK-Ser(P)-181 antibody and markedly stained with the 12E8 antibody. CA1, CA1 segment of the hippocampus; Sub, subiculum; TOG, temporo-occipital gyrus. Scale bars, 200 μm in A, panels a and b; 50 μm in A, panels c and d; and 100 μm in A, panels e and f, and B, panels a–f.
We next performed a semiquantitative analysis of the intensity of staining by PSK-Ser(P)-181 and 12E8 antibodies of tau-positive structures in brain. We found that in AD pretangles, NFTs, neuropil threads, and neuritic plaques were strongly immunoreactive toward the 12E8 tau-Ser(P)-262/356 antibody and moderately immunoreactive toward the PSK-Ser(P)-181 antibody (Table 2). Granulovacuolar degeneration bodies were also strongly immunoreactive with the phospho-PSK antibody and moderately immunoreactive with the phosphotau antibody (Table 2). In some control brain tissue sections, rare NFTs and neuropil threads were also strongly immunoreactive toward the phosphotau antibody and moderately immunoreactive toward the phospho-PSK antibody (Table 2).
TABLE 2.
Semiquantitative analysis of the intensity of PSK-Ser(P)-181 or 12E8 antibody staining of tau-positive structures in brain tissue sections
The intensity of antibody immunostaining and reactivity of tau-positive structures was scored from 0 to 3+ for eight brain samples, and case details and results are shown. PT, pretangles; T, tangles; NT, neuropil threads; NP, neuritic plaques; GVDB, granulovacuolar degeneration bodies; HP-tau, hyperphosphorylated tau.
| Case details | 12E8 |
PSK-Ser(P)-181 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PT | T | NT | NP | GVDB | PT | T | NT | NP | GVDB | |
| Normal, tau-negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Braak score I-II | 2+ | 2+ | 2+ | 0 | 1+ | 1+ | 1+ | 1+ | 0 | 2+ |
| HP-tau stage + | 1+ | 1+ | 1+ | 0 | 0 | 0 | 0 | 0 | 0 | 1+ |
| Normal, tau-negative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1+ |
| AD, Braak VI | 3+ | 3+ | 3+ | 3+ | 1+ | 2+ | 2+ | 2+ | 2+ | 3+ |
| AD, Braak VI | 3+ | 3+ | 3+ | 2+ | 2+ | 2+ | 2+ | 2+ | 1+ | 3+ |
| AD, Braak VI | 3+ | 3+ | 2+ | 1+ | 0 | 1+ | 1+ | 1+ | 0 | 2+ |
| AD, Braak VI | 3+ | 3+ | 2+ | 1+ | 0 | 1+ | 1+ | 1+ | 0 | 2+ |
Double immunofluorescence staining experiments were also carried out to determine whether catalytically active PSKs colocalize with Ser-262/Ser-356 phosphorylated tau in AD hippocampus. As reported previously, the PSK-Ser(P)-181 antibody exhibited punctate staining, and we found that sites that were immunopositive for activated PSK were also immunoreactive with 12E8 (Fig. 7) (17, 31). These results demonstrate that catalytically active PSK and phosphorylated tau (Ser-262/Ser-356) are present at the same sites in AD brain.
FIGURE 7.
Catalytically active PSKs colocalize with phosphorylated tau-Ser(P)-262/356 in AD brains. AD brain sections (Braak stage VI) were double immunostained with antibodies to detect catalytically active PSKs (PSK-Ser(P)-181 and goat anti-rabbit 568 antibodies) and phosphorylated tau-Ser(P)-262/356 (12E8 and goat anti-mouse 488 antibodies). DNA was stained with DAPI (blue) and is shown in the merged panel. The hippocampal region of AD brain sections was analyzed, and images were captured using a confocal microscope (×63). Scale bars, 10 μm.
