Abstract
In 2011, a joint World Health Organization (WHO) and United Nations Environment Programme (UNEP) expert consultation took place, during which the possible inclusion of brominated analogues of the dioxin-like compounds in the WHO Toxicity Equivalency Factor (TEF) scheme was evaluated. The expert panel concluded that polybrominated dibenzo-p-dioxins (PBDDs), dibenzofurans (PBDFs), and some dioxin-like biphenyls (dl-PBBs) may contribute significantly in daily human background exposure to the total dioxin toxic equivalencies (TEQs). These compounds are also commonly found in the aquatic environment. Available data for fish toxicity were evaluated for possible inclusion in the WHO-UNEP TEF scheme (van den Berg et al., 1998). Because of the limited database, it was decided not to derive specific WHO-UNEP TEFs for fish, but for ecotoxicological risk assessment, the use of specific relative effect potencies (REPs) from fish embryo assays is recommended. Based on the limited mammalian REP database for these brominated compounds, it was concluded that sufficient differentiation from the present TEF values of the chlorinated analogues (van den Berg et al., 2006) was not possible. However, the REPs for PBDDs, PBDFs, and non-ortho dl-PBBs in mammals closely follow those of the chlorinated analogues, at least within one order of magnitude. Therefore, the use of similar interim TEF values for brominated and chlorinated congeners for human risk assessment is recommended, pending more detailed information in the future.
Key Words: dioxin, halogenated hydrocarbon, persistent organic chemicals, polychlorinated biphenyls, regulatory/policy, biomarkers.
The Toxicity Equivalency Factor (TEF) methodology has been evolving since it was first proposed by the Ontario Ministry of the Environment in 1983. The World Health Organization (WHO) has been the lead regulatory agency involved in the development of this methodology (Ahlborg et al., 1994; van den Berg et al., 1998, 2006). Initially, this TEF method included only polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs). As exposure, mechanistic, and toxicological data accumulated, the dioxin-like polychlorinated biphenyls (dl-PCBs) were included in the TEF approach for risk assessment. When the TEF methodology was refined further, other individual chemicals or chemical classes were evaluated or proposed for inclusion. The polybrominated dioxins (PBDDs) and dibenzofurans (PBDFs) are classes of chemicals that were recently proposed for inclusion (van den Berg et al., 2006). This review represents the outcome of a joint WHO and United Nations Environment Programme (UNEP) expert consultation. It discusses the available information on exposure, mechanism of action, and toxicity of the brominated analogues of the PCDDs, PCDFs, and PCBs and provides arguments for including these compounds in the existing WHO TEF scheme.
OCCURRENCE AND EXPOSURE
Over the last decade, there has been accumulating evidence that PBDDs and PBDFs can be found in a variety of biological matrices in addition to their well-known presence in abiotic matrices such as fly ash. One of the most noticeable occurrences is their presence as impurities in commercial mixtures of brominated flame retardants (BFRs), such as polybrominated diphenylethers (PBDEs), and subsequent occurrence in household products and house dust. PBDFs are major contaminants of PBDEs with congener profiles dependent on the degree of bromination of the commercial mixture. PBDDs are usually not detected above the analytical limit of detection in these flame retardant mixtures, and based on the production of these commercial mixtures, the potential global emissions of PBDFs in 2001 were estimated to be several thousands of kilograms (Hanari et al., 2006).
In addition, during combustion processes, such as in poorly run municipal waste incinerators, significant amounts of PBDDs and PBDFs can be formed, including those with the most toxic dioxin-like properties (Tu et al., 2011). Open burning of domestic waste and accidental fires can produce similar or higher amounts of PBDDs and PBDFs compared with their chlorinated congeners. The origin of these PBDFs may partly be explained by their presence as contaminants in the commercial PBDE flame retardant mixtures in existing (household) materials, but de novo synthesis can not be excluded (Gullett et al., 2010; Lundstedt et al., 2011). Furthermore, thermal degradation, photochemical transformation, sunlight exposure, and recycling of (other) BFRs, such as tetrabromobisphenol A, can also yield significant amounts of PBDFs (Ortuño et al., 2011).
The formation of TrBDDs and TeBDDs in the marine environment via natural processes is also well established, and reactive bromophenols and hydroxylated PBDEs are assumed to be precursors of the naturally occurring PBDDs. Although several TeBDD isomers have been measured in the marine environment, the highly toxic 2,3,7,8-TeBDD has not been detected, and only congeners with bromine atoms on two or three lateral positions have been observed (Haglund, 2010; Haglund et al., 2007). TrBDDs have been detected in mussels, algae, and sponges (Löfstrand et al., 2010; Malmvärn et al., 2005a,b, 2008; Unger et al., 2009). In view of their common occurrence in the marine food chain, more toxicological studies are required to determine whether these lower brominated congeners also meet the criteria for inclusion in the WHO TEFs approach for dioxin-like compounds (van den Berg et al., 1998). The congener-specific patterns of PBDDs and PBDFs from the marine environment and combustion processes are quite distinct with higher chlorinated and more toxic congeners dominating from the latter source (Tu et al., 2011; Wyrzykowska et al., 2009). Another source of direct human exposure to PBDDs and PBDFs is house and office dust, which may originate from wear and tear processes of common household products, e.g., polyurethane foam, TV sets, computers, and other electronic and electrical equipment, containing flame retardants such as PBDEs. 2,3,7,8-Substituted PBDDs and PBDFs are detected at significant quantities, e.g., in house dust and sewage sludge (Brorstrom-Lunden et al., 2010; Suzuki et al., 2010). If similar TEF values are applied for the 2,3,7,8-substituted PBDDs and PBDFs as for the chlorinated congeners, the brominated congeners can contribute up to 17% of the total amount of TEQs in Japanese house dust (Suzuki et al., 2010). Due to the widespread occurrence of 2,3,7,8-substituted PBDDs and PBDFs, particularly in the aquatic environment, it is not surprising that these compounds were detected in seafood for human consumption. Recent studies from various countries indicate that PBDDs and PBDFs can contribute significantly to the total amount of TEQ in seafood items compared with their chlorinated congeners (Ashizuka et al., 2005; Fernandes et al., 2008, 2009a; Lam et al., 2008). A comparable situation is observed for the presence of 2,3,7,8-substituted PBDFs in dairy products and meat (Fernandes et al., 2009b). In the present WHO TEF/TEQ scheme, certain dl-PCBs contribute significantly to the total TEQs in many biological or food samples (van den Berg et al., 1998, 2006). The TEQ contribution of dioxin-like polybrominated biphenyls (dl-PBBs) is yet not clarified sufficiently; however, limited recent data indicate that non-ortho dl-PBBs (i.e., 81, 77, 126, and 169) are found in human milk and shellfish, albeit at very low concentrations (M. Rose (personal communication)). Future studies must show whether these dl-PBB congeners indeed contribute significantly to the total amount of TEQ in human milk and food. As a result of their presence in the human food chain and abiotic environment, e.g., house dust and combustion particles, it could be predicted that 2,3,7,8-substituted PBDDs and PBDFs are found in human tissues. Although this information is still limited, dioxin-like PBDDs and PBDFs have indeed been found at concentrations, which warrant further studies with respect to their contribution to the total amount of TEQ in the human body (Choi et al., 2003; Ericson Jogsten et al., 2010).
KINETICS AND METABOLISM
Absorption
A limited number of studies have been conducted with PBDDs and PBDFs, providing comparative data with their chlorinated congeners. 2,3,7,8-TeBDD and -TeBDF have been studied in some detail. Based on these data, it can be assumed that gastrointestinal, pulmonary, and dermal uptake does not vary significantly between the chlorinated and brominated congeners (Banks and Birnbaum, 1991; Brewster et al., 1989; Diliberto et al., 1993; Nagao et al., 1996). As observed with chlorinated congeners, the amount of absorption/bioavailability in the body depends significantly on the number of bromine substituents.
