ABSTRACT
The alarming rise in antibiotic resistance has led to an increase in patient mortality and health care costs. This problem is compounded by the absence of new antibiotics close to regulatory approval. Acinetobacter baumannii is a human pathogen that causes infections primarily in patients in intensive care units (ICUs) and is highly antibiotic resistant. Colistin is one of the last-line antibiotics for treating A. baumannii infections; however, colistin-resistant strains are becoming increasingly common. This cationic antibiotic attacks negatively charged bacterial membranes in a manner similar to that seen with cationic antimicrobials of the innate immune system. We therefore set out to determine if the increasing use of colistin, and emergence of colistin-resistant strains, is concomitant with the generation of cross-resistance to host cationic antimicrobials. We found that there is indeed a positive correlation between resistance to colistin and resistance to the host antimicrobials LL-37 and lysozyme among clinical isolates. Importantly, isolates obtained before and after treatment of individual patients demonstrated that colistin use correlated with increased resistance to cationic host antimicrobials. These data reveal the overlooked risk of inducing cross-resistance to host antimicrobials when treating patients with colistin as a last-line antibiotic.
IMPORTANCE
Increased use of the cationic antibiotic colistin to treat multidrug-resistant Acinetobacter baumannii has led to the development of colistin-resistant strains. Here we report that treatment of patients with colistin can induce not only increased resistance to colistin but also resistance to host cationic antimicrobials. This worrisome finding likely represents an example of a broader trend observed in other bacteria against which colistin is used therapeutically such as Pseudomonas aeruginosa and Klebsiella pneumoniae. Furthermore, these data suggest that the possible future use of an array of cationic antimicrobial peptides in development as therapeutics may have unintended negative consequences, eventually leading to the generation of hypervirulent strains that are resistant to innate host defenses. The potential for the induction of cross-resistance to innate immune antimicrobials should be considered during the development of new therapeutics.
Observation
Due to overuse and misuse of antibiotics and the ability of bacteria to rapidly gain resistance, we are now in an age of multidrug-resistant (MDR) and pan-drug-resistant (PDR) bacterial pathogens and face a possible return to the pre-antibiotic era. The burden of antimicrobial resistance has increased the length of hospital stays, patient mortality, and health care costs (1–5). Acinetobacter baumannii is a Gram-negative and highly antibiotic-resistant bacterial pathogen (6, 7). Due to its ability to persist on surfaces for weeks, its ability to colonize humans, and its increasing rate of antibiotic resistance, A. baumannii has become an emerging health care problem (6, 8).
Polymyxin antibiotics, including colistin, are currently used as last-line drugs to treat MDR A. baumannii infections (9). Colistin is a cationic antimicrobial peptide that disrupts both the outer and inner membranes of Gram-negative bacteria (10), a trait shared by the host cationic antimicrobials LL-37 and lysozyme (11–16). LL-37 is a human antimicrobial peptide typically found at sites of inflammation, where it is a primary defense against Gram-negative bacteria (17). Lysozyme is a host antimicrobial found within multiple immune cells as well as in secretions such as tears, breast milk, and mucus and is important for their activity against invading microbes. Importantly, the highly cationic, nonenzymatic, C-terminal portion of lysozyme has very potent antimicrobial activity (12–14).
Given the increasing prevalence of colistin resistance among A. baumannii clinical isolates (9), we set out to test whether there was a correlation with cross-resistance to host cationic antimicrobials. We assembled a panel of A. baumannii isolates (CI-1, CI-2, CI-3, 17978, CI-4, ARLC, MU134, MU215, MU181, and MU52) and first determined their colistin MICs (see Table S1 in the supplemental material) and susceptibilities to multiple other antibiotics (Table S2). Bacterial suspensions were prepared using a PROMPT (3M Company, St. Paul, MN) inoculation system, and antibiotic susceptibilities were determined using Neg Breakpoint Combo Panel type 41 on a Microscan WalkAway Plus automated system (Siemens Healthcare Diagnostics Inc., West Sacramento, CA). Additionally, the MICs for colistin were measured using Etest strips (bioMérieux, Durham, NC), following the inoculation and reading instructions of the manufacturer. The strains exhibited a range of MIC values. Using Clinical and Laboratory Standards Institute (CLSI) interpretive criteria, we found that strains CI-1, CI-2, CI-3, and 17978 were sensitive to colistin whereas CI-4, ARLC, MU134, MU215, MU181, and MU52 were resistant (Table S1).