DISCUSSION
An important objective for dementia research is the identification of kinases that are activated catalytically in AD and contribute to the abnormal phosphorylation of PHF-tau. Here we have shown that PSKs are activated in tangle-bearing neurons in AD brain tissues and that tau is a substrate for these kinases. Mass spectrometric analysis of phosphorylated tau has identified 41 and 44 tau residues that are targeted by PSK1-α/β and PSK2, respectively, and 28 of these residues are phosphorylated in PHF-tau extracted from AD brain tissues (Table 3) (30). These results demonstrate that PSKs are strong candidate kinases for the abnormal phosphorylation of PHF-tau and may contribute to the development of AD pathology and tau-associated neurodegeneration.
TABLE 3.
Comparison of tau residues phosphorylated by PSK1-α/β and PSK2 in vitro or phosphorylated in control and AD brain extracts
Tau sites are numbered according to 2N4R nomenclature (column 1). N/A indicates residues for which sequence coverage was not obtained.
| Tau site | Recombinant tau |
Tau extracted from brain |
||
|---|---|---|---|---|
| Tau + PSK1α/β | Tau + PSK2 | Control tau | PHF-tau | |
| Thr-17 | a | a | ||
| Thr-30 | a | a | ||
| Ser-61 | a | |||
| Thr-63 | a | |||
| Thr-76 | a | a | ||
| Thr-123 | a | a | ||
| Ser-129 | N/A | N/A | ||
| Thr-149 | a | |||
| Thr-169 | a | a | ||
| Thr-175 Thr-Pro | a | a | ||
| Ser-184 | a | a | a | |
| Ser-185 | a | a | a | |
| Ser-191 | a | a | a | |
| Ser-195 | a | a | ||
| Ser-198 | a | a | a | |
| Ser-199 Ser-Pro | a | a | a | |
| Thr-205 Thr-Pro | a | a | a | a |
| Thr-212 Thr-Pro | a | a | a | |
| Ser-214 | a | a | a | |
| Thr-220 | a | |||
| Thr-231 Thr-Pro | a | a | a | a |
| Ser-237 | a | a | a | |
| Ser-238 | a | a | ||
| Ser-241 | a | a | ||
| Thr-245 | a | a | ||
| Ser-258 | a | a | a | |
| Ser-262 KXGS | a | a | a | |
| Thr-263 | a | a | ||
| Ser-285 | a | a | ||
| Ser-289 | a | a | a | |
| Ser-293 | N/A | N/A | ||
| Ser-305 | a | a | ||
| Thr-319 | N/A | a | ||
| Ser-320 | N/A | N/A | ||
| Ser-324 KXGS | a | a | ||
| Ser-352 | a | |||
| Ser-356 KXGS | a | a | a | |
| Thr-361 | a | a | ||
| Thr-373 | a | a | ||
| Thr-377 | a | a | ||
| Thr-386 | a | a | ||
| Ser-400 | a | a | a | a |
| Thr-403 | a | a | a | |
| Ser-409 | a | a | a | |
| Ser-412 | a | b | a | |
| Ser-413 | a | a | b | a |
| Thr-414 | a | a | b | a |
| Ser-416 | a | a | a | a |
| Ser-422 Ser-Pro | a | a | a | |
| Thr-427 | a | a | a | |
| Ser-433 | a | a | a | |
| Ser-435 | a | a | a | |
a Phosphorylated tau residues detected in this study using mass spectrometry and where recombinant human tau was incubated in the presence of purified PSK1-α/β (column 2) or purified PSK2 (column 3). For comparison, sites that are phosphorylated on tau extracted from control adult brain (column 4) or PHF-tau extracted from AD brain (column 5) are shown using data published previously (Ref. 30 and D. Hanger, personal communication).
b Two of three closely located phosphorylation sites (Ser-412, Ser-413, and/or Thr-414) in tau extracted from control brain (30).