Tissue Distribution
PBDDs and PBDFs are primarily retained in liver and adipose tissue as reported earlier for chlorinated analogues. Distribution between these tissues is dose dependent and similar for 2,3,7,8-TeBDD and 2,3,7,8-TeCDD. Differences observed after dermal absorption are attributed to the higher lipophilicity and/or molecular size of 2,3,7,8-TeBDD (Diliberto et al., 1993; Kedderis et al., 1991a, b, 1992, 1993, 1994). Comparative studies between 2,3,7,8-TeCDD and TeBDD indicate comparable liver/adipose tissue ratios (Nagao et al., 1996; WHO, 1998). The tissue distribution of 1,2,3,7,8-PeCDD and -PeBDD in the rat is also comparable (Golor et al., 1993). Toxicokinetic information for PBDFs is even more limited than for the PBDDs. A study with 1,2,7,8-TeBDF indicated an affinity for the liver and adipose tissue in the rat comparable with TeBDD (Kedderis et al., 1994). The tissue distribution of 2,3,4,7,8-PeBDF was also studied in the marmoset monkey with liver and adipose tissue being major storage sites (Schulz et al., 1993). However, 2,3,4,7,8-PeBDF and -PeCDF have a much higher affinity for the liver than 2,3,7,8-TeCDD or -TeBDD. This indicates similar congener-specific differences in hepatic affinity for the brominated and chlorinated congeners (van den Berg et al., 1994).
Metabolism
Like 2,3,7,8-TeCDD, elimination of 2,3,7,8-TeBDD is relatively slow in rodents, and metabolic transformation to mono- and dihydroxy metabolites occurs for both congeners, but oxygen bridge cleavage appears predominant for TeCDD (De Jongh et al., 1993; Poiger and Buser, 1984). At present, information regarding metabolic pathways of 2,3,7,8-substituted PBDFs is lacking, and limited information is only available for 1,2,7,8-TeBDF (Kedderis et al., 1994). 1,2,7,8-TeBDF was rapidly metabolized in the rat, which is consistent with the presence of two adjacent unsubstituted carbons facilitating rapid metabolism of PCDF (van den Berg et al., 1994).
Elimination and Excretion
Tissue-specific and whole-body half-life has only been reported for 2,3,7,8-TeBDD in rodents. Comparable with 2,3,7,8-TeCDD, there are significant differences in elimination from the liver and adipose tissue, with more rapid elimination from the liver. Half-lives of 2,3,7,8-TeBDD in liver, adipose tissue, and whole body of the rat varied between 15–25, 40–55, and 15–20 days, respectively (Kedderis et al., 1991b; Nagao et al., 1996). Thus, elimination rates are comparable with those reported in rodents for 2,3,7,8-TeCDD (van den Berg et al., 1994). Nevertheless, differences between the disposition of 2,3,7,8-TeBDD and -TeCDD in mice following subchronic exposure have also been reported with TeBDD retained less than TeCDD, i.e., 6.9 versus 23.5% of the dose, respectively. In addition, dose-dependent hepatic sequestration of TeBDD was observed in these mice, with a four- to fivefold induction of CYP1A2 activity (DeVito et al., 1998). The elimination rate of 1,2,3,7,8-PeCDD from the liver and adipose tissue in the rat was similar to 2,3,7,8-TeBDD (Golor et al., 1993) and is comparable with that observed for 2,3,7,8-TeCDD and 1,2,3,7,8-PeCDD (van den Berg et al., 1994).
Blood from an industrial cohort has provided information about the half-lives of 2,3,7,8-TeBDD and 2,3,7,8-TeBDF in humans, which was reported to be 2.9–10 (mean value 5.9) and 1.1–1.9 (mean value 1.5) years, respectively (Zober et al., 1992). Thus, in humans, the half-life of 2,3,7,8-TeBDD is comparable to 2,3,7,8-TeCDD (5–11 years) (Pirkle et al., 1989; Poiger and Schlatter, 1986; Wong et al., 2011). Furthermore, observed toxicokinetic differences between TeBDD and TeBDF are similar to those observed for both chlorinated congeners in several laboratory species and humans (van den Berg et al., 1994).
In conclusion, the available toxicokinetic information for PBDDs and PBDFs indicates properties comparable with the chlorinated congeners. This applies to tissue distribution, metabolism, and excretion. However, the number of brominated congeners that has been studied remains limited. These results suggest that the weaker carbon-bromine bond compared with the carbon-chlorine bond does not lead to more rapid elimination of the brominated congeners in experimental animals and humans. Thus, experimental data provide evidence that a comparable persistence in biota, including humans, for the 2,3,7,8-substituted brominated and chlorinated congeners exists.
BIOLOGICAL AND TOXIC EFFECTS IN MAMMALS AND FISH
Because PBDDs, PBDFs, and PBBs occur in the abiotic environment and in the human food chain, risks should be determined for both human and wildlife populations. Such an approach is consistent with the 1998 WHO TEF reassessment, which differentiated TEF values for humans (mammals), birds, and fish (van den Berg et al., 1998). A similar approach was chosen for this joint WHO-UNEP evaluation to determine TEF values that are useful for human and ecotoxicological risk assessment. As expected, the majority of the data is available from mammalian in vivo and in vitro studies and the expert panel thought that reliable, but limited, data are available for relative potencies of PBDDs and PBDFs in fish. However, information for avian models is lacking. It was also found that information for birds and dl-PBBs is not sufficient to determine REPs. Therefore, this WHO-UNEP consultation only evaluated relative potencies for humans and fish.
Structure-Activity Relationships in Mammalian Models
The structure-activity relationships (SARs) of PCDDs and PCDFs are among the best examined from any group of environmental contaminants. Numerous publications have summarized these SARs for various toxicological, biochemical, and molecular endpoints using a wide variety of in vivo and in vitro systems, including human tissues. From these studies, there is scientific consensus that congeners with a 2,3,7,8-substituted chlorine pattern can elicit dioxin-like toxicity (Safe, 1986, 1990, 1994). Based on this scientific information, the WHO developed consensus TEFs for the 2,3,7,8-substituted PCDDs, PCDFs, and some PCBs based upon a similar dioxin-like mechanism of action in humans and wildlife (Ahlborg et al., 1994; van den Berg et al., 1998, 2006). There is general agreement that similar SARs can be applied for the 2,3,7,8-substituted PBDDs and PBDFs (Birnbaum et al., 2003; Weber and Greim, 1997; WHO, 1998). Information that supports this view follows in the paragraphs below.
Biochemical Effects in Mammalian Models
Exposure to 2,3,7,8-TeCDD, the prototypical and most potent persistent dioxin-like polyhalogenated aromatic hydrocarbon (dl-PHAH), and related congeners like PCDDs, PCDFs, and dl-PCBs produce a wide variety of species and tissue-specific toxic and biological effects (Denison et al., 2011; Furness et al., 2007; Hankinson, 1995; Poland and Knutson, 1982; Safe, 1990). Furthermore, the majority, if not all, of the biological and toxic effects of these compounds in vertebrates are mediated by the Ah receptor (AhR), a soluble intracellular ligand-activated transcription factor. AhR-dependent (“dioxin”) toxicity involves persistent activation of the AhR signaling pathway by metabolically stable (persistent) dl-PHAHs and subsequent alterations in gene expression (Denison et al., 2011; Poland and Knutson, 1982; Safe, 1990). There is much experimental evidence that demonstrates the ability of 2,3,7,8-substituted PBDDs and PBDFs to bind to the AhR, activate the AhR signaling pathway, and produce classical dioxin-like toxic and biological effects (Andres et al., 1983; Dannan et al., 1983; Render et al., 1982; Robertson et al., 1982, 1983). The routine detection of 2,3,7,8-substituted PBDDs and PBDFs in biological and environmental matrices, including those of the human food chain, by itself warrants inclusion in the WHO-UNEP TEF concept. For this WHO-UNEP evaluation, the scientific literature was reviewed for published relative potencies (REPs) of PBDDs, PBDFs, and dl-PBBs and compared with their chlorinated congeners.