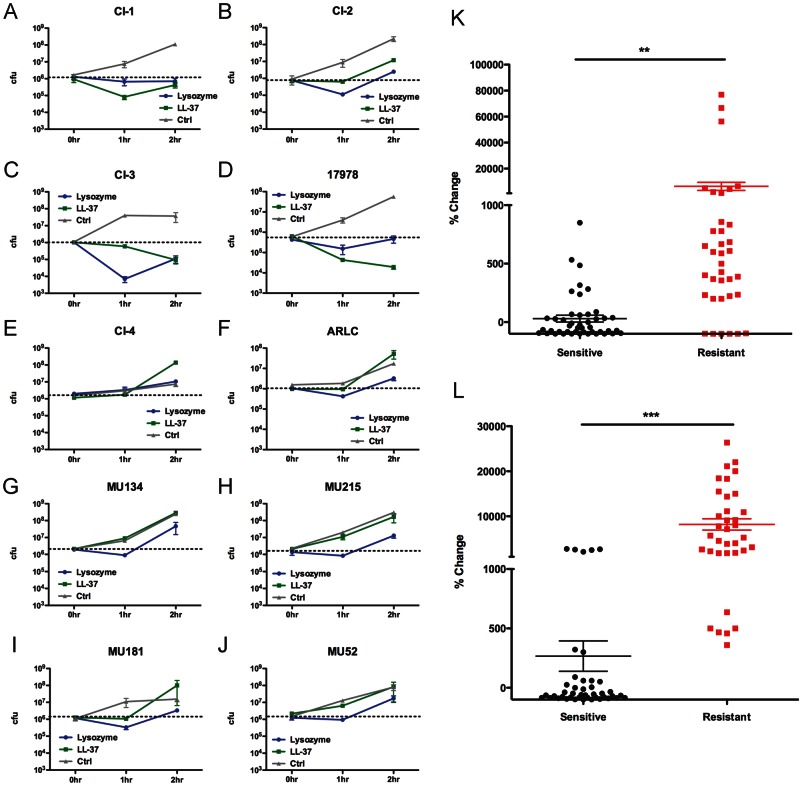
We treated the colistin-sensitive and -resistant isolates with LL-37 or lysozyme to determine their sensitivities, as previously described (18). Briefly, overnight cultures were grown from frozen stock in lysogeny broth (LB; also known as Luria broth) (BD Biosciences, Sparks, MD) at 37°C with aeration and then diluted to a final concentration of ~10e6 CFU/ml in 25% LB. Bacteria were treated with host antimicrobials (LL-37, 6.25 µg/ml; lysosome, 2.5 mg/ml) and incubated with aeration at 37°C, and aliquots were plated at 0 h, 1 h, and 2 h for enumeration of CFU. The colistin-sensitive clinical isolates CI-1 (Fig. 1A) and CI-2 (Fig. 1B) were inhibited from replicating to wild-type levels in the presence of LL-37, while CI-3 (Fig. 1C) and 17978 (Fig. 1D) were killed. In contrast, the colistin-resistant clinical isolates CI-4 (Fig. 1E), ARLC (Fig. 1F), MU134 (Fig. 1G), MU215 (Fig. 1H), MU181 (Fig. 1I), and MU52 (Fig. 1J) each replicated roughly 100- to 200-fold after 2 h in the presence of LL-37. These data suggest that colistin resistance correlates with increased resistance to the host cationic antimicrobial peptide LL-37, which was further clearly demonstrated when results from 4 experiments performed with the aforementioned strains were pooled (Fig. 1K). We observed similar phenotypes following lysozyme treatment, with the exception of the colistin-susceptible 17978 isolate (Fig. 1D), which was able to persist in the presence of lysozyme instead of being killed, and CI-2 (Fig. 1B), which exhibited very limited replication. These results were again further illustrated when data from several experiments were pooled (Fig. 1L) and suggest that colistin resistance is highly correlated with resistance to lysozyme.
FIG 1 .