In previous studies, phosphorylated tau residues have been divided into two types according to their location (37). The first type includes residues located in the tau microtubule-binding domain (amino acids 244–368) and are involved in regulating tau-microtubule interactions, and the second type includes residues located in the regions flanking this domain that are also abnormally phosphorylated in AD (37). The phosphorylation and detachment of tau from microtubules are likely to contribute to the aggregation of insoluble tau into PHFs, and we have shown here that PSK1-α/β and PSK2 can phosphorylate nine and 12 sites, respectively, within the tau microtubule-binding domain. Both kinases phosphorylate tau on the KXGS motif residues Ser-262, Ser-324, and Ser-356, and the phosphorylation of these residues is known to strongly inhibit tau-microtubule binding and microtubule stability (20). Furthermore, PSK1-α/β and/or PSK2 can also phosphorylate tau on Ser-214 and Thr-231, and the modification of these sites also reduces tau-microtubule interactions (38, 39). Tau phosphorylation on Ser-262/Ser-356 is an early event in the development of AD and is detectable during the pretangle stage in which neurons exhibit nonfibrillar punctate tau accumulations in the cytoplasm and when aggregated filamentous tau first appears in the cytoplasm (26). Likewise, in transgenic pR5 mice expressing the FTDP-17T tau mutation P301L in neurons, tau phosphorylated at Ser-262/Ser-356 is detectible after 3 months and precedes the appearance of NFTs at 6 months (40). To date, the main candidate kinase for the phosphorylation of tau on Ser-262/Ser-356 is MARK, but the ability of PSKs to target these KXGS motifs either directly and/or indirectly via the stimulation of MARK suggests a potential role for PSKs in the phosphorylation of tau on these sites (14, 41). Either way, although the phosphorylation of KXGS motifs and the dissociation of tau from microtubules appear to be important in AD pathogenesis, they are not sufficient by themselves to generate PHF-tau in vitro (37). It has been suggested that cytosolic tau may be more accessible than microtubule-bound tau to phosphorylation on additional sites and that the hyperphosphorylation of tau may be required to form PHFs (37). The detachment of phosphorylated tau (Ser-262/Ser-356) from microtubules may also disrupt microtubule integrity and axonal transport and contribute to neuronal dysfunction and neurodegeneration in this way (37).
As well as the Ser-262/Ser-356 sites, the phosphorylation of several additional tau residues located in the regions flanking the microtubule-binding domain has also been correlated with increasing AD severity, and the majority of these sites are also phosphorylated by PSKs (26). PSKs can target Ser-262 and Thr-231, which are phosphorylated during the formation of pretangles; Thr-175, Ser-214, Ser-262, Ser-356, and Ser-422, which are phosphorylated when intraneuronal NFTs appear; and Ser-199, Thr-205, Thr-212, and Thr-214, which are detected in extracellular NFTs (26). PSK1-α/β and PSK2 can also phosphorylate different residues on tau; all of the sites phosphorylated by PSK1-α/β alone occur in the N-terminal half of tau (e.g. Thr-63, Thr-123, Ser-198, Thr-212, and Thr-220), whereas the sites targeted by PSK2 alone are located throughout tau (e.g. Ser-61, Thr-149, Thr-175, Ser-199, Ser-238, Thr-319, Ser-352, and Ser-412) (Table 3). Although PSK1-α/β and PSK2 phosphorylate 33 and 34 sites, respectively, in the tau flanking regions, these kinases only target four and five of the possible 17 Ser-Pro or Thr-Pro motifs available (Table 3). However, PSK1-α/β and PSK2 can stimulate JNK and p38, and these proline-directed kinases are able to phosphorylate 10 and 15 tau sites, respectively, predominantly on Ser-Pro or Thr-Pro motifs (7, 12, 42, 43). PSKs may therefore generate hyperphosphorylated tau either directly or indirectly via the stimulation of additional kinases such as JNK, p38, and MARK (7, 8, 12, 14). JNK, p38, and MARK have been shown to be activated catalytically in tangle-bearing AD neurons, and each of these kinases is stimulated by PSKs (29, 41, 44). The coordinated action of several tau kinases during the progression of Alzheimer disease appears to be likely because PHF-tau extracted from AD brain is phosphorylated on more than 45 serine or threonine residues, and no single kinase can currently account for all of these modified sites (30). PSK1-α/β and PSK2 can phosphorylate tau on 28 of the residues that are modified in PHF-tau, and it is plausible that additional stimulation of JNK and/or p38 by PSKs could phosphorylate tau on additional sites (e.g. Ser-46, Thr-181, Ser-202, Thr-217, Ser-235, Ser-396, and Ser-404) (Table 3) (30, 43).
