Abstract
Risk factors have not been identified that determine susceptibility for development of diisocyanate-induced occupational asthma (DA). We hypothesized that diisocyanate (DI) exposure could modify gene promoter regions regulating transcription of cytokine mediators and thereby influence expression of DA. A cross-sectional study was designed to investigate the promoter methylation status of candidate genes in DI-exposed workers. Subjects consisted of 131 workers in three groups: 40 cases with DA confirmed by a positive specific inhalation challenge (SIC) (DA+), 41 exposed workers with lower respiratory symptoms and negative SIC (DA−), and 50 asymptomatic exposed workers (AWs). We studied four candidate genes (GSTM1, DUSP22, IFN-γ, and IL-4) for which altered promoter methylation has been previously investigated for relationships with a variety of other environmental exposures. Methylation status was determined using methylation-specific quantitative PCR performed on genomic DNA extracted from whole blood. Results showed that relative methylation of IFN-γ promoter was significantly increased in DA+ in comparison with both comparator groups (DA− and AW), and it exhibited good sensitivity (77.5%) and specificity (80%) for identifying DA workers in a multivariate predictive model after adjusting for type of DI exposure, smoking status, methacholine PC20, and gender. IL-4 promoter was slightly less methylated only in DA+ compared with AW among nonsmoking workers. Both GSTM1 and DUSP22 promoter methylations were found not associated with DA. Our finding suggests that exposure to occupational chemicals could play a heretofore undefined mechanistic role via epigenetic modification of specific genes in the promoter region.
Key Words: DNA methylation, IFN-γ, environmental exposure, diisocyanate, asthma, occupational asthma.
Diisocyanate (DI) chemicals, widely utilized in a variety of industries, are the most common chemicals causing occupational asthma (OA) (Bakerly et al., 2008). Among these, hexamethylene DI (HDI) is widely used as a spray paint hardener. Methylene diphenyl DI (MDI) and toluene DI (TDI) are used in production of polyurethane products. DIs account for most new cases of OA, affecting about 2–5% of chronically exposed workers (Bernstein et al., 1993). Despite recognition of diisocyanate asthma (DA) for over 60 years, investigators have been unable to define key risk factors predicting susceptibility for DA in chronically exposed workers (Bakerly et al., 2008). Because clinical manifestations of DA resemble allergic asthma, immune mechanisms have been extensively studied. There has been, however, little evidence supporting Th2-directed immune responses in the majority of affected workers. Serum-specific IgE for DI-protein antigens has been identified in only 20–30% of workers with confirmed DA (Bernstein et al., 2002; Campo et al., 2007; Cartier et al., 1989).
In an attempt to further define risk for DA, numerous investigators have conducted candidate gene association studies among workers with DA including those assessing immune response genes (i.e., HLA class II alleles), candidate single nucleotide polymorphism (SNP) variants of antioxidant enzyme genes (e.g., glutathione transferase polymorphisms), and Th2 cytokine genes (Balboni et al., 1996; Bernstein, 2011; Mapp et al., 2002). Although significant SNP associations have been identified, in association with DA, most have not been replicated in other DI worker populations (Choi et al., 2009; Rihs et al., 1997) with the exception of two polymorphisms of the CTNNA3 (α-catenin) gene reported to be significantly associated with DA in both Caucasian and South Korean DI-exposed workers (Bernstein et al., 2012; Kim et al., 2009).
Epigenetic modification of gene expression is a novel mechanism by which environmental exposures may influence disease expression through modification of promoter regions regulating gene transcription. In a previous study, we reported methylation studies of promoter regions of multiple candidate genes including glutathione S-transferase Mu 1 (GSTM1), dual specificity protein phosphatase 22 (DUSP22), and interferon-γ (IFN-γ) conducted in professional firefighters chronically exposed to smoke-related toxicants (Ouyang et al., 2012). We found significantly reduced DUSP22 promoter methylation relative to nonfirefighters. In a birth cohort study, maternal exposure to traffic-related polycyclic aromatic hydrocarbons was found to be associated with promoter hypermethylation in both the acyl-CoA synthetase long-chain family member 3 (ACSL3) and the IFN-γ gene in umbilical cord blood DNA from offspring, although no such effect was observed in the interleukin-4 (IL-4) gene (Perera et al., 2009; Tang et al., 2012). Finally, exposure of young children to traffic-related, diesel exhaust particles was found associated with forkhead box P3 (FOXP3) promoter hypermethylation in saliva DNA of children that developed wheezing and asthma (Brunst et al., 2013).
We posited that specific epigenetic changes induced by DI in genomic DNA of exposed workers could be associated with DA. To test this hypothesis, we investigated methylation status using four candidate gene promoters (GSTM1, DUSP22, IFN-γ, and IL-4), which have been studied in firefighters or traffic-related offspring asthma (Ouyang et al., 2012; Perera et al., 2009; Tang et al., 2012) and assessed their associations with DA, through a cross-sectional study of DI-exposed workers. The choice of these genes was based on prior observations that the methylation status of their promoters is susceptible to alteration upon exposure to complex mixtures of environmental agents.