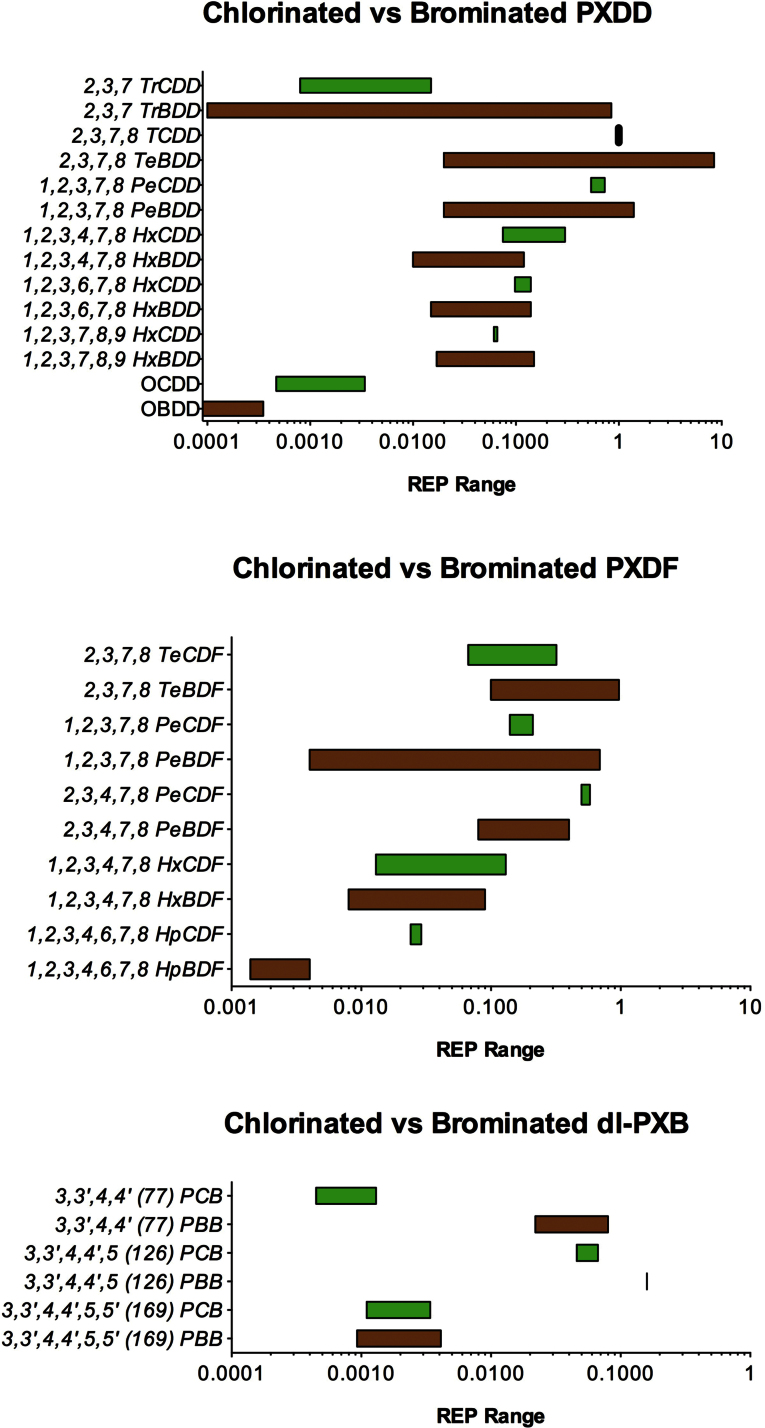
Figure 1 shows REP ranges for brominated compounds compiled from a range of in vitro and in vivo studies and compares them with their respective chlorinated compounds, and the numerical value ranges for these REPs are presented in Supplementary table 1. These values have been calculated on a molar basis to correct for the large differences in molecular weight that are associated with the brominated congeners. In general, REP ranges of the chlorinated versus brominated congeners reveal some minor differences, but a general overlap is present for the most toxic and relevant congeners. One of the most noticeable differences observed is the fact that PBB 77 appears significantly more potent than PCB 77.
FIG. 1.
Relative potency ranges of PXDDs, PXDFs, and dl-PXBs compiled from in vitro and in vivo studies. Polychlorinated compounds are indicated in green bars and polybrominated compounds indicated in brown bars.
For mono-ortho dl-PBBs, very limited information is available and does not allow quantitative evaluation of their REP ranges compared with their chlorinated analogues. However, these studies indicated that the SARs may be comparable with their chlorinated analogues (Dannan et al., 1983; Parkinson et al., 1983; Robertson et al., 1984).
Toxicity in Mammalian Models
In vivo toxicity studies with PBDDs and PBDFs are limited compared with those with PCDDs and PCDFs. However, there is general consensus from a qualitative point of view that the 2,3,7,8-substituted brominated congeners produce a similar toxic spectrum as TeCDD, albeit with congener-specific differences in potency (Kimbrough and Jensen, 1989; WHO, 1998). In rodents, the major overt toxic effects of 2,3,7,8-TeBDD are observed on thymus, body weight, and liver, whereas teratogenic effects such as hydronephrosis and cleft palate are identical to that caused by 2,3,7,8-TeCDD.
Toxicity studies have been reported for different brominated congeners using oral, ip, and sc administration (Ao et al., 2009; Haijima et al., 2010; Ivens et al., 1992 ,1993; Mason et al., 1987a; Moore et al., 1979; Nagao et al., 1990; White et al., 2012). These studies indicate that the toxic effects in rodents for 2,3,7,8-TeBDD are in the same dose range as that of 2,3,7,8-TeCDD, indicating a comparable potency for both congeners. Furthermore, the relative potencies of PBDDs for reduction in body weight gain and thymic atrophy followed the order 2,3,7,8-TeBDD > 1,2,3,7,8-PeBDD > 1,2,4,7,8- PeBDD > 1,3,7,8-TeBDD, similar to that of the chlorinated congeners (Mason et al., 1987a). A study with mice examined the relative teratogenic effects of 2,3,7,8-TeBDD, 2,3,7,8-TeBDF, 1,2,3,7,8-PeBDF, and 2,3,4,7,8-PeBDF and compared the effects with their chlorinated congeners. Although the ranking order was similar for the brominated and chlorinated congeners, relative potencies were different between the groups of congeners (Birnbaum et al., 1991). In a subchronic mouse study, the potency of 2,3,7,8-TeBDD for enzyme induction compared with TeCDD was also evaluated. Based on administered dose, the relative potencies for CYP1A1 induction in liver, lung, and skin ranged from 0.03 to 0.15 for 2,3,7,8-TeBDD compared with 2,3,7,8-TeCDD. However, if this difference was evaluated based on tissue concentrations, the relative potencies increased from 0.63 to 4.5 (DeVito et al., 1997). Possible toxic effects of PBDDs and PBDFs on immune functions and developmental brain functions have recently been studied in more detail (Ao et al., 2009; Haijima et al., 2010; White et al., 2012). In mice, 2,3,7,8-TeCDD or 2,3,7,8-TeBDD had nearly identical potencies for immunological endpoints, such as thymus weight and cell numbers, spleen weight and cell numbers, and IL-5 production (Ao et al., 2009). Another recent study presented relative potencies for immune suppression in mice of several PBDDs and their chlorinated congeners. The PBDDs produced a comparable suppression of the plaque-forming cell response in mice compared with their chlorinated congeners. Of note is that the relative potency of the 2,3,7,8-TeBDF was significantly higher than its chlorinated analogue, whereas the 2,3,7-TrBDD did not cause this immunosuppression at all (White et al., 2012). Furthermore, mice exposed to TeCDD or TeBDD in utero and via lactation showed nearly identical responses to fear conditioning tests and had deficits in contextual and auditory fear memory, indicating that both TeCDD and TeBDD disrupt memory and emotional functions (Haijima et al., 2010). A total of 32 different in vivo and in vitro REP values of 2,3,7,8-TeBDD were evaluated. From this wide range of experiments, an average REP value of 1.02 (median: 0.64, min: 0.02, max: 8.45) could be derived. Large differences in outcome of 2,3,7,8-TeBDD studies were observed compared with results for 2,3,7,8-TeCDD. Nevertheless, average and median REPs of 2,3,7,8-TeBDD are similar or close to 2,3,7,8-TeCDD. Thus, use of similar potencies for both congeners (TEF = 1) is suggested as an interim approach pending more detailed chronic studies with 2,3,7,8-TeBDD.
If the one order of magnitude estimated uncertainty of the assigned WHO TEF values is taken into consideration (van den Berg et al., 2006), relative potencies between brominated and chlorinated congeners are in general comparable. With respect to differences in species sensitivity to acute toxicity, brominated and chlorinated congeners also induce comparable effects in the guinea pig. The similar toxicity of 2,3,7,8-TeBDD and 2,3,7,8-TeBDF as previously observed for 2,3,7,8-TeCDD and 2,3,7,8-TeCDF in the guinea pig is associated with the inability of this species to rapidly metabolize and eliminate the 2,3,7,8-halogenated dibenzofurans (van den Berg et al., 1994).
Toxicity in Fish Models
There is only one detailed in vivo toxicity study examining the relative potencies (REPs) of PBDD, PBDF, and dl-PBB congeners in fish (Hornung et al., 1996a). In this study, the early life stage toxicity was assessed at the larval stage of development in different strains of rainbow trout. The toxic endpoints observed were yolk sac edema, pericardial edema, hemorrhages, craniofacial malformation, and growth retardation prior to death. This pattern of toxic effects is referred to as blue sac syndrome and is commonly observed in the larval stage of rainbow trout exposed to individual dioxin-like PCDDs, PCDFs, and non-ortho dl-PCBs. Relative potencies for individual dioxin-like PBDD, PBDF, and dl-PBB congeners determined in these rainbow trout studies are presented in Table 1 (Hornung et al., 1996a; Walker et al., 1996; Zabel et al., 1995a,c).