To date, the only other kinases capable of phosphorylating tau on a similar number of residues to PSKs in vitro are GSK3 and casein kinase 1. GSK3 and casein kinase 1 phosphorylate tau on 37 and 33 residues, respectively, and 26 and 15 of these respective sites are modified in PHF-tau (28, 30, 33). Moreover, GSK3 phosphorylates nine sites on tau that are modified in PHF-tau but do not appear to be targeted by PSKs (e.g. Thr-69, Thr-153, Thr-181, Ser-202, Ser-210, Thr-217, Ser-235, Ser-396, and Ser-404) (Table 3) (30). Whether GSK3 is activated in tangle-bearing neurons is unclear. Activated GSK3-Tyr(P)-216 has been detected on NFTs, but additional studies were unable to show colocalization of GSK3 with NFTs or have found inactive GSK3-Ser(P)-9 on NFTs and in neuropil threads (45–48). In transgenic mice, GSK3 is an efficient kinase, and it increases the phosphorylation of wild type tau without inducing tau fibrillization, but NFT numbers are enhanced when GSK3 is coexpressed with mutated tau (P301L) (49–51). These studies suggest that GSK3 activity alone may not be sufficient to phosphorylate and aggregate tau into fibrils and tangles in transgenic mice and that additional factors may be required. In AD brain, tau is not only highly phosphorylated but also fragmented, and tau cleavage by proteolytic enzymes may contribute to the aggregation of insoluble tau. Caspases can cleave tau to produce C-terminally truncated tau (Δtau; amino acids 1–420), and this tau fragment has been detected on NFTs and is toxic to neurons (52–54). PSK1-α/β and PSK2 are known to stimulate caspase activity via JNK and induce apoptosis, and it is plausible that PSKs may also promote tau cleavage by caspases to further promote the generation of tau aggregates and NFTs to cause tau-associated neurodegeneration in AD (8, 9).
Increased tau phosphorylation also occurs during early neuronal development when changes in microtubule dynamics and organization are required for axon and dendrite formation and the growth and shrinkage of exploratory neurites. Mass spectrometric analysis of tau extracted from fetal rat brain has identified 16 phosphorylated tau residues to date, and we have shown here that eight of these sites are targeted by PSKs (Table 3).3 These observations suggest potential roles for PSKs in the regulation of tau phosphorylation and microtubules during neuronal differentiation and early brain development. In support of this notion, PSKs are activated catalytically in embryonic cortical neurons, and PSK1-α/β is required to transduce signals from semaphorin 3A and the neuropilin 1 receptor to regulate dendrite arborization and axon elongation in the developing cerebral cortex (55). PSK2 is also essential for cultured neurons to undergo neuritogenesis and differentiate (14). Whether PSKs exert their effects on microtubules and neuronal differentiation via the phosphorylation and regulation of tau remains to be determined, but MARK phosphorylates tau on Ser-262/Ser-356, and this modification is required for neurite outgrowths and the establishment of neuronal polarity (20, 21, 23). PSKs may also phosphorylate these tau residues either directly or indirectly via the stimulation of MARK (14).