MATERIALS AND METHODS
Subjects.
Subjects are the workers with occupational exposure to one of the common DIs, MDI, TDI, or HDI, in the workplace. They were recruited from occupational pulmonary disease clinics located in Canada (Sacre Coeur Hospital, Montreal, and Laval Hospital, Sainte-Foy, Quebec). To confirm or exclude DA, all subjects underwent specific inhalation challenge (SIC) with the appropriate work-relevant DI chemicals according to previously described protocols (Malo et al., 1999; Sastre et al., 2003). Three groups of subjects were studied, consisting of: 40 workers with DA confirmed by a positive SIC test and were categorized in the DA+ group, 41 workers presenting with work-related lower respiratory symptoms and negative SICs and were categorized in the DA− group, and 50 asymptomatic HDI-exposed spray painters were recruited as AWs. Ten workers in each group were current smokers, whereas the remainder had never smoked. Demographic characteristics of the three study groups are listed in Table 1. All participants gave signed informed consent using protocols approved by the ethics committee at each respective institution.
Table 1.
Demographic and Clinical Characteristics of Subjects
| Characteristics | Category | DA+a (N = 40) | DA−b (N = 41) | AWc (N = 50) | p |
|---|---|---|---|---|---|
| Age (year)d | All | 34 (20, 57) | 36 (19, 64) | 28 (24, 61) | 0.006 |
| Gender (%) | Male | 34 (85.0%) | 36 (87.8%) | 45 (90.0%) | NS |
| DI exposed (%) | HDI | 19 (47.5%) | 28 (68.3%) | 50 (100%) | < 0.001 |
| MDI | 10 (25.0%) | 7 (17.1%) | 0 (0.0%) | ||
| TDI | 11 (27.5%) | 6 (14.6%) | 0 (0.0%) | ||
| Smoking (never versus others) | Never | 30 (75.0%) | 30 (73.2%) | 40 (80.0%) | NS |
| Atopy | Positive | 30 (78.9%) | 27 (65.9%) | 28 (56.0%) | NS |
| Methacholine PC20 (mg/ml) | < 2 | 17 (42.5%) | 6 (15.0%) | 2 (4.0%) | < 0.001 |
| ≥ 2 but < 32 | 16 (40.0%) | 16 (40.0%) | 17 (34.0%) | ||
| ≥ 32 | 7 (17.5%) | 18 (45.0%) | 31 (62.0%) | ||
| Duration of DI exposure (month)* | All | 60 (1, 456) | 48 (3, 384) | 77 (5, 113) | 0.044 |
| HDI only | 84 (3, 465) | 78 (3, 364) | 77 (5, 113) | NS |
Note. NS, no statistical significant with a p value > 0.05.
aDI-exposed workers presenting with lower respiratory symptoms and positive SIC.
bDI-exposed workers presenting with lower respiratory symptoms and negative SIC.
cDI-exposed workers presenting no lower respiratory symptoms.
dAge (year), median (minimum, maximum).
Five–ten milliliters of EDTA-anticoagulated blood was collected from each subject and stored frozen at −80°C prior to DNA extraction. Skin prick testing was performed in participants with a panel of common aeroallergens; atopy was defined as a positive skin test (wheal ≥ 3 mm) to at least one aeroallergen. Airway hyperresponsiveness was evaluated by methacholine inhalation challenge testing performed prior to SIC testing and expressed as the provocative concentration of methacholine eliciting a fall in forced expiratory volume in 1 s (FEV1) of ≥ 20% from postsaline challenge baseline (PC20).
DNA methylation.
DNA was purified using Flexigene DNA kits from Qiagen, according to the manufacturer’s instructions. Methylation status of gene promoters was analyzed using methylation-specific quantitative PCR (MS-qPCR) (Ng et al., 2011). For DNA bisulfite conversion, 500ng genomic DNA from each sample was bisulfite converted and eluted in 50 ul of elution solution using the EZ Methylation Kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. Primers for MS-qPCR of GSTM1, DUSP22, and IFN-γ were described in previous report (Ouyang et al., 2012) and primers for MS-qPCR of IL-4 were designed based on sequence from GenBank NC_000005.9: forward methylated: 5′-GATGGAGATTATTTTGGTTAATACG; reverse methylated: 5′-GCCTCCTAAATTCATACCGTT; forward unmethylated: 5′- GATGGAGATTATTTTGGTTAATATGG; reverse unmethylated: 5′-TCCA CCTCCTAAATTCATACCATT. MS-qPCR was carried out in a total volume of 10 μl, containing 5 μl 2× Power SYBR Green Master Mix (Invitrogen), 0.5 μl 10μM “methylation-specific” or “unmethylated-specific” primers, 0.2 μl ROX, 2.3 μl H2O, and 2 μl of bisulfite-converted DNA template in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) cycled at 50°C × 2min, 95°C × 10min and followed by 45 cycles of 95°C × 15 s and 60°C × 1min. Data were analyzed using Sequence Detector v2.3 Analysis Software (Applied Biosystems). Each sample was analyzed in duplicate. DNA from the prostate cell line, LNCaP, was used in each set reaction as the internal control. Relative level of methylation of a gene promoter in each sample was calculated using the equation: % methylation = 100/[1 + 2ΔCt(meth-unmeth)]% and was used as the primary measure in this article (Ng et al., 2011). Hypermethylation was defined as ≥ 50% methylated.