Colistin resistance correlates with resistance to host cationic antimicrobials in A. baumannii clinical isolates. (A to J) Colistin-sensitive (A to D) and colistin-resistant (E to J) A. baumannii clinical isolates were treated with 6.25 µg/ml of LL-37 (green) or 2.5 mg/ml of lysozyme (blue) for the indicated times and plated for enumeration of CFU. Time zero CFU is indicated by dashed black lines. (K to L) Percent changes of CFU from time zero to 2 h after treatment with LL-37 (K) or lysozyme (L) from 4 individual experiments with 3 to 4 samples of each isolate represented in panels A to J. Data were analyzed for significance using the unpaired Student’s t test. **, P < 0.001; ***, P < 0.0001. Error bars represent the standard deviations of the results determined for triplicate samples.
We next investigated the basis for the resistance phenotypes observed. The A. baumannii PmrAB two-component regulatory system induces phosphoethanolamine modifications to the lipid A component of lipopolysaccharide (LPS) in response to the presence of polymyxins (19, 20). Arroyo et al. showed that point mutations within the PmrB periplasmic domain, histidine kinase (HisK) dimerization/phosphoacceptor domain, or C-terminal ATP binding domain (ATPB) can confer constitutive polymyxin resistance through these lipid A modifications (20). We sequenced the pmrB genes in the colistin-resistant isolates used in this study and found that each harbored nonsynonymous mutations in the sequences encoding one or more of these three domains compared to the pmrB sequence from the colistin-sensitive 17978 strain (see Table S1 in the supplemental material). In contrast, only synonymous mutations were identified in the sequences of pmrB from the colistin-sensitive strains. Nonsynonymous point mutations conferring resistance can be selected rapidly and likely explain the ability of some of the colistin-sensitive strains to eventually acquire resistance during extended exposure to LL-37 or lysozyme (see Fig. S1A to D in the supplemental material) (20). Taken together, these data suggest that mutations within pmrB confer not only colistin resistance but also cross-resistance to host cationic antimicrobial peptides.
Since the clinical isolates used here were very diverse (isolated from different anatomical sites, from different areas of the United States, and exhibiting differing antibiotic susceptibility patterns; see Table S1 and S2 in the supplemental material), it is not surprising that there was heterogeneity in their patterns of resistance to host antimicrobials; specifically, the colistin-sensitive CI-2 isolate displayed low-level resistance to LL-37 and lysozyme (Fig. 1B) that was not observed with the other colistin-sensitive isolates (Fig. 1A and C to D). In order to overcome the complications of comparing diverse strains, and to directly determine whether colistin treatment can induce cross-resistance to host cationic antimicrobials, we obtained 2 pairs of A. baumannii clinical isolates from patients pre- and post-colistin treatment (21). Each pair of serial isolates (Ab6266 and Ab6267, and Ab3527 and Ab3941) was from a distinct patient, under 35 years of age, who had sustained severe trauma overseas (21). Ab6267 was collected after 3 weeks of patient treatment with colistin, and Ab3941 was collected after 6 weeks of patient treatment with colistin. Both pre-treatment isolates (Ab6266 and Ab3527) were colistin sensitive, while the post-treatment isolates (Ab6267 and Ab3941) displayed increased resistance to colistin (see Table S1 in the supplemental material). In addition, the transition to increased colistin resistance was not accompanied by changes in susceptibility to the other antibiotics tested (Table S2). While we cannot specify the event(s) (an induced mutational event, selection for a preexisting mutant, or reinfection) that led to the selection for the post-treatment resistant isolates, the pre- and post-treatment isolates were shown to be highly similar by pulse-field gel electrophoresis (PFGE), using a 90% similarity cutoff, compared to 1,500 A. baumannii strains collected from 24 geographically separate hospitals (21). Additionally, optical genome mapping (OGM) analysis showed that the two sets of pre- and post-treatment isolates shared >99% homology (21).