In summary, we have shown that tau is a substrate for PSK1-α/β and PSK2 and that these kinases can target multiple sites on tau, many of which are also phosphorylated in Alzheimer brain. Following immunostaining of AD brain tissues, catalytically active PSKs were detected on NFTs, in neuropil threads, in dystrophic neurites surrounding neuritic plaques, and in granulovacuolar degeneration bodies, and each of these structures is characteristic for AD. Activated PSKs and phosphorylated tau were not detected in age-matched control brains but were present on rare NFTs and in neuropil threads that occur due to normal aging. PSK activity is therefore associated with the appearance of NFTs in control as well as AD neurons. The deposition of insoluble and phosphorylated tau in the brain is also characteristic of a number of other neurological disorders, including Pick disease, FTDP-17, progressive supranuclear palsy, argyrophilic grain disease, Guam Parkinsonism dementia, and corticobasal degeneration (25, 28, 56). Collectively these neurological disorders are known as tauopathies, and it remains to be determined whether PSK activity correlates with the appearance of phosphorylated and aggregated tau in each of these diseases as well. However, an analysis of tau extracted from brains with progressive supranuclear palsy has identified 17 phosphorylated tau residues, and 10 of these sites can be phosphorylated by PSKs (57). In addition to these tauopathies, the PSK1 gene is located on chromosome 16p11.2, and microdeletions or microduplications at this site are commonly associated with increased susceptibility to autism and schizophrenia (58–60). Moreover, PSKs can be phosphorylated by the leucine-rich repeat kinase 2, and this molecule is causal for autosomal dominant Parkinson disease (61, 62). Taken together these observations and the results presented here suggest important roles for these relatively uncharacterized protein kinases in contributing to the abnormal phosphorylation of tau and the pathogenesis of AD and potentially other neurological disorders as well. Further work is now needed to investigate the activity and biological roles of PSKs during the development of AD and the mechanisms by which PSKs may contribute to neurodegeneration.
Acknowledgments
We thank the MRC London Neurodegenerative Diseases Brain Bank (King's College London) and Brains for Dementia Research for providing tissues. We also thank Costas Mitsopoulos, Ayodeji Asuni, Pahini Pandya, Chris Miller, and Brian Anderton for many helpful discussions during this study.
This work was supported by the Medical Research Council (to D. H. and W. N.), the Alzheimer's Association and British United Provident Association Limited (to R. K.), and the King's Medical Research Trust and a donation from Laura Price (to J. M.).
D. Hanger, personal communication.
- STE20
- sterile 20
- PSK
- prostate-derived sterile 20-like kinase
- PSK-Ser(P)-181
- catalytically active PSK phosphorylated on serine 181
- tau-Ser(P)-262/356
- tau phosphorylated on serine 262 and/or 356
- NFT
- neurofibrillary tangle
- AD
- Alzheimer disease
- TAOK
- thousand and one amino acid kinase
- MARK
- microtubule affinity-regulating kinase
- GSK3
- glycogen synthase kinase-3
- FTDP-17
- familial frontotemporal dementia with Parkinsonism linked to chromosome 17
- PHF
- paired helical filament.
REFERENCES
- 1. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 2. Dan I., Watanabe N. M., Kusumi A. (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230 [DOI] [PubMed] [Google Scholar]
- 3. Delpire E. (2009) The mammalian family of sterile 20p-like protein kinases. Pflugers Arch. 458, 953–967 [DOI] [PubMed] [Google Scholar]
- 4. Bokoch G. M. (2003) Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 [DOI] [PubMed] [Google Scholar]
- 5. Arias-Romero L. E., Chernoff J. (2008) A tale of two Paks. Biol. Cell 100, 97–108 [DOI] [PubMed] [Google Scholar]
- 6. Eswaran J., Soundararajan M., Kumar R., Knapp S. (2008) UnPAKing the class differences among p21-activated kinases. Trends Biochem. Sci. 33, 394–403 [DOI] [PubMed] [Google Scholar]