Data analysis.
Relative methylation levels of four candidate genes were considered primary measures of interest and they are all numerical variables. Each of the primary measures was assessed of its association to the DA status, a categorical variable of three groups (DA+, DA−, and AW) using a univariate fixed effect (or one-way ANOVA) model. Post hoc means were compared between groups under the fixed effect model framework. Considering more than two groups were compared simultaneously, a correction method called Tukey’s method was used to account for multiple comparison and ensure an overall type I error of 0.05. In order to identify confounders or adjusting covariates for multivariate analyses, the following methods were used on characteristic variables listed in Table 1: (1) each of the characteristic variable was assessed of its association to the DA status using a chi-square test if it was a categorical variable or an ANOVA model if it was a numerical variable and (2) each of the primary measures was assessed of its association to a categorized characteristic variable using an ANOVA model. A characteristic variable showing significance in any of proposed analyses was considered an adjusting covariate. The associations between primary measures and the DA status were then reassessed using multivariate fixed effect models after including the adjusting covariates as independent variables. In addition, using each adjusting covariate as strata, both univariate and multivariate fixed effect models were repeated in subgroups stratified by this variable. For example, as smoking status (smoking vs. nonsmoking) was identified as an adjusting covariate, subanalyses were performed among smokers and on smokers individually. Since in the end, both GSTM1 and DUSP22 showed no significant associations to the DA status in any analysis using either univariate models or multivariate models and either the overall method including all subjects or subset methods including a proportion of subjects only; their results were not reported in the article. IL-4 showed limited association to the DA status, specifically among the nonsmokers only, hence its results were only reported in Supplementary table 1. IFN-γ showed significance in most of analyses and its results were fully presented in the article. Moreover, in the next step of predictive analysis, IFN-γ was used to predict DA (a binary variable of DA+ vs. DA−/AW) using univariate and multivariate logistical regression models, respectively. A propensity score (or the predicted probability) of DA+ was generated from each of the logistical regression models and used to construct the receiver operating characteristic (ROC) curve to evaluate the performance of identifying or predicting DA+ using the methylation of IFN-γ promoter. The accuracy of identification was assessed using the area of the ROC curve (area under the curve [AUC]), and the sensitivity and specificity corresponding to a cut of the propensity score. All statistical analyses were performed using SAS 9.3 (SAS, Cary, NC). p Values < 0.05 were considered statistically significant.
RESULTS
Subjects and Characteristics
Group descriptive characteristics are shown in Table 1. One hundred and thirty-one Caucasian subjects with median age of 31 (range 19–64) years and a male:female ratio of 115:16 were studied. Subjects were categorized into three test groups: 40 with SIC confirmed DA (DA+), 41 symptomatic workers in whom DA was excluded (DA−) based on a negative SIC test with a work-relevant DI chemical, and 50 asymptomatic exposed workers (AWs). Because we anticipated that cigarette smoking may impact the DNA methylation status of candidate genes, 10 currently smoking workers in each group were recruited. Workers were classified by specific chemical exposure at work (i.e., MDI, TDI, and HDI). All AWs were exposed to HDI at work, whereas 47% of the DA+ subjects were exposed to HDI, 25% to MDI, and 27.5% to TDI. In the DA− group, 68.3% were exposed to HDI, 17.1% were exposed to MDI, and 14.6% to TDI. The overall duration of DI exposure was significantly higher in the AW group (p = 0.044); however, there was no difference in duration of exposure to HDI among the three study groups (p = 0.893). The DA+ group had significantly greater number of subjects with hyperresponsiveness to methacholine defined by a PC20 (mg/ml) levels < 2mg/ml than other groups (p < 0.001).
DNA Methylation Studies
Relative promoter methylation levels of four candidate genes (GSTM1, DUSP22, IFN-γ, and IL-4) were assessed in 131 DI-exposed workers. The GSTM1 promoter was sparsely methylated in all samples (mean ± SE = 8.1 ± 2.9%), whereas the DUSP22 promoter region was heavily methylated in all samples (mean ± SE = 83.2 ± 2.5%). Both showed no statistical associations to the DA status (DUSP22: F-statistic = 0.86, pF = 0.43; GSTM1: F-statistic = 0.38, pF = 0.69). The IL-4 methylation was not significantly associated to the DA status in overall analyses when all workers were included (see Supplementary table 1 in the Online Supplementary Data). However, in a subset including nonsmokers only, the DA+ group showed slightly lower methylation level (mean ± SE = 5.0 ± 1.8%) than the AW group (mean ± SE = 12.1 ± 1.5%, p’s < 0.05 using univariate and multivariate models, respectively). Interestingly, higher methylation level was found in the DA+ group in a univariate model but not in multivariate model when only smokers were studied.