Table 1.
Comparison of Relative Potencies of PXDD, PXDF, and dl-PXB Congeners in Causing Larval Mortality in Different Strains of Rainbow Trouta,b
| Congener | REPs chlorinated congeners |
REPs brominated congeners |
Trout strain |
|---|---|---|---|
| PXDD | |||
| 2,3,7 | 0.017c | Erwin | |
| 0.018c | McConoughy | ||
| 1,3,7,8 | 0.013c | Erwin | |
| 2,3,7,8 | 1d | Shasta | |
| 1e | 2.54c | Arlee | |
| 1d | 2.22c | Erwin | |
| 1d | 1.90c | Eagle Lake | |
| 1.14c | Eagle Lake | ||
| 1,2,3,7,8 | 0.730e | 0.082c | Arlee |
| 0.140c | Eagle Lake | ||
| 1,2,3,4,7,8 | 0.319e | 0.009c | Arlee |
| 1,2,3,6,7,8 | 0.024d | Shasta | |
| 1,2,3,4,6,7,8 | 0.002d | Shasta | |
| PXDF | |||
| 2,3,7,8 | 0.028e | 0.250c | Erwin |
| 1,2,3,7,8 | 0.034e | 0.041c | Erwin |
| 2,3,4,7,8 | 0.359e | 0.071c | Erwin |
| 1,2,3,4,7,8 | 0.280e | 0.002c | Erwin |
| dl-PXB | |||
| 3,3′,4,4′ (77) | 0.0002e | 0.002c | Erwin |
| 0.001c | Eagle Lake | ||
| 0.001c | Shasta | ||
| 0.002c | Arlee | ||
| 3,4,4′,5 (81) | 0.001d | Eagle Lake | |
| 3,3′,4,4′,5 (126) | 0.005e,f | Arlee | |
| 3,3′,4,4′,5,5′ (169) | 0.00004d | Erwin | |
| 0.0001c | Arlee | ||
Note. a2,3,7,8-TeCDD-like endpoints of early life–stage toxicity, grossly identical to blue sac syndrome and characterized by yolk sac edema, pericardial edema, multifocal hemorrhages, craniofacial malformation, and growth retardation prior to mortality, were observed during the larval stage of development for all congeners shown in this table.
bCalculated as ratio of LD50 of 2,3,7,8-TeCDD (pmol/g egg) to LD50 of a PBDD, PBDF, or dl-PBB or PCDD, PCDF, or dl-PCB congener.
cHornung et al. (1996a).
dZabel et al. (1995a).
eWalker and Peterson (1991).
fPCB 126 relative potency in causing larval mortality in lake trout is 0.0030 (Zabel et al., 1995c).
The mechanism of blue sac syndrome associated mortality in fish larvae, exposed as embryos to dioxin-like chemicals, is mediated by the AhR2 and ARNT1 proteins and induces alterations in gene expression comparable with the Ah-receptor mechanism in mammals (Heideman et al., 2004; Peterson et al., 2003, 2006). All polychlorinated and -brominated congeners shown in Table 1 produced this blue sac syndrome–related mortality and share the same AhR2-mediated mechanism and identical endpoints of toxicity across several different fish species (King-Heiden et al., 2011).
Table 1 compares the “fish-specific” REPs of PCDDs, PCDFs, and dl-PCBs with those of PBDDs, PBDFs, and dl-PBBs based on larval mortality in rainbow trout. The REP of each congener is expressed as the ratio of LDegg 50 of 2,3,7,8-TeCDD (pmol/g egg) to LDegg 50 of a PCDD, PDCF, non-ortho dl-PCB, PBDD, PBDF, or non-ortho dl-PBB congener (pmol/g egg). For the polychlorinated congeners, their REPs follow the classic SAR with PCDDs and PCDFs having greater REP values than non-ortho dl-PCBs. This is also the case for PBDDs and PBDFs compared with non-ortho dl-PBBs. When individual congeners within each class (PCDDs to PBDDs; PCDFs to PBDFs; non-ortho dl-PCBs to non-ortho dl-PBBs) are compared, the REPs for many brominated congeners with similar substitution to the chlorine congeners are distinctly different. 2,3,7,8-TeBDD, 1,3,7,8-TeBDD, and PBB 77 caused a higher mortality in the rainbow trout early life stage assay than their corresponding chlorinated congeners. Thus, SARs for the brominated compounds appear to be different from that observed for their chlorinated analogues in the same fish bioassay. The most notable difference is the observation that trilateral substituted PBDDs are weak AhR2 agonists in fish, providing the rationale to include these congeners in future aquatic risk assessment.
In conclusion, the present information for relative potencies of PBDDs, PBDFs, and dl-PBBs in fish is more or less limited to one particular bioassay system but with multiple species. The REPs provided in Table 1 provide a basis for ecotoxicological risk assessment in the aquatic environment and are clearly different from those of mammals. However, the (limited) data for fish derived from this bioassay show a significant deviation in REPs between the brominated and chlorinated congeners in fish. In fact, these data provide evidence that the SARs for PBDDs, PBDFs, and dl-PBBs may be different from the chlorinated congeners. Due to the limited database available for the brominated compounds, a recommendation for general fish-specific TEFs for the brominated congeners can not be made. However, if regulatory agencies need to determine ecotoxicological risks for aquatic organisms, the relative potencies presented in Table 1 may be used to estimate the total toxicity equivalency of PBDDs, PCDFs, and dl-PBBs with or without the PCDDs, PCDFs, and dl-PCBs.
Mixture Interaction Between PBDDs, PBDFs, Non-ortho dl-PBBs, and Their Chlorinated Congeners
Although there is a wealth of information regarding the additivity of PCDDs, PCDFs, and dl-PCBs (van den Berg et al., 1998, 2006), there is a paucity of experimental data involving mixture toxicity studies with the brominated congeners. Most information is available from mixture experiments using early life stage assays in fish. Using fish-specific REPs, the hypothesis was tested if pairs of PBDD, PBDF, and dl-PBB congeners interact additively to cause mortality in fish embryos. Four congener pairs were tested across a wide range of different dose combinations (2,3,7,8-TeBDD/1,2,3,7,8-PeBDD), (2,3,7,8-TeBDD/1,2,3,7,8-PeBDF), (1,2,3,7,8-PeBDD/2,3,4,7,8-PeBDF), and (3,3′,4,4′- TBB/2,3,4,7,8-PeBDF). In all cases, interactions between these congener pairs were additive (Hornung et al., 1996b), which is comparable with earlier observations made for pairs of chlorinated congeners in the same fish larvae bioassay. Nevertheless, these mixture toxicity experiments showed some deviations from additivity for mixtures of 2,3,7,8-TeCDD/PCB 77 and 2,3,7,8-TeCDD/PCB 126. However, these limited deviations from additivity toward synergism are not sufficient to warrant a change from the TEF prerequisite of additivity (Zabel et al., 1995c). The same fish larvae bioassay has been used to test a complex and toxicologically relevant mixture of PCDDs, PCDFs, and PCBs, as measured in Great Lakes fish and found not to act in a strictly additive manner. However, these data are still within the one order of magnitude uncertainty factor estimated for individual TEF values (van den Berg et al., 2006; Walker et al., 1996).
In conclusion, the fish bioassays provide sufficient support for the TEF additivity model for 2,3,7,8-substituted PBDDs, PBDFs, and non-ortho dl-PBBs. In addition, it should be recognized that the common mechanism of a single receptor-mediated effect for both the chlorinated and brominated congeners warrants the use of additivity for mixtures. Based on common mechanistic considerations from mammals and fish, it can be assumed that these conclusions can be generalized to other classes of vertebrates, including humans. However, it should be noted that quantitative differences in REPs and TEFs for individual brominated congeners can differ between classes of vertebrates, as concluded earlier by WHO for the chlorinated congeners (van den Berg et al., 1998).