- 7. Moore T. M., Garg R., Johnson C., Coptcoat M. J., Ridley A. J., Morris J. D. (2000) PSK, a novel STE20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J. Biol. Chem. 275, 4311–4322 [DOI] [PubMed] [Google Scholar]
- 8. Zihni C., Mitsopoulos C., Tavares I. A., Ridley A. J., Morris J. D. (2006) Prostate-derived sterile 20-like kinase 2 (PSK2) regulates apoptotic morphology via c-Jun N-terminal kinase and Rho kinase-1. J. Biol. Chem. 281, 7317–7323 [DOI] [PubMed] [Google Scholar]
- 9. Zihni C., Mitsopoulos C., Tavares I. A., Baum B., Ridley A. J., Morris J. D. (2007) Prostate-derived sterile 20-like kinase 1-α induces apoptosis. JNK- and caspase-dependent nuclear localization is a requirement for membrane blebbing. J. Biol. Chem. 282, 6484–6493 [DOI] [PubMed] [Google Scholar]
- 10. Tassi E., Biesova Z., Di Fiore P. P., Gutkind J. S., Wong W. T. (1999) Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor. J. Biol. Chem. 274, 33287–33295 [DOI] [PubMed] [Google Scholar]
- 11. Hutchison M., Berman K. S., Cobb M. H. (1998) Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 273, 28625–28632 [DOI] [PubMed] [Google Scholar]
- 12. Chen Z., Hutchison M., Cobb M. H. (1999) Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J. Biol. Chem. 274, 28803–28807 [DOI] [PubMed] [Google Scholar]
- 13. Mitsopoulos C., Zihni C., Garg R., Ridley A. J., Morris J. D. (2003) The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 278, 18085–18091 [DOI] [PubMed] [Google Scholar]
- 14. Timm T., Li X. Y., Biernat J., Jiao J., Mandelkow E., Vandekerckhove J., Mandelkow E. M. (2003) MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 22, 5090–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timm T., Matenia D., Li X. Y., Griesshaber B., Mandelkow E. M. (2006) Signaling from MARK to tau: regulation, cytoskeletal crosstalk, and pathological phosphorylation. Neurodegener. Dis. 3, 207–217 [DOI] [PubMed] [Google Scholar]
- 16. Timm T., Balusamy K., Li X., Biernat J., Mandelkow E., Mandelkow E. M. (2008) Glycogen synthase kinase (GSK) 3β directly phosphorylates Serine 212 in the regulatory loop and inhibits microtubule affinity-regulating kinase (MARK) 2. J. Biol. Chem. 283, 18873–18882 [DOI] [PubMed] [Google Scholar]
- 17. Wojtala R. L., Tavares I. A., Morton P. E., Valderrama F., Thomas N. S., Morris J. D. (2011) Prostate-derived sterile 20-like kinases (PSKs/TAOKs) are activated in mitosis and contribute to mitotic cell rounding and spindle positioning. J. Biol. Chem. 286, 30161–30170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3, 519–526 [DOI] [PubMed] [Google Scholar]
- 19. Mandelkow E. M., Mandelkow E. (2012) Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2, a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11, 153–163 [DOI] [PubMed] [Google Scholar]
- 21. Biernat J., Mandelkow E. M. (1999) The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains. Mol. Biol. Cell 10, 727–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drewes G., Ebneth A., Mandelkow E. M. (1998) MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci. 23, 307–311 [DOI] [PubMed] [Google Scholar]
- 23. Biernat J., Wu Y. Z., Timm T., Zheng-Fischhöfer Q., Mandelkow E., Meijer L., Mandelkow E. M. (2002) Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol. Biol. Cell 13, 4013–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., Andreadis A., Wiederholt W. C., Raskind M., Schellenberg G. D. (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43, 815–825 [DOI] [PubMed] [Google Scholar]
- 25. Lee V. M., Goedert M., Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 26. Augustinack J. C., Schneider A., Mandelkow E. M., Hyman B. T. (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 103, 26–35 [DOI] [PubMed] [Google Scholar]
- 27. Drewes G., Trinczek B., Illenberger S., Biernat J., Schmitt-Ulms G., Meyer H. E., Mandelkow E. M., Mandelkow E. (1995) Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 270, 7679–7688 [DOI] [PubMed] [Google Scholar]
- 28. Hanger D. P., Anderton B. H., Noble W. (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119 [DOI] [PubMed] [Google Scholar]