Associations of the IFN-γ methylation to demographic and clinical characteristics of interest were presented in Table 2. There were higher relative methylation levels among smokers and workers with lower mean baseline methacholine PC20. In addition, the relative methylation of IFN-γ promoter was also found significantly associated with type of DI exposure and gender. Four variables, smoking status, methacholine PC20, type of DI exposure, and gender, were used as adjusting covariates in the multivariate models for evaluating associations between methylation of IFN-γ promoter and DA status. Table 3 summarized mean ± SE of IFN-γ methylation among three DA groups and their comparisons using univariate and multivariate models, respectively. In particular, the mean ± SE of IFN-γ methylation was 59.2 ± 4.1% in the DA+, higher than 38.1 ± 4.0% and 40.1 ± 3.6% in the DA− and AW groups, respectively, using the univariate model. Similar conclusions could be found from the multivariate model except that the difference between DA+ and AWs was not significant (p = 0.126). Subset analyses showed the IFN-γ methylation was significantly associated to the DA status in subgroups such as nonsmokers, or workers with absence of airway hyperreponsiveness (defined as PC20 32mg/ml), or male participants, or workers with MDI exposure only.
Table 2.
IFN-γ Methylation Level (%) to Demographic and Clinical Characteristics
| Characteristics | Category | IFN-γ | |
|---|---|---|---|
| Mean ± SE | p | ||
| Age (year) | < 30 | 42.9±3.5 | NS |
| ≥ 30 | 47.4±3.2 | ||
| Gender | Female | 58.0±6.7 | 0.045 |
| Male | 43.6±2.5 | ||
| DI exposed | HDI | 41.7±2.7 | 0.017 |
| MDI | 61.1±6.4 | ||
| TDI | 50.3±6.4 | ||
| Smoking status | Current | 57.5±4.7 | 0.004 |
| Never | 41.5±2.6 | ||
| Atopy | Negative | 47.5±4.1 | NS |
| Positive | 44.0±2.9 | ||
| Methacholine PC20 (mg/ml) | < 2 | 52.6±5.3 | 0.035 |
| ≥ 2 | 49.9±3.8 | ||
| ≥ 32 | 38.6±3.6 | ||
| Duration of DI exposure (month) | < 72 | 44.9±3.5 | NS |
| ≥ 72 | 45.7±3.3 | ||
Note. NS, no statistical significant with a p value > 0.05.
Table 3.
IFN-γ Methylation Level (%) Among DA+, DA−, and AWs
| Stratification variable | Category | Mean ± SE | p valuea | |||
|---|---|---|---|---|---|---|
| (1) DA+ | (2) DA− | (3) AW | (1) versus (2) | (1) versus (3) | ||
| All | — | 59.2±4.1 | 38.1±4.0 | 40.1±3.6 | 0.001/0.007 | 0.002/0.126 |
| DI exposed | HDI | 53.4±5.9 | 36.5±4.9 | 40.1±3.7 | 0.077/0.221 | 0.143/0.555 |
| MDI | 73.1±6.0 | 43.9±7.1 | — | 0.007/0.007 | — | |
| TDI | 56.6±9.1 | 38.8±12.4 | — | 0.265/0.097 | — | |
| Smoking status | Never | 58.6±4.4 | 31.3±4.4 | 36.5±3.8 | 0.000/0.002 | 0.001/0.076 |
| Current | 61.0±8.4 | 56.9±8.0 | 54.8±8.4 | 0.935/0.987 | 0.866/0.935 | |
| Methacholine PC20 (mg/ml) | ≥ 32 | 64.3±8.5 | 33.7±5.3 | 35.7±4.1 | 0.010/0.034 | 0.011/0.217 |
| ≥ 2 and < 32 | 57.8±6.4 | 42.6±6.4 | 49.5±6.2 | 0.226/0.191 | 0.627/0.768 | |
| < 2 | 58.5±7.9 | 43.9±13.3 | 29.2±23.0 | 0.620/0.616 | 0.461/0.487 | |
| Gender | Female | 68.6±9.8 | 55.1±10.8 | 48.4±10.8 | 0.636/0.439 | 0.378/0.775 |
| Male | 57.6±4.4 | 35.8±4.3 | 39.2±3.8 | 0.002/0.026 | 0.006/0.332 | |
Note . p Values were obtained from both univariate/multivariate fixed effect model. In the univariate model, the group (DA+ vs. DA− vs. AW) factor was the only independent variable of interest, whereas in the multivariate method, other factors such as DI exposed, smoking, methacholine PC20 (mg/ml), and gender were used as controlling covariate or independent variables. Both p values were accounted for multiple comparisons using a Tukey’s method to ensure an overall 5% type I error.
aUnivariate/multivariate.