DISCUSSION
In 1998, the WHO published an extensive review of the biological and toxic properties of PBDDs and PBDFs and concluded that brominated and chlorinated 2,3,7,8-substituted congeners elicit a similar spectrum of effects (WHO, 1998). As with PCDDs, PCDFs, and some non-ortho dl-PCBs, the brominated congeners show comparable biological and toxic effects, such as immune suppression, enzyme induction, thyroid hormone and vitamin A perturbations, antiestrogenicity, teratogenicity, and neurobehavioral deficits. These effects of brominated congeners are found at similar low doses as 2,3,7,8-substituted PCDDs and PCDFs, indicating comparable potencies for the 2,3,7,8-brominated congeners (for review, see WHO (1998)). Because the 1998 WHO review of PBDDs and PBDFs, several other studies determined the relative potencies of these compounds in various experimental systems (Ao et al., 2009; Behnisch et al., 2003; Guruge et al., 2009; Haijima et al., 2010; Olsman et al., 2007; Samara et al., 2009). Studies performed for this purpose used either AhR-mediated induction of CYP1A1/1B1 activity or luciferase expression, developmental effects in fish, or immunoassays. On the other hand, changes in immune, memory, and emotional functions have also been studied in vivo. If the assumed uncertainty of one order of magnitude in WHO TEFs is taken into account, it can be concluded that the relative potencies of 2,3,7,8-substituted PBDDs and PBDFs are comparable to their corresponding chlorinated congeners in mammalian systems. However, it should also be noted that the limited mammalian database for REPs of PBDDs, PBDFs, and dl-PBBs does not allow sufficient differentiation for a TEF compared with their chlorinated congeners. In Figure 1, REP ranges for PBDDs and PBDFs are presented and can be compared directly with their chlorinated counterparts. The results in this figure also show differences in the REP ranges between some chlorinated and brominated congeners, with dl-PBBs significantly different from the dl-PCBs. Depending on the relevance in the present exposure of humans to dl-PBBs, it must be decided whether more detailed chronic studies with these congeners are warranted.
Consequently, the limited in vivo and in vitro data from mammalian systems for PBDDs, PBDFs, and non-ortho dl-PBBs support the use of similar TEFs as interim values for human risk assessment, pending more research with (semi)chronic studies. Clearly, dissimilarities between both groups of congeners exist, but possible consequences should again be placed in the context of the assumed uncertainty of one order of magnitude for WHO mammalian TEFs of the chlorinated congeners. Based on some of the limited data, it is possible that 2,3,7,8-TeBDF and 1,2,3,7,8-PeBDF may have higher relative potencies than their chlorinated analogues. This could be due to the longer half-lives of both brominated congeners in experimental animals and humans. New toxicity studies can provide more clarity on this issue in the future. For further comparison, the present WHO TEFs for chlorinated congeners and data from recent publications with the range of REP values for both the chlorinated and brominated congeners are given in Supplementary table 1 (Behnisch et al., 2003; Olsman et al., 2007; Samara et al., 2009).
The expert panel also reviewed the available evidence with respect to human exposure to PBDDs, PBDFs, and dl-PBBs. It was concluded that these compounds may contribute significantly to the total TEQs in daily human background exposure. As a result, inclusion of 2,3,7,8-substituted PBDDs and PBDFs in the WHO-UNEP TEF concept is essential for improving human risk assessment for dioxin-like compounds. Although, very little information is available on dl-PBBs, there are indications that the more toxic non-ortho dl-PBBs are still present in the environment and human food chain. Therefore, from a precautionary principle, it is recommended that the non-ortho dl-PBBs 77, 81, 126, and 169 are also included in the WHO-UNEP TEF concept for dioxin-like compounds. In contrast, there seems to be an almost complete lack of exposure and toxicity information concerning the mono-ortho dl-PPBs. Future studies may provide more clarity about the brominated mono-ortho dl-PCBs with respect to inclusion in the WHO-UNEP TEF concept. In view of the significant uncertainty already present in the TEF values for the mono-ortho PCBs (van den Berg et al., 2006), it appears premature to assign similar values to the mono-ortho PBBs until more information about REPs has become available. Similarly, very limited information is available for the polybrominated and polychlorinated naphthalenes (PBNs and PCNs) and their occurrence in the human food chain (Isosaari et al., 2006; Schiavone et al., 2009), in spite of the fact that these compounds are known to have dioxin-like activity. The latter aspect by itself could warrant inclusion in the TEF method if future studies show substantial occurrence in the human food chain (Behnisch et al., 2001, 2003).
The mixed halogenated dibenzo-p-dioxins (PXDDs), diben zofurans (PXDFs), and biphenyls (PXBs) are other dl- compounds that may be considered in the WHO-UNEP TEF concept in the near future. During the last decade, their occurrence in the environment and human food chain has been studied only to a limited extent. However, it was concluded that PXDDs, PXDFs, and non-ortho dl-PXBs may significantly contribute to overall dioxin-like exposure. Unfortunately, complicated analytical methodology at present hampers a regular and adequate human and environmental exposure assessment. With respect to de novo formation of PXDDs, PXDFs, and PXBs, it is noticeable that these compounds may be formed during combustion processes (Falandysz et al., 2012; Gómara et al., 2011; Ohta et al., 2008; Myers et al., 2012; Weber and Kuch, 2003; Terauchi et al., 2009).
Many different PBDD, PBDF, and dl-PBB congeners have now been detected in the aquatic environment. For this reason, the available data for fish and wildlife were evaluated for possible inclusion in the WHO-UNEP TEF concept, along with the chlorinated congeners as published earlier (van den Berg et al., 1998). For this purpose, only REPs from fish larvae studies are available for the brominated compounds. These indicate that there may be significant differences between the SARs of REPs for fish early life stage toxicity for both brominated and chlorinated congeners. Because of this limited database, it was decided not to derive specific WHO-UNEP TEFs for fish. Instead, it is recommended that regulatory agencies consider the use of REPs provided in Table 1 for the use of ecotoxicological risk assessment in the aquatic environment.
CONCLUSIONS
At present, there is sufficient evidence to conclude that concentrations of 2,3,7,8-substituted PBDDs, PBDFs, and possibly some non-ortho dl-PBBs in human food, tissue, and milk can contribute significantly to the total amount of TEQ. In mammalian systems, the mechanism of action and type of toxicity of 2,3,7,8-substituted PBDDs, PBDFs, and non-ortho dl-PBBs are similar to their chlorinated congeners. The available evidence also demonstrates that many REPs for PBDDs and PBDFs in mammals are similar to those of their chlorinated analogues or at least within one order of magnitude (see Fig. 1). This is comparable to the inherent uncertainty of one order of magnitude in the present WHO TEF approach for dl-like chlorinated compounds. Based on the considerations discussed above, the use of similar interim TEF values for brominated and chlorinated congeners for human risk assessment is recommended by the WHO and UNEP, pending availability of future studies on these compounds.