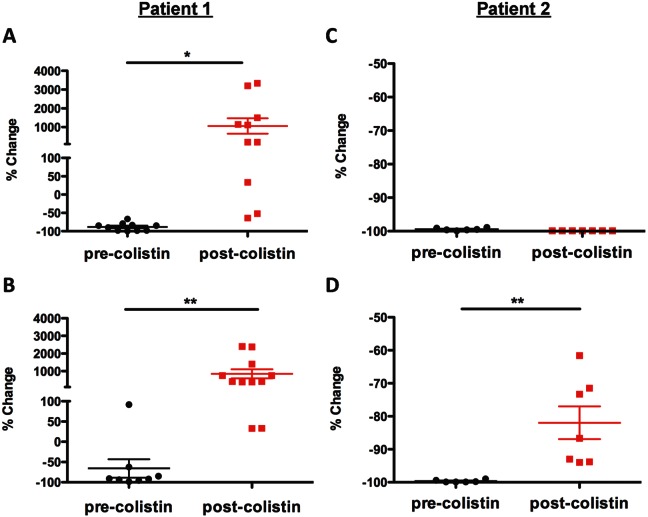
When treated with LL-37 or lysozyme, the post-colistin treatment Ab6267 isolate displayed a significant increase in resistance compared to the corresponding pre-colistin treatment isolate (Ab6266) (Fig. 2A and B). Ab3941 displayed a significant increase in resistance to killing by lysozyme compared to Ab3527 (Fig. 2D), although there was no change in its level of resistance to LL-37 (Fig. 2C). Neither pre-colistin treatment isolate demonstrated resistance to LL-37 or lysozyme over 22 h of treatment (see Fig. S1E and F in the supplemental material). As with the colistin-resistant isolates tested above, the post-colistin treatment strains both harbored nonsynonymous mutations in critical PmrB domains (Table S1). These data from sequentially isolated strains pre- and post-colistin treatment strongly support the conclusion that clinical use of colistin induces cross-resistance to host cationic antimicrobial peptides.
FIG 2 .
Clinical treatment with colistin can induce increased resistance to host cationic antimicrobials. Sequential A. baumannii isolates were collected from 2 patients (Ab6266 and Ab6267, patient 1; Ab3527 and Ab3941, patient 2) pre-colistin treatment (Ab6266 and Ab3527) and post-colistin treatment (Ab6267 and Ab3941). Isolates were treated with 6.25 µg/ml of LL-37 (A and C) or 2.5 mg/ml of lysozyme (B and D). Percent changes from time zero to 2 h after treatment with host antimicrobials from 3 experiments with 3 to 4 samples of each isolate are represented. Data were analyzed for significance using the unpaired Student’s t test. *, P < 0.05; **, P < 0.001. Error bars represent the standard deviations of the results determined for triplicate samples.
Implications.
The rising prevalence of antibiotic resistance is linked to increases in mortality, morbidity, length of hospitalization, and health care costs (1–5). Understanding the mechanisms and consequences of antimicrobial resistance is critical to our ability to combat this problem. Here we have determined that there is a strong correlation between colistin resistance and cross-resistance to the host antimicrobials LL-37 and lysozyme (Fig. 1 and 2). This is likely a phenomenon that occurs broadly and is relevant to a range of nosocomial pathogens, including Pseudomonas aeruginosa and Klebsiella pneumoniae, against which colistin is used as a therapy. This is also critical information in light of the fact that numerous cationic antimicrobial peptides are currently in development as novel therapeutics (22–24). Our data caution that the use of such agents in the clinic may have the unintended negative consequence of inducing resistance to host antimicrobials, which could lead to the selection of hypervirulent strains in the long term.
SUPPLEMENTAL MATERIAL
Colistin-sensitive A. baumannii isolates grown in the presence of host cationic antimicrobials for 22 h. The indicated colistin-sensitive A. baumannii isolates were grown in 25% LB in the presence of LL-37 (10 µl/ml) or lysozyme (6 mg/ml) for 22 h. Download
Origin and colistin sensitivity of Acinetobacter baumannii strains.
MICs for all A. baumannii strains used in this study. (S), sensitivity; (I), intermediate sensitivity; (R), resistance.
ACKNOWLEDGMENTS
We thank Brandi Limbago (CDC) for generously providing clinical isolates CI-1, CI-2, CI-3, CI-4, and ARLC. The MU134, MU215, MU181, and MU52 isolates were collected by the Georgia Emerging Infections Program (GaEIP) as part of the active population-based surveillance for multidrug-resistant Gram-negative bacteria. Additionally, we would like to thank Chui-Yoke Chin, Phil Rather, Tim Sampson, and William Shafer for critical review of the manuscript.
The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
This work was supported by NIH grant R21-AI098800. Its contents are solely our responsibility and do not necessarily represent the official views of the NIH.
Footnotes
Citation Napier BA, Burd EM, Satola SW, Cagle SM, Ray SM, McGann P, Pohl J, Lesho EP, Weiss DS. 2013. Clinical use of colistin induces cross-resistance to host antimicrobials in Acinetobacter baumannii. mBio 4(3):e00021-13. doi:10.1128/mBio.00021-13.