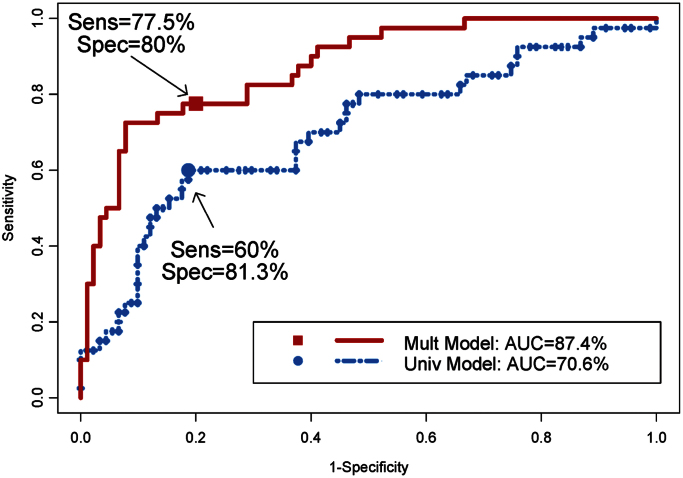
In the predictive analyses, we evaluated the performance of the IFN-γ promoter methylation as a predictor of DA+ phenotype using AUC from the ROC analysis. Figure 1 and Table 4 showed the moderate accuracy of identifying DA+ using the IFN-γ promoter methylation (univariate model), with an AUC of 0.71. In a multivariate logistical regression model, DA+ phenotype was predicted by IFN-γ promoter methylation, along with other covariates (or predictors) of gender, smoking status, exposure, and methacholine PC20. Contributions of the covariates were assessed using the Wald statistics and they were 7.87 (p = 0.005), 2.1 (p = 0.147), 2.5 (p = 0.114), 15.2 (p = 0.001), and 18.9 (p < 0.001) for the predictors of IFN-γ promoter methylation, gender, smoking status, exposure, and methacholine PC20, respectively. The AUC from this multivariate model increased (0.71–0.87) as did sensitivity (60–77.5%). Interestingly, IFN-γ promoter methylation seemed to have the best prediction of DA for the workers with methacholine PC20 of ≥ 32, with AUCs reaching 0.81 and 0.94 in the univariate and multivariate models, respectively.
Fig. 1.
ROC curves of identifying DA+ (against DA− and asymptomatic) using IFN-γ promoter methylation status as a predictor. Univariate model: The ROC curve was estimated from a univariate logistical model using IFN-γ promoter methylation status as the only predictor. Multivariate model: The ROC curve was estimated from a multivariate logistical model using IFN-γ promoter methylation status as the predictor and DI exposed, smoking status, methacholine PC20 (mg/ml), and sex as controlling covariates. Cutoff points for derived sensitivities and specificities are provided in Table 4.
Table 4.
AUC, Sensitivity, and Specificity of Identifying DA+ Using IFN-γ Promoter Methylation Level as a Predictor
| Stratification variable | Category | Univariate modela | Multivariate model | ||||
|---|---|---|---|---|---|---|---|
| AUC | Sensitivityb | Specificityb | AUC | Sensitivityb | Specificityb | ||
| — | — | 70.6% | 60.0% | 81.3% | 87.4%c | 77.5% | 80.0% |
| DI exposed | HDI | 65.3% | 52.6% | 82.1% | 83.7%d | 63.2% | 81.8% |
| Smoking status | Never | 74.4% | 60.0% | 87.1% | 88.1%e | 80.0% | 84.1% |
| Methacholine PC20 (mg/ml) | ≥ 32 | 81.3% | 71.4% | 85.7% | 93.6%f | 85.7% | 89.8% |
| Gender | Male | 70.2% | 55.9% | 84.0% | 87.4%g | 76.5% | 88.8% |
Note. NS, no statistical significant with a p value > 0.05.
aROC curves were drawn from univariate logistical regression models using DA status (DA+ vs. not) as the dependent variable and the IFN-γ promoter methylation level as the only predictor.
bSensitivity and specificity were calculated from an “optimal cut” that maximized its corresponding average of sensitivity and specificity.
cThe ROC curve was drawn from a multivariate logistical model using DA status (DA+ vs. not) as the dependent variable and the following covariates: the IFN-γ promoter methylation level, DI exposed, smoking status, methacholine PC20 (mg/ml), and gender.
dThe ROC curve was drawn from a multivariate logistical model using DA status (DA+ vs. not) as the dependent variable and the following covariates: the IFN-γ promoter methylation level, smoking status, methacholine PC20 (mg/ml), and gender. The analysis was performed to subjects who had HDI exposure only.
eThe ROC curve was drawn from a multivariate logistical model using DA status (DA+ vs. not) as the dependent variable and the following covariates: the IFN-γ promoter methylation level, DI exposed, methacholine PC20 (mg/ml), and gender. The analysis was performed to nonsmokers only.
fThe ROC curve was drawn from a multivariate logistical model using DA status (DA+ vs. not) as the dependent variable and the following covariates: the IFN-γ promoter methylation level, DI exposed, smoking status, and gender. The analysis was performed to subjects whose methacholine PC20 ≥ 32mg/ml only.
gThe ROC curve was drawn from a multivariate logistical model using DA status (DA+ vs. not) as the dependent variable and the following covariates: the IFN-γ promoter methylation level, DI exposed, smoking status, and methacholine PC20 (mg/ml). The analysis was performed to male workers only.