FUNDING
This research has been supported by the United Nations Environment Programme, Chemicals Branch of Division of Technology, Industry and Economics, and the Intramural Research Program of the National Institutes of Health, NIEHS, and NCI.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Ahlborg U. G., Becking G. C., Birnbaum L. S., Brouwer A., Derks H. J., Feeley M., Golor G., Hanberg A., Larsen J. C., Liem A. K. D., et al. (1994). Toxic equivalency factors for dioxin-like PCBs—Report on a WHO-ECEH and IPCS consultation. Chemosphere 28, 1049–1067 [Google Scholar]
- Andres J., Lambert I., Robertson L., Bandiera S., Sawyer T., Lovering S., Safe S. (1983). The comparative biologic and toxic potencies of polychlorinated biphenyls and polybrominated biphenyls. Toxicol. Appl. Pharmacol. 70, 204–215 [DOI] [PubMed] [Google Scholar]
- Ao K., Suzuki T., Murai H., Matsumoto M., Nagai H., Miyamoto Y., Tohyama C., Nohara K. (2009). Comparison of immunotoxicity among tetrachloro-, pentachloro-, tetrabromo- and pentabromo-dibenzo-p-dioxins in mice. Toxicology 256, 25–31 [DOI] [PubMed] [Google Scholar]
- Ashizuka Y., Nakagawa R., Tobiishi K., Hori T., Iida T. (2005). Determination of polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins/dibenzofurans in marine products. J. Agric. Food Chem. 53, 3807–3813 [DOI] [PubMed] [Google Scholar]
- Banks Y. B., Birnbaum L. S. (1991). Absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) after low dose dermal exposure. Toxicol. Appl. Pharmacol. 107, 302–310 [DOI] [PubMed] [Google Scholar]
- Behnisch P. A., Hosoe K., Sakai S. (2001). Combinatorial bio/chemical analysis of dioxin and dioxin-like compounds in waste recycling, feed/food, humans/wildlife and the environment. Environ. Int. 27, 495–519 [DOI] [PubMed] [Google Scholar]
- Behnisch P. A., Hosoe K., Sakai S. (2003). Brominated dioxin-like compounds: In vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ. Int. 29, 861–877 [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S., Morrissey R. E., Harris M. W. (1991). Teratogenic effects of 2,3,7,8-tetrabromodibenzo-p-dioxin and three polybrominated dibenzofurans in C57BL/6N mice. Toxicol. Appl. Pharmacol. 107, 141–152 [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S., Staskal D. F., Diliberto J. J. (2003). Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs). Environ. Int. 29, 855–860 [DOI] [PubMed] [Google Scholar]
- Brewster D. W., Banks Y. B., Clark A. M., Birnbaum L. S. (1989). Comparative dermal absorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin and three polychlorinated dibenzofurans. Toxicol. Appl. Pharmacol. 97, 156–166 [DOI] [PubMed] [Google Scholar]
- Brorstrom-Lunden E., Remberger M., Kaj L., Hansson K., Palm-Cousins A., Anderson H., Haglund P., Ghebremeskel M., Schlabach M. (2010). Results From the Swedish National Screening Programme 2008—Subreport 4: Screening of Unintentionally Produced Organic Contaminants. I.S.E.R. Institute: Goteborg, Sweden: [Google Scholar]
- Choi J. W., Fujimaki T. S., Kitamura K., Hashimoto S., Ito H., Suzuki N., Sakai S., Morita M. (2003). Polybrominated dibenzo-p-dioxins, dibenzofurans, and diphenyl ethers in Japanese human adipose tissue. Environ. Sci. Technol. 37, 817–821 [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Guengerich F. P., Kaminsky L. S., Aust S. D. (1983). Regulation of cytochrome P-450. Immunochemical quantitation of eight isozymes in liver microsomes of rats treated with polybrominated biphenyl congeners. J. Biol. Chem. 258, 1282–1288 [PubMed] [Google Scholar]
- Denison M. S., Soshilov A. A., He G., DeGroot D. E., Zhao B. (2011). Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh J., Buser H. R., Poiger H. (1993). The metabolism of 2,3,7,8-tetrabromodibenzodioxin in the rat. Xenobiotica. 23, 19–26 [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Diliberto J. J., Ross D. G., Menache M. G., Birnbaum L. S. (1997). Dose-response relationships for polyhalogenated dioxins and dibenzofurans following subchronic treatment in mice. I. CYP1A1 and CYP1A2 enzyme activity in liver, lung, and skin. Toxicol. Appl. Pharmacol. 147, 267–280 [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Ross D. G., Dupuy A. E., Jr, Ferrario J., McDaniel D., Birnbaum L. S. (1998). Dose-response relationships for disposition and hepatic sequestration of polyhalogenated dibenzo-p-dioxins, dibenzofurans, and biphenyls following subchronic treatment in mice. Toxicol. Sci. 46, 223–234 [DOI] [PubMed] [Google Scholar]
- Diliberto J. J., Kedderis L. B., Jackson J. A., Birnbaum L. S. (1993). Effects of dose and routes of exposure on the disposition of 2,3,7,8-[3H]tetrabromodibenzo-p-dioxin (TBDD) in the rat. Toxicol. Appl. Pharmacol. 120, 315–326 [DOI] [PubMed] [Google Scholar]
- Ericson Jogsten I., Hagberg J., Lindström G., Bavel B. v. (2010). Analysis of POPs in human samples reveal a contribution of brominated dioxin of up to 15% of the total dioxin TEQ. Chemosphere 78, 113–120 [DOI] [PubMed] [Google Scholar]
- Falandysz J., Rose M., Fernandes A. R. (2012). Mixed poly-brominated/chlorinated biphenyls (PXBs): Widespread food and environmental contaminants. Environ. Int. 44, 118–127 [DOI] [PubMed] [Google Scholar]
- Fernandes A., Dicks P., Mortimer D., Gem M., Smith F., Driffield M., White S., Rose M. (2008). Brominated and chlorinated dioxins, PCBs and brominated flame retardants in Scottish shellfish: Methodology, occurrence and human dietary exposure. Mol. Nutr. Food Res. 52, 238–249 [DOI] [PubMed] [Google Scholar]
- Fernandes A., Mortimer D., Gem M., Dicks P., Smith F., White S., Rose M. (2009a). Brominated dioxins (PBDD/Fs) and PBDEs in marine shellfish in the UK. Food Addit. Contam. Part A. Chem. Anal. Control. Expo. Risk Assess. 26, 918–927 [DOI] [PubMed] [Google Scholar]
- Fernandes A. R., Mortimer D., Gem M., Dicks P., Smith F., White S., Rose M. (2009b). Polybrominated diphenylethers (PBDEs) and brominated dioxins (PBDD/Fs) in Irish food of animal origin. Food Add. Contam. Part B-Surveillance. 2, 86–94 [DOI] [PubMed] [Google Scholar]
- Furness S. G., Lees M. J., Whitelaw M. L. (2007). The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett. 581, 3616–3625 [DOI] [PubMed] [Google Scholar]
- Golor G., Yamashita K., McLachlan M. S., Hutzinger O. (1993). Comparison of the kinetics of chlorinated and brominated dioxins and furans in the rat. Organohal. Cmpds. 13, 203–206 [Google Scholar]
- Gómara B., Herrero L., Pacepavicius G., Ohta S., Alaee M., González M. J. (2011). Occurrence of co-planar polybrominated/chlorinated biphenyls (PXBs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk of women from Spain. Chemosphere 83, 799–805 [DOI] [PubMed] [Google Scholar]
- Gullett B. K., Wyrzykowska B., Grandesso E., Touati A., Tabor D. G., Ochoa G. S. (2010). PCDD/F, PBDD/F, and PBDE emissions from open burning of a residential waste dump. Environ. Sci. Technol. 44, 394–399 [DOI] [PubMed] [Google Scholar]
- Guruge K. S., Yamanaka N., Hasegawa J., Miyazaki S. (2009). Differential induction of cytochrome P450 1A1 and 1B1 mRNA in primary cultured bovine hepatocytes treated with TCDD, PBDD/Fs and feed ingredients. Toxicol. Lett. 185, 193–196 [DOI] [PubMed] [Google Scholar]
- Haglund P. (2010). On the identity and formation routes of environmentally abundant tri- and tetrabromodibenzo-p-dioxins. Chemosphere 78, 724–730 [DOI] [PubMed] [Google Scholar]
- Haglund P., Malmvärn A., Bergek S., Bignert A., Kautsky L., Nakano T., Wiberg K., Asplund L. (2007). Brominated dibenzo-p-dioxins: A new class of marine toxins? Environ. Sci. Technol. 41, 3069–3074 [DOI] [PubMed] [Google Scholar]
- Haijima A., Endo T., Zhang Y., Miyazaki W., Kakeyama M., Tohyama C. (2010). In utero and lactational exposure to low doses of chlorinated and brominated dioxins induces deficits in the fear memory of male mice. Neurotoxicology 31, 385–390 [DOI] [PubMed] [Google Scholar]
- Hanari N., Kannan K., Miyake Y., Okazawa T., Kodavanti P. R., Aldous K. M., Yamashita N. (2006). Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environ. Sci. Technol. 40, 4400–4405 [DOI] [PubMed] [Google Scholar]