REFERENCES
- 1. Brusselaers N, Vogelaers D, Blot S. 2011. The rising problem of antimicrobial resistance in the intensive care unit. Ann. Intensive Care 1:47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, Nagler J, Icket C, Kalenic S, Horvatic J, Seifert H, Kaasch AJ, Paniara O, Argyropoulou A, Bompola M, Smyth E, Skally M, Raglio A, Dumpis U, Kelmere AM, Borg M, Xuereb D, Ghita MC, Noble M, Kolman J, Grabljevec S, Turner D, Lansbury L, Grundmann H. 2011. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob. Agents Chemother. 55:1598–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maragakis LL, Perencevich EN, Cosgrove SE. 2008. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 6:751–763 [DOI] [PubMed] [Google Scholar]
- 4. Salgado CD, O’Grady N, Farr BM. 2005. Prevention and control of antimicrobial-resistant infections in intensive care patients. Crit. Care Med. 33:2373–2382 [DOI] [PubMed] [Google Scholar]
- 5. Vandijck DM, Depaemelaere M, Labeau SO, Depuydt PO, Annemans L, Buyle FM, Oeyen S, Colpaert KE, Peleman RP, Blot SI, Decruyenaere JM. 2008. Daily cost of antimicrobial therapy in patients with intensive care unit-acquired, laboratory-confirmed bloodstream infection. Int. J. Antimicrob. Agents 31:161–165 [DOI] [PubMed] [Google Scholar]
- 6. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 7. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 67:1607–1615 [DOI] [PubMed] [Google Scholar]
- 10. Hancock RE. 1997. Peptide antibiotics. Lancet 349:418–422 [DOI] [PubMed] [Google Scholar]
- 11. Bucki R, Leszczyńska K, Namiot A, Sokołowski W. 2010. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch. Immunol. Ther. Exp. (Warsz) 58:15–25 [DOI] [PubMed] [Google Scholar]
- 12. Düring K, Porsch P, Mahn A, Brinkmann O, Gieffers W. 1999. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 449:93–100 [DOI] [PubMed] [Google Scholar]
- 13. Ibrahim HR, Thomas U, Pellegrini A. 2001. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 276:43767–43774 [DOI] [PubMed] [Google Scholar]
- 14. Laible NJ, Germaine GR. 1985. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: inhibition by chitin oligosaccharides. Infect. Immun. 48:720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thennarasu S, Tan A, Penumatchu R, Shelburne CE, Heyl DL, Ramamoorthy A. 2010. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys. J. 98:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaara M, Vaara T. 1981. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob. Agents Chemother. 19:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandamme D, Landuyt B, Luyten W, Schoofs L. 2012. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell. Immunol. 280:22–35 [DOI] [PubMed] [Google Scholar]
- 18. Mai J, Tian XL, Gallant JW, Merkley N, Biswas Z, Syvitski R, Douglas SE, Ling J, Li YH. 2011. A novel target-specific, salt-resistant antimicrobial peptide against the cariogenic pathogen Streptococcus mutans. Antimicrob. Agents Chemother. 55:5205–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. 2009. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 53:3628–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 55:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lesho E, Yoon E, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman P. Emergence of colistin-resistance in Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of extremely-drug-resistant wound infections. J. Infect. Dis, in press [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Wang D, Cong Y, Wang J, Zhu J, Yang J, Hu Z, Hu X, Tan Y, Hu F, Rao X. 2011. Recombinant antimicrobial peptide hPAB-β expressed in pichia pastoris, a potential agent active against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 89:281–291 [DOI] [PubMed] [Google Scholar]
- 23. Lamb HM, Wiseman LR. 1998. Pexiganan acetate. Drugs 56:1047–1052; discussion 1053–1054 [DOI] [PubMed] [Google Scholar]
- 24. Peters BM, Shirtliff ME, Jabra-Rizk MA. 2010. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 6:e1001067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colistin-sensitive A. baumannii isolates grown in the presence of host cationic antimicrobials for 22 h. The indicated colistin-sensitive A. baumannii isolates were grown in 25% LB in the presence of LL-37 (10 µl/ml) or lysozyme (6 mg/ml) for 22 h. Download
Origin and colistin sensitivity of Acinetobacter baumannii strains.
MICs for all A. baumannii strains used in this study. (S), sensitivity; (I), intermediate sensitivity; (R), resistance.