DISCUSSION
OA is defined as “variable airflow limitation and/or airway hyperresponsiveness due to exposure to a specific causal agent present in a particular work environment and not to stimuli encountered outside the workplace” (Tarlo et al., 2008). IgE-mediated OA due to a workplace respiratory sensitizer is more often attributable to protein sensitizers rather than reactive chemicals. Allergic asthma is a disorder of airway inflammation and airway hyperresponsiveness driven by Th2 cytokines and specific IgE induced by exposure and sensitization to aeroallergens. Enhanced expression of IL-4 has been identified in bronchial biopsies of patients with mild asthma but not moderate or severe asthma (Siddiqui et al., 2010). DI chemicals including HDI, TDI, and MDI are prototypic reactive chemicals known to cause DA. Despite clinical features suggesting an allergic mechanism, serum-specific IgE antibodies for DI-HSA antigens have been demonstrated in less than 30% of workers with confirmed DA (Bernstein et al., 2002). In addition, although IL-5 is enhanced, IL-4 and IgE expression is not increased in bronchial biopsy tissue of workers with DA following SIC with TDI (Jones et al., 2006). The latter observation suggests that alternate non-IgE, non-IL-4-dependent immune mechanisms may contribute to airway inflammation in DA. We and others have reported that DIs enhance release of proinflammatory cytokines and chemokines (e.g., monocyte chemoattractant protein-1) from monocytes and macrophages. Nonimmune direct oxidative stress effects of DIs on respiratory epithelium may also play an important role in the pathogenesis of DA (Bernstein et al., 2002; Lummus et al., 2011; Wisnewski et al., 2005, 2008).
In this study, we found significantly higher levels of IFN-γ promoter methylation in DA+ workers than in DA− and AWs. In contrast, methylation of IL-4 promoter was slightly decreased in nonsmoking workers with DA+, indicating that epigenetic modification of IL-4 was not significant in these workers. The actual biologic role of IFN-γ, however, in development and perpetuation of DA is unknown. This data would suggest that decreased production of IFN-γ via hypermethylation and silencing of its gene promoter could enhance Th2 differentiation and Th2 cytokine-directed airway inflammation. Although we did not investigate the entire epigenome in this study, it is probable that epigenetic modifications of multiple genes could also be associated with DA.
Overall, IFN-γ promoter percent methylation was generally higher in smoking workers than nonsmoking workers (see Table 2). When levels of methylation of IFN-γ promoter were compared among DA groups, it was interesting to find they were only significant among workers who never smoked, whereas for smokers, the levels of methylation of IFN-γ promoter were similar among the various groups (Table 3). These data suggested that environmental tobacco smoke exposure had a substantial effect on IFN-γ promoter methylation, which is similar to the report that secondhand smoke in combination with ambient air pollution exposure is associated with increased CpG methylation and decreased expression of IFN-γ in T effector cells and Foxp3 in T regulatory cells in children (Kohli et al., 2012). However, for nonsmoking subjects, the DA (or group) effect was noticeably a significant factor contributing to IFN-γ promoter methylation.
Currently, there are no validated diagnostic tests that accurately identify workers with DA. Our data showed that IFN-γ promoter methylation could be promising marker to enhance the accuracy in distinguishing DA+ from DA-exposed workers. Obviously, smoking status and female gender are also associated with hypermethylation of IFN-γ promoter, which could confound the ability of this assay to differentiate DA+ and non-DA workers. However, using a multivariate model to adjust for other covariate predictors (Table 3) sufficiently improved test characteristics to achieve 77.5% sensitivity and 80% specificity. Our initial data also suggest that the IFN-γ hypermethylation test should be further evaluated in expanded worker cohorts as a potential biomarker of risk for DA.
There are several limitations intrinsic to the design of this type of a cross-sectional study. Hypermethylation of IFN-γ (defined as ≥ 50% methylated) was by no means specific for DA and was identified in 17% of symptomatic exposed workers (DA−) as well as AWs (23%) in our comparator groups. These findings suggest that other environmental toxicants, encountered in both occupational and nonoccupational settings, might also contribute to IFN-γ promoter methylation. This finding may not be specific for OA. Runyon et al. (2012) reported that T cells collected from adult discordant asthmatic twins demonstrated increased methylation of the IFN-γ. We have recently reported that IFN-γ promoter hypermethylation of leukocytes obtained from human neonates participating in a birth cohort study was associated with maternal exposure to polycyclic aromatic hydrocarbons. Another limitation is the unknown effect of different frequencies of exposure to MDI and TDI versus HDI between the DA+ group and the two comparator groups (see Table 1). Finally, because this is a cross-sectional study, we were unable to assess dose-response effects of DI exposure on IFN-γ promoter methylation.