- 29. Vingtdeux V., Davies P., Dickson D. W., Marambaud P. (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol. 121, 337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanger D. P., Noble W. (2011) Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int. J. Alzheimers Dis. 2011, 352805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu T., Rohn J. L., Picone R., Kunda P., Baum B. (2010) Tao-1 is a negative regulator of microtubule plus-end growth. J. Cell Sci. 123, 2708–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seubert P., Mawal-Dewan M., Barbour R., Jakes R., Goedert M., Johnson G. V., Litersky J. M., Schenk D., Lieberburg I., Trojanowski J. Q., Lee V. M. (1995) Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J. Biol. Chem. 270, 18917–18922 [DOI] [PubMed] [Google Scholar]
- 33. Hanger D. P., Byers H. L., Wray S., Leung K. Y., Saxton M. J., Seereeram A., Reynolds C. H., Ward M. A., Anderton B. H. (2007) Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J. Biol. Chem. 282, 23645–23654 [DOI] [PubMed] [Google Scholar]
- 34. Caušević M., Farooq U., Lovestone S., Killick R. (2010) β-Amyloid precursor protein and tau protein levels are differently regulated in human cerebellum compared to brain regions vulnerable to Alzheimer's type neurodegeneration. Neurosci. Lett. 485, 162–166 [DOI] [PubMed] [Google Scholar]
- 35. Zhou T., Raman M., Gao Y., Earnest S., Chen Z., Machius M., Cobb M. H., Goldsmith E. J. (2004) Crystal structure of the TAO2 kinase domain: activation and specificity of a Ste20p MAP3K. Structure 12, 1891–1900 [DOI] [PubMed] [Google Scholar]
- 36. Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider A., Mandelkow E. (2008) Tau-based treatment strategies in neurodegenerative diseases. Neurotherapeutics 5, 443–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho J. H., Johnson G. V. (2003) Glycogen synthase kinase 3β phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 278, 187–193 [DOI] [PubMed] [Google Scholar]
- 39. Schneider A., Biernat J., von Bergen M., Mandelkow E., Mandelkow E. M. (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38, 3549–3558 [DOI] [PubMed] [Google Scholar]
- 40. Deters N., Ittner L. M., Götz J. (2008) Divergent phosphorylation pattern of tau in P301L tau transgenic mice. Eur. J. Neurosci. 28, 137–147 [DOI] [PubMed] [Google Scholar]
- 41. Matenia D., Mandelkow E. M. (2009) The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem. Sci. 34, 332–342 [DOI] [PubMed] [Google Scholar]
- 42. Raman M., Earnest S., Zhang K., Zhao Y., Cobb M. H. (2007) TAO kinases mediate activation of p38 in response to DNA damage. EMBO J. 26, 2005–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reynolds C. H., Betts J. C., Blackstock W. P., Nebreda A. R., Anderton B. H. (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3β. J. Neurochem. 74, 1587–1595 [DOI] [PubMed] [Google Scholar]
- 44. Ferrer I., Blanco R., Carmona M., Puig B. (2001) Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies. J. Neural Transm. 108, 1397–1415 [DOI] [PubMed] [Google Scholar]
- 45. Pei J. J., Braak E., Braak H., Grundke-Iqbal I., Iqbal K., Winblad B., Cowburn R. F. (1999) Distribution of active glycogen synthase kinase 3β (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 58, 1010–1019 [DOI] [PubMed] [Google Scholar]
- 46. Leroy K., Yilmaz Z., Brion J. P. (2007) Increased level of active GSK-3β in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 33, 43–55 [DOI] [PubMed] [Google Scholar]
- 47. Ferrer I., Barrachina M., Puig B. (2002) Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 104, 583–591 [DOI] [PubMed] [Google Scholar]
- 48. Harr S. D., Hollister R. D., Hyman B. T. (1996) Glycogen synthase kinase 3α and 3β do not colocalize with neurofibrillary tangles. Neurobiol. Aging 17, 343–348 [DOI] [PubMed] [Google Scholar]
- 49. Spittaels K., Van den Haute C., Van Dorpe J., Geerts H., Mercken M., Bruynseels K., Lasrado R., Vandezande K., Laenen I., Boon T., Van Lint J., Vandenheede J., Moechars D., Loos R., Van Leuven F. (2000) Glycogen synthase kinase-3β phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 275, 41340–41349 [DOI] [PubMed] [Google Scholar]