- Hankinson O. (1995). The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35, 307–340 [DOI] [PubMed] [Google Scholar]
- Heideman W., Carney S. A., Peterson R. E. (2004). 2,3,7,8-tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Molec. Pharmacol. 66, 512–521 [DOI] [PubMed] [Google Scholar]
- Hornung M. W., Zabel E. W., Peterson R. E. (1996a). Toxic equivalency factors of polybrominated dibenzo-p-dioxin, dibenzofuran, biphenyl, and polyhalogenated diphenyl ether congeners based on rainbow trout early life stage mortality. Toxicol. Appl. Pharmacol. 140, 227–234 [DOI] [PubMed] [Google Scholar]
- Hornung M. W., Zabel E. W., Peterson R. E. (1996b). Additive interactions between pairs of polybrominated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners in a rainbow trout early life stage mortality bioassay. Toxicol. Appl. Pharmacol. 140, 345–355 [DOI] [PubMed] [Google Scholar]
- Isosaari P., Hallikainen A., Kiviranta H., Vuorinen P. J., Parmanne R., Koistinen J., Vartiainen T. (2006). Polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, naphthalenes and polybrominated diphenyl ethers in the edible fish caught from the Baltic Sea and lakes in Finland. Environ. Pollut. 141, 213–225 [DOI] [PubMed] [Google Scholar]
- Ivens I. A., Löser E., Rinke M., Schmidt U., Neupert M. (1992). Toxicity of 2,3,7,8-tetrabromodibenzo-p-dioxin in rats after single oral administration. Toxicology 73, 53–69 [DOI] [PubMed] [Google Scholar]
- Ivens I. A., Löser E., Rinke M., Schmidt U., Mohr U. (1993). Subchronic toxicity of 2,3,7,8-tetrabromodibenzo-p-dioxin in rats. Toxicology 83, 181–201 [DOI] [PubMed] [Google Scholar]
- Kedderis L. B., Diliberto J. J., Birnbaum L. S. (1991a). Disposition and excretion of intravenous 2,3,7,8-tetrabromodibenzo-p-dioxin (TBDD) in rats. Toxicol. Appl. Pharmacol. 108, 397–406 [DOI] [PubMed] [Google Scholar]
- Kedderis L. B., Diliberto J. J., Jackson J. A., Linko P., Goldstein J. A., Birnbaum L. S. (1992). Effects of dose and route of exposure on dioxin disposition. Chemosphere 25, 7–10 [Google Scholar]
- Kedderis L. B., Diliberto J. J., Linko P., Goldstein J. A., Birnbaum L. S. (1991b). Disposition of 2,3,7,8-tetrabromodibenzo-p-dioxin and 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat: Biliary excretion and induction of cytochromes CYP1A1 and CYP1A2. Toxicol. Appl. Pharmacol. 111, 163–172 [DOI] [PubMed] [Google Scholar]
- Kedderis L. B., Jackson J. A., Patterson D. G., Jr, Grainger J., Diliberto J. J., Birnbaum L. S. (1994). Chemical characterization and disposition studies with 1,2,7,8-tetrabromodibenzofuran in the rat. J. Toxicol. Environ. Health. 41, 53–69 [DOI] [PubMed] [Google Scholar]
- Kedderis L. B., Mills J. J., Andersen M. E., Birnbaum L. S. (1993). A physiologically based pharmacokinetic model for 2,3,7,8-tetrabromodibenzo-p-dioxin (TBDD) in the rat: Tissue distribution and CYP1A induction. Toxicol. Appl. Pharmacol. 121, 87–98 [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D., Jensen A. A. (1989). Halogenated biphenyls, terphenyls, naphtalenes, dibenzodioxins and related products. In Topics in Environmental Health. (Kimbrough R. D., Jensen A. A. Eds.). Elsevier Publishers: Amsterdam: [Google Scholar]
- King-Heiden T. C., Mehta V., Xiong K. M., Lanham K. A., Antkiewicz D. S., Ganser A., Heideman W., Peterson R. E. (2011). Reproductive and developmental toxicity of dioxin in fish. Mol. Cell. Endocrinol. 354, 121–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P. K. S., Miyake Y., Jiang Q., Yuan W., Hanari N., Okazawa T., Wyrzykowska B., So M. K., Yamashita N. (2008). Preliminary health risk assessment for polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins/furans in seafood from Guangzhou and Zhoushan, China. Marine Pollut. Bull. 57, 357–364 [DOI] [PubMed] [Google Scholar]
- Löfstrand K., Malmvärn A., Haglund P., Bignert A., Bergman A., Asplund L. (2010). Brominated phenols, anisoles, and dioxins present in blue mussels from the Swedish coastline. Environ. Sci. Pollut. Res. Int. 17, 1460–1468 [DOI] [PubMed] [Google Scholar]
- Lundstedt S., Haglund P., Marklund S. (2011). Emissions of brominated dioxins during accidental fires in flame retarded materials. Organohal. Cmpds. 73, 436–439 [Google Scholar]
- Malmvärn A., Marsh G., Kautsky L., Athanasiadou M., Bergman A., Asplund L. (2005a). Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environ. Sci. Technol. 39, 2990–2997 [DOI] [PubMed] [Google Scholar]
- Malmvärn A., Zebühr Y., Jensen S., Kautsky L., Greyerz E., Nakano T., Asplund L. (2005b). Identification of polybrominated dibenzo-p-dioxins in blue mussels (Mytilus edulis) from the Baltic Sea. Environ. Sci. Technol. 39, 8235–8242 [DOI] [PubMed] [Google Scholar]
- Malmvärn A., Zebühr Y., Kautsky L., Bergman K., Asplund L. (2008). Hydroxylated and methoxylated polybrominated diphenyl ethers and polybrominated dibenzo-p-dioxins in red alga and cyanobacteria living in the Baltic Sea. Chemosphere 72, 910–916 [DOI] [PubMed] [Google Scholar]
- Mason G., Denomme M. A., Safe L., Safe S. (1987a). Polybrominated and chlorinated dibenzo-p-dioxins: Synthesis biologic and toxic effects and structure-activity relationships. Chemosphere 16, 1729–1731 [DOI] [PubMed] [Google Scholar]
- Mason G., Zacharewski T., Denomme M. A., Safe L., Safe S. (1987b). Polybrominated dibenzo-p-dioxins and related compounds: Quantitative in vivo and in vitro structure-activity relationships. Toxicology 44, 245–255 [DOI] [PubMed] [Google Scholar]
- Moore J. A., McConnell E. E., Dalgard D. W., Harris M. W. (1979). Comparative toxicity of three halogenated dibenzofurans in guinea pigs, mice, and rhesus monkeys. Ann. N. Y. Acad. Sci. 320, 151–163 [DOI] [PubMed] [Google Scholar]
- Myers A. L., Mabury S. A., Reiner E. J. (2012). Analysis of mixed halogenated dibenzo-p-dioxins and dibenzofurans (PXDD/PXDFs) in soil by gas chromatography tandem mass spectrometry (GC-MS/MS). Chemosphere 87, 1063–1069 [DOI] [PubMed] [Google Scholar]
- Nagao T., Neubert D., Löser E. (1990). Comparative studies on the induction of ethoxyresorufin O-deethylase by 2,3,7,8-TCDD and 2,3,7,8-TBrDD. Chemosphere 20, 1189–1192 [Google Scholar]
- Nagao T., Yamashita K., Golor G., Bittmann H., Körner W., Hagenmaier H., Neubert D. (1996). Tissue distribution after a single subcutaneous administration of 2,3,7,8-tetrabromodibenzo-p-dioxin in comparison with toxicokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Wistar rats. Life Sci. 58, 325–336 [DOI] [PubMed] [Google Scholar]
- Ohta S., Tokusawa H., Nakao T., Aozasa O., Miyata H., Alaee M. (2008). Global contamination of coplanar polybrominated/chlorinated biphenyls (Co-PXBs) in the market fishes from Japan. Chemosphere 73(1 Suppl)S31–S38 [DOI] [PubMed] [Google Scholar]
- Olsman H., Engwall M., Kammann U., Klempt M., Otte J., Bavel B. V., Hollert H. (2007). Relative differences in aryl hydrocarbon receptor-mediated response for 18 polybrominated and mixed halogenated dibenzo-p-dioxins and -furans in cell lines from four different species. Environ. Toxicol. Chem. 26, 2448–2454 [DOI] [PubMed] [Google Scholar]
- Ortuño N., Font R., Moltó J., Conesa J. A. (2011). Thermal degradation of tetrabromobisphenol A: Emission of polybrominated dibenzo-p-dioxins and dibenzofurans and other organic compounds. Organohal. Cmpds. 73, 511–514 [Google Scholar]
- Parkinson A., Safe S. H., Robertson L. W., Thomas P. E., Ryan D. E., Reik L. M., Levin W. (1983). Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J. Biol. Chem. 258, 5967–5976 [PubMed] [Google Scholar]
- Peterson R. E., Prasch A. L., Tanguay R. L., Mehta V., Heideman W. (2006). Identification of zebrafish ARNT1 homologs: 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Molec. Pharmacol. 69, 776–787 [DOI] [PubMed] [Google Scholar]
- Peterson R. E., Prasch A. L., Teraoka H., Carney S. A., Dong W., Hiraga T., Stegeman J. J., Heideman W. (2003). Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 76, 138–150 [DOI] [PubMed] [Google Scholar]