The role of hypermethylation of the IFN-γ promoter in the pathogenesis or clinical expression of DA remains to be determined in future studies. It has been demonstrated that in vivo and in vitro exposure to DIs, specifically MDI, readily form adducts with nuclear DNA. Thus, although unproven, it is feasible that DIs or aromatic diamine derivatives reacting with DNA could directly bind to CpG sites in the IFN-γ gene promoter region (Bolognesi et al., 2001). The finding of hypermethylation in the majority of DA+ patients is consistent with a hypothesis that exposure to occupational chemicals could play a heretofore undefined role in enhancing airway inflammation potentially via epigenetic modification of the IFN-γ promoter region. If the relatively high sensitivity and specificity demonstrated in this study is replicated in future respective longitudinal studies, IFN-γ hypermethylation could become a biomarker for predicting risk of DA.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (P50ES01590, U01ES019480, U01ES020988, R01ES015584, R01ES022071, P30ES006096 to S.-M.H.); U.S. Department of Veterans Affairs (I01BX000675 to S.-M.H.); National Institute of Occupational Safety and Health (R01OH008795 to D.I.B.).
Supplementary Material
ACKNOWLEDGMENTS
Stacy Langmeyer and Banurekha Kesavalu provided excellent technical assistance.
REFERENCES
- Bakerly N. D., Moore V. C., Vellore A. D., Jaakkola M. S., Robertson A. S., Burge P. S. (2008). Fifteen-year trends in occupational asthma: Data from the Shield surveillance scheme. Occup. Med. (Lond). 58, 169–174 [DOI] [PubMed] [Google Scholar]
- Balboni A., Baricordi O. R., Fabbri L. M., Gandini E., Ciaccia A., Mapp C. E. (1996). Association between toluene diisocyanate-induced asthma and DQB1 markers: A possible role for aspartic acid at position 57. Eur. Respir. J. 9, 207–210 [DOI] [PubMed] [Google Scholar]
- Bernstein D. I. (2011). Genetics of occupational asthma. Curr. Opin. Allergy Clin. Immunol. 11, 86–89 [DOI] [PubMed] [Google Scholar]
- Bernstein D. I., Cartier A., Côté J., Malo J. L., Boulet L. P., Wanner M., Milot J., L’Archevéque J., Trudeau C., Lummus Z. (2002). Diisocyanate antigen-stimulated monocyte chemoattractant protein-1 synthesis has greater test efficiency than specific antibodies for identification of diisocyanate asthma. Am. J. Respir. Crit. Care Med. 166, 445–450 [DOI] [PubMed] [Google Scholar]
- Bernstein D. I., Kashon M., Lummus Z. L., Johnson V. J., Fluharty K., Gautrin D., Malo J. L., Cartier A., Boulet L. P., Sastre J., et al. (2012). CTNNA3 (α-catenin) gene variants are associated with diisocyanate asthma: A replication study in a Caucasian worker population. Toxicol. Sci. 131, 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. I., Korbee L., Stauder T., Bernstein J. A., Scinto J., Herd Z. L., Bernstein I. L. (1993). The low prevalence of occupational asthma and antibody-dependent sensitization to diphenylmethane diisocyanate in a plant engineered for minimal exposure to diisocyanates. J. Allergy Clin. Immunol. 92, 387–396 [DOI] [PubMed] [Google Scholar]
- Bolognesi C., Baur X., Marczynski B., Norppa H., Sepai O., Sabbioni G. (2001). Carcinogenic risk of toluene diisocyanate and 4,4’-methylenediphenyl diisocyanate: Epidemiological and experimental evidence. Crit. Rev. Toxicol. 31, 737–772 [DOI] [PubMed] [Google Scholar]
- Brunst K. J., Leung Y. K., Ryan P. H., Khurana Hershey G. K., Levin L., Ji H., Lemasters G. K., Ho S. M. (2013). Forkhead box protein 3 (FOXP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma. J. Allergy Clin. Immunol. 131, 592–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo P., Wisnewski A. V., Lummus Z., Cartier A., Malo J. L., Boulet L. P., Bernstein D. I. (2007). Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDI-exposed workers. Clin. Exp. Allergy. 37, 1095–1102 [DOI] [PubMed] [Google Scholar]
- Cartier A., Grammer L., Malo J. L., Lagier F., Ghezzo H., Harris K., Patterson R. (1989). Specific serum antibodies against isocyanates: association with occupational asthma. J. Allergy Clin. Immunol. 84(4 Pt 1)507–514 [DOI] [PubMed] [Google Scholar]
- Choi J. H., Lee K. W., Kim C. W., Park C. S., Lee H. Y., Hur G. Y., Kim S. H., Hong C. S., Jang A. S., Park H. S. (2009). The HLA DRB1*1501-DQB1*0602-DPB1*0501 haplotype is a risk factor for toluene diisocyanate-induced occupational asthma. Int. Arch. Allergy Immunol. 150, 156–163 [DOI] [PubMed] [Google Scholar]