- 50. Terwel D., Muyllaert D., Dewachter I., Borghgraef P., Croes S., Devijver H., Van Leuven F. (2008) Amyloid activates GSK-3β to aggravate neuronal tauopathy in bigenic mice. Am. J. Pathol 172, 786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kremer A., Louis J. V., Jaworski T., Van Leuven F. (2011) GSK3 and Alzheimer's disease: facts and fiction. Front. Mol. Neurosci. 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gamblin T. C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A. L., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., Miller R., Berry R. W., Binder L. I., Cryns V. L. (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 100, 10032–10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung C. W., Song Y. H., Kim I. K., Yoon W. J., Ryu B. R., Jo D. G., Woo H. N., Kwon Y. K., Kim H. H., Gwag B. J., Mook-Jung I. H., Jung Y. K. (2001) Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis. 8, 162–172 [DOI] [PubMed] [Google Scholar]
- 54. Hanger D. P., Wray S. (2010) Tau cleavage and tau aggregation in neurodegenerative disease. Biochem. Soc. Trans. 38, 1016–1020 [DOI] [PubMed] [Google Scholar]
- 55. de Anda F. C., Rosario A. L., Durak O., Tran T., Gräff J., Meletis K., Rei D., Soda T., Madabhushi R., Ginty D. D., Kolodkin A. L., Tsai L. H. (2012) Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat. Neurosci. 15, 1022–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hernández F., Avila J. (2007) Tauopathies. Cell. Mol. Life Sci. 64, 2219–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hanger D. P., Seereeram A., Noble W. (2009) Mediators of tau phosphorylation in the pathogenesis of Alzheimer's disease. Expert Rev. Neurother. 9, 1647–1666 [DOI] [PubMed] [Google Scholar]
- 58. Weiss L. A., Shen Y., Korn J. M., Arking D. E., Miller D. T., Fossdal R., Saemundsen E., Stefansson H., Ferreira M. A., Green T., Platt O. S., Ruderfer D. M., Walsh C. A., Altshuler D., Chakravarti A., Tanzi R. E., Stefansson K., Santangelo S. L., Gusella J. F., Sklar P., Wu B. L., Daly M. J. (2008) Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 358, 667–675 [DOI] [PubMed] [Google Scholar]
- 59. McCarthy S. E., Makarov V., Kirov G., Addington A. M., McClellan J., Yoon S., Perkins D. O., Dickel D. E., Kusenda M., Krastoshevsky O., Krause V., Kumar R. A., Grozeva D., Malhotra D., Walsh T., Zackai E. H., Kaplan P., Ganesh J., Krantz I. D., Spinner N. B., Roccanova P., Bhandari A., Pavon K., Lakshmi B., Leotta A., Kendall J., Lee Y. H., Vacic V., Gary S., Iakoucheva L. M., Crow T. J., Christian S. L., Lieberman J. A., Stroup T. S., Lehtimäki T., Puura K., Haldeman-Englert C., Pearl J., Goodell M., Willour V. L., Derosse P., Steele J., Kassem L., Wolff J., Chitkara N., McMahon F. J., Malhotra A. K., Potash J. B., Schulze T. G., Nöthen M. M., Cichon S., Rietschel M., Leibenluft E., Kustanovich V., Lajonchere C. M., Sutcliffe J. S., Skuse D., Gill M., Gallagher L., Mendell N. R., Wellcome Trust Case Control Consortium, Craddock N., Owen M. J., O'Donovan M. C., Shaikh T. H., Susser E., Delisi L. E., Sullivan P. F., Deutsch C. K., Rapoport J., Levy D. L., King M. C., Sebat J. (2009) Microduplications of 16p11.2 are associated with schizophrenia. Nat. Genet. 41, 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar R. A., Marshall C. R., Badner J. A., Babatz T. D., Mukamel Z., Aldinger K. A., Sudi J., Brune C. W., Goh G., Karamohamed S., Sutcliffe J. S., Cook E. H., Geschwind D. H., Dobyns W. B., Scherer S. W., Christian S. L. (2009) Association and mutation analyses of 16p11.2 autism candidate genes. PLoS One 4, e4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zach S., Felk S., Gillardon F. (2010) Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKCζ that modulate neuronal plasticity. PLoS One 5, e13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kawakami F., Yabata T., Ohta E., Maekawa T., Shimada N., Suzuki M., Maruyama H., Ichikawa T., Obata F. (2012) LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS One 7, e30834. [DOI] [PMC free article] [PubMed] [Google Scholar]