- Pirkle J. L., Wolfe W. H., Patterson D. G., Needham L. L., Michalek J. E., Miner J. C., Peterson M. R., Phillips D. L. (1989). Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. J. Toxicol. Environ. Health. 27, 165–171 [DOI] [PubMed] [Google Scholar]
- Poiger H., Buser H. R. (1984). The metabolism of TCDD in dog and rat. In Biological Mechanisms of Dioxin Action. (Poland A., Kimbrough R. D, Eds.), pp. 39–47 Cold Spring Harbor Laboratory: Cold Spring Harbor, NY: [Google Scholar]
- Poiger H., Schlatter C. (1986). Pharmacokinetics of 2,3,7,8-TCDD in man. Chemosphere 15, 1489–1494 [Google Scholar]
- Poland A., Knutson J. C. (1982). 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: Examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 22, 517–554 [DOI] [PubMed] [Google Scholar]
- Render J. A., Aust S. D., Sleight S. D. (1982). Acute pathologic effects of 3,3’,4,4’,5,5’-hexabromobiphenyl in rats: Comparison of its effects with Firemaster BP-6 and 2,2’,4,4’,5,5’-hexabromobiphenyl. Toxicol. Appl. Pharmacol. 62, 428–444 [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Andres J. L., Safe S. H., Lovering S. L. (1983). Toxicity of 3,3’,4,4’- and 2,2’,5,5’-tetrabromobiphenyl: Correlation of activity with aryl hydrocarbon hydroxylase induction and lack of protection by antioxidants. J. Toxicol. Environ. Health. 11, 81–91 [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Bandiera S., Lambert I., Merrill J., Safe S. H. (1984). PCBs and PBBs: Biologic and toxic effects on C57BL/6J and DBA/2J inbred mice. Toxicology 31, 191–206 [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Campbell M. A., Safe S. (1982). Polybrominated biphenyls as aryl hydrocarbon hydroxylase inducers: Structure-activity correlations. Chem. Biol. Interact. 42, 53–66 [DOI] [PubMed] [Google Scholar]
- Safe S. (1990). Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 21, 51–88 [DOI] [PubMed] [Google Scholar]
- Safe S. H. (1986). Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu. Rev. Pharmacol. Toxicol. 26, 371–399 [DOI] [PubMed] [Google Scholar]
- Safe S. H. (1994). Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 24, 87–149 [DOI] [PubMed] [Google Scholar]
- Samara F., Gullett B. K., Harrison R. O., Chu A., Clark G. C. (2009). Determination of relative assay response factors for toxic chlorinated and brominated dioxins/furans using an enzyme immunoassay (EIA) and a chemically-activated luciferase gene expression cell bioassay (CALUX). Environ. Int. 35, 588–593 [DOI] [PubMed] [Google Scholar]
- Schiavone A., Kannan K., Horii Y., Focardi S., Corsolini S. (2009). Occurrence of brominated flame retardants, polycyclic musks, and chlorinated naphthalenes in seal blubber from Antarctica: Comparison to organochlorines. Mar. Pollut. Bull. 58, 1415–1419 [DOI] [PubMed] [Google Scholar]
- Schulz T., Koerner W., Hagemaier H., Neubert D. (1993). Comparitive study on enzyme induction and tissue distribution of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,4,7,8-pentachlorodibenzofuran and 2,3,4,7,8-pentabromodibenzofuran in marmoset monkeys (Callithrix jacchus). Organohal. Cmpds. 13, 145–148 [Google Scholar]
- Suzuki G., Someya M., Takahashi S., Tanabe S., Sakai S., Takigami H. (2010). Dioxin-like activity in Japanese indoor dusts evaluated by means of in vitro bioassay and instrumental analysis: Brominated dibenzofurans are an important contributor. Environ. Sci. Technol. 44, 8330–8336 [DOI] [PubMed] [Google Scholar]
- Terauchi H., Takahashi S., Lam P. K., Min B. Y., Tanabe S. (2009). Polybrominated, polychlorinated and monobromo-polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in marine surface sediments from Hong Kong and Korea. Environ. Pollut. 157, 724–730 [DOI] [PubMed] [Google Scholar]
- Tu L.-K., Wu Y.-L., Wang L.-C., Chang-Chien G.-P. (2011). Distribution of polybrominated dibenzo-p-dioxins and dibenzofurans and polybrominated diphenyl ethers in a coal-fired power plant and two municipal solid waste incinerators. Aerosol Air Qual. Res. 11, 596–615 [Google Scholar]
- Unger M., Asplund L., Haglund P., Malmvärn A., Arnoldsson K., Gustafsson O. (2009). Polybrominated and mixed brominated/chlorinated dibenzo-p-dioxins in sponge (Ephydatia fluviatilis) from the Baltic Sea. Environ. Sci. Technol. 43, 8245–8250 [DOI] [PubMed] [Google Scholar]
- van den Berg M., Birnbaum L., Bosveld A. T., Brunström B., Cook P., Feeley M., Giesy J. P., Hanberg A., Hasegawa R., Kennedy S. W., et al. (1998). Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 106, 775–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M., Birnbaum L. S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., et al. (2006). The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 93, 223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M., De Jongh J., Poiger H., Olson J. R. (1994). The toxicokinetics and metabolism of polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) and their relevance for toxicity. Crit. Rev. Toxicol. 24, 1–74 [DOI] [PubMed] [Google Scholar]
- Walker M. K., Cook P. M., Butterworth B. C., Zabel E. W., Peterson R. E. (1996). Potency of a complex mixture of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin in causing fish early life stage mortality. Fundam. Appl. Toxicol. 30, 178–186 [DOI] [PubMed] [Google Scholar]
- Walker M. K., Peterson R. E. (1991). Potencies of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners, relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin, for producing early life stage mortality in rainbow-trout (oncorhynchus-Mykiss). Aquat. Toxicol. 21, 219–238 [Google Scholar]
- Weber L. W., Greim H. (1997). The toxicity of brominated and mixed-halogenated dibenzo-p-dioxins and dibenzofurans: An overview. J. Toxicol. Environ. Health. 50, 195–215 [DOI] [PubMed] [Google Scholar]
- Weber R., Kuch B. (2003). Relevance of BFRs and thermal conditions on the formation pathways of brominated and brominated-chlorinated dibenzodioxins and dibenzofurans. Environ. Int. 29, 699–710 [DOI] [PubMed] [Google Scholar]
- White K., Smith M., Walker N., Birnbaum L., DeVito M., Germolec D. R., Frawley R. (2012). Immunological evaluation of the relative potency of a single oral administration of brominated and chlorinated dioxins and furans in female B6C3F1 mice. In Society of Toxicology Annual Meeting 2012, San Francisco, USA: [Google Scholar]
- WHO (1998). Polybrominated Dibenzo-p-Dioxins and Dibenzofurans. Environmental Health Criteria, Vol. 205. World Health Organization: Geneva [Google Scholar]
- Wong M. H., Leung A. O. W., Zheng J. S., Yu C. K., Liu W. K., Wong C. K. C., Cai Z. W. (2011). Polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in surface dust at an E-waste processing site in southeast China. Environ. Sci. Technol. 45, 5775–5782 [DOI] [PubMed] [Google Scholar]
- Wyrzykowska B., Tabor D., Gullett B. K. (2009). Same-sample determination of ultratrace levels of polybromodiphenylethers, polybromodibenzo-p-dioxins/furans, and polychlorodibenzo-p-dioxins/furans from combustion flue gas. Anal. Chem. 81, 4334–4342 [DOI] [PubMed] [Google Scholar]
- Zabel E. W., Cook P. M., Peterson R. E. (1995a). Toxic equivalency factors of polychlorinated dibenzo-p-dioxin, dibenzofuran and biphenyl congeners based on early-life stage mortality in rainbow-trout (Oncorhynchus-mykiss). Aquat. Toxicol. 31, 315–328 [Google Scholar]
- Zabel E. W., Cook P. M., Peterson R. E. (1995b). Potency of 3,3’,4,4’,5-Pentachlorobiphenyl (PCB 126), alone and in combination with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), to produce lake trout early life-stage mortality. Environ. Toxicol. Chem. 14, 2175–2179 [Google Scholar]
- Zabel E. W., Walker M. K., Hornung M. W., Clayton M. K., Peterson R. E. (1995c). Interactions of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners for producing rainbow trout early life stage mortality. Toxicol. Appl. Pharmacol. 134, 204–213 [DOI] [PubMed] [Google Scholar]
- Zober M. A., Ott M. G., Päpke O., Senft K., Germann C. (1992). Morbidity study of extruder personnel with potential exposure to brominated dioxins and furans. I. Results of blood monitoring and immunological tests. Br. J. Ind. Med. 49, 532–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.