- Jones M. G., Floyd A., Nouri-Aria K. T., Jacobson M. R., Durham S. R., Taylor A. N., Cullinan P. (2006). Is occupational asthma to diisocyanates a non-IgE-mediated disease? J. Allergy Clin. Immunol. 117, 663–669 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Cho B. Y., Park C. S., Shin E. S., Cho E. Y., Yang E. M., Kim C. W., Hong C. S., Lee J. E., Park H. S. (2009). Alpha-T-catenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin. Exp. Allergy. 39, 203–212 [DOI] [PubMed] [Google Scholar]
- Kohli A., Garcia M. A., Miller R. L., Maher C., Humblet O., Hammond S. K., Nadeau K. (2012). Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-γ in T effector cells and Foxp3 in T regulatory cells in children. Clin. Epigenetics. 4, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummus Z. L., Wisnewski A. V., Bernstein D. I. (2011). Pathogenesis and disease mechanisms of occupational asthma. Immunol. Allergy Clin. North Am. 31, 699–716, vi [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo J. L., Ghezzo H., Elie R. (1999). Occupational asthma caused by isocyanates: Patterns of asthmatic reactions to increasing day-to-day doses. Am. J. Respir. Crit. Care Med. 159, 1879–1883 [DOI] [PubMed] [Google Scholar]
- Mapp C. E., Fryer A. A., De Marzo N., Pozzato V., Padoan M., Boschetto P., Strange R. C., Hemmingsen A., Spiteri M. A. (2002). Glutathione S-transferase GSTP1 is a susceptibility gene for occupational asthma induced by isocyanates. J. Allergy Clin. Immunol. 109, 867–872 [DOI] [PubMed] [Google Scholar]
- Ng E. K., Leung C. P., Shin V. Y., Wong C. L., Ma E. S., Jin H. C., Chu K. M., Kwong A. (2011). Quantitative analysis and diagnostic significance of methylated SLC19A3 DNA in the plasma of breast and gastric cancer patients. PLoS One. 6, e22233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang B., Baxter C. S., Lam H. M., Yeramaneni S., Levin L., Haynes E., Ho S. M. (2012). Hypomethylation of dual specificity phosphatase 22 promoter correlates with duration of service in firefighters and is inducible by low-dose benzo[a]pyrene. J. Occup. Environ. Med. 54, 774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F., Tang W. Y., Herbstman J., Tang D., Levin L., Miller R., Ho S. M. (2009). Relation of DNA methylation of 5’-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 4, e4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs H. P., Barbalho-Krölls T., Huber H., Baur X. (1997). No evidence for the influence of HLA class II in alleles in isocyanate-induced asthma. Am. J. Ind. Med. 32, 522–527 [DOI] [PubMed] [Google Scholar]
- Runyon R. S., Cachola L. M., Rajeshuni N., Hunter T., Garcia M., Ahn R., Lurmann F., Krasnow R., Jack L. M., Miller R. L., et al. (2012). Asthma discordance in twins is linked to epigenetic modifications of T cells. PLoS One. 7, e48796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre J., Fernández-Nieto M., Novalbos A., De Las Heras M., Cuesta J., Quirce S. (2003). Need for monitoring nonspecific bronchial hyperresponsiveness before and after isocyanate inhalation challenge. Chest. 123, 1276–1279 [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Mistry V., Doe C., Stinson S., Foster M., Brightling C. (2010). Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest. 137, 797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. Y., Levin L., Talaska G., Cheung Y. Y., Herbstman J., Tang D., Miller R. L., Perera F., Ho S. M. (2012). Maternal exposure to polycyclic aromatic hydrocarbons and 5’-CpG methylation of interferon-γ in cord white blood cells. Environ. Health Perspect. 120, 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlo S. M., Balmes J., Balkissoon R., Beach J., Beckett W., Bernstein D., Blanc P. D., Brooks S. M., Cowl C. T., Daroowalla F., et al. (2008). Diagnosis and management of work-related asthma: American College Of Chest Physicians Consensus Statement. Chest. 134(3 Suppl), 1S–41S [DOI] [PubMed] [Google Scholar]
- Wisnewski A. V., Liu Q., Liu J., Redlich C. A. (2005). Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin. Exp. Allergy. 35, 352–357 [DOI] [PubMed] [Google Scholar]
- Wisnewski A. V., Liu Q., Liu J., Redlich C. A. (2008). Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI-albumin conjugates. Clin. Exp. Allergy. 38, 957–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.