ABSTRACT
The Mycobacterium tuberculosis gene Rv3232c/MT3329 (ppk2) encodes a class II polyphosphate kinase, which hydrolyzes inorganic polyphosphate (poly P) to synthesize GTP. We assessed the role of ppk2 in M. tuberculosis poly P regulation, antibiotic tolerance, and virulence. A ppk2-deficient mutant (ppk2::Tn) and its isogenic wild-type (WT) and complemented (Comp) strains were studied. For each strain, the intrabacillary poly P content, MIC of isoniazid, and growth kinetics during infection of J774 macrophages were determined. Multiplex immunobead assays were used to evaluate cytokines elaborated during macrophage infection. The requirement of ppk2 for M. tuberculosis virulence was assessed in the murine model. The ppk2::Tn mutant was found to have significantly increased poly P content and a 4-fold increase in the MIC of isoniazid relative to the WT and Comp strains. The ppk2::Tn mutant showed reduced survival at day 7 in activated and naive J774 macrophages relative to the WT. Naive ppk2::Tn mutant-infected macrophages showed increased expression of interleukin 2 (IL-2), IL-9, IL-10, IL-12p70, and gamma interferon (IFN-γ) relative to WT-infected macrophages. The ppk2::Tn mutant exhibited significantly lower lung CFU during acute murine infection compared to the control groups. ppk2 is required for control of intrabacillary poly P levels and optimal M. tuberculosis growth and survival in macrophages and mouse lungs.
IMPORTANCE
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is a highly successful human pathogen because it has developed mechanisms to multiply and survive in the lungs by circumventing the immune system. Identification of virulence factors responsible for M. tuberculosis growth and persistence in host tissues may assist in the development of novel strategies to treat TB. In this study, we found that the mycobacterial enzyme polyphosphate kinase 2 (PPK2) is required for controlling intracellular levels of important regulatory molecules and for maintaining susceptibility to the first-line anti-TB drug isoniazid. In addition, PPK2 was found to be required for M. tuberculosis growth in the lungs of mice, at least in part by suppressing the expression of certain key cytokines and chemokines by inactivated lung macrophages.
Introduction
Tuberculosis (TB) remains a major global health problem (1, 2). One of the major obstacles to global TB eradication efforts is the prolonged treatment course required to cure active TB, which is believed to reflect the organism’s ability to remain in a nonreplicating persistent state with reduced susceptibility to conventional antituberculous drugs.
Inorganic polyphosphate (poly P), a linear polymer of many tens or hundreds of inorganic phosphate residues linked by high-energy phosphoanhydride bonds, appears to play an important regulatory role in the transition to bacterial persistence (3, 4). Bacteria accumulate poly P intracellularly when they encounter growth-limiting conditions, such as phosphate depletion, amino acid starvation, or osmotic stress (3, 5). Poly P accumulation has been shown to modulate many different bacterial processes, including protein synthesis, nucleotide balance, lipid metabolism, energy utility, and susceptibility to antibiotics (3, 4). Bacterial polyphosphate kinases are of two classes. Polyphosphate kinase 1 (PPK1) is responsible for poly P synthesis through hydrolysis of ATP (3). Although PPK2 enzymes synthesize poly P, they also function as GTP synthases, using poly P as a substrate (3, 6–9). Deletion of the ppk2B gene in Corynebacterium glutamicum reduced PPK activity and intrabacterial poly P content, while overexpression of ppk2B increased PPK activity and poly P content (10). Conversely, ppk2 deficiency in Campylobacter jejuni led to decreased poly P-dependent GTP synthesis and increased intracellular ATP/GTP ratio (9). Mutation of ppk2 attenuates bacterial survival under different growth-limiting conditions (7, 9, 10). In addition, PPK2 plays an important role in invasion and intracellular survival of C. jejuni in human intestinal epithelial cells (9).
The Mycobacterium tuberculosis genome contains genes that encode both PPK1 (Rv2984) and PPK2 (Rv3232c), which play a role in poly P synthesis (7, 11). PPK1 is involved in the stress-induced mprAB-sigE-rel signaling pathway in mycobacteria (11) and appears to be encoded by an essential gene in M. tuberculosis (12). M. tuberculosis PPK2 is an octamer that catalyzes poly P-dependent phosphorylation of ADP to ATP at a rate >800-fold higher than that of poly P synthesis (13). PPK2 also regulates the intracellular nucleotide pool and is required for survival of M. smegmatis under acidic, heat, and hypoxic conditions (7). In addition, deficiency of ppk2 or ppk1 is associated with decreased mycobacterial survival during macrophage infection (7, 11). However, the role of PPK2 in M. tuberculosis virulence is unknown.
In this study, we investigated the role of ppk2 on M. tuberculosis poly P homeostasis and susceptibility to the cell wall-acting agent isoniazid. Next, we determined the impact of ppk2 deficiency on M. tuberculosis pathogenesis in the animal host by studying the growth, survival, and pathology of a ppk2-deficient mutant in the murine model of TB infection. To investigate host factors responsible for growth restriction of the ppk2-deficient mutant during acute infection in mouse lungs, we used multiplex immunobead assays to evaluate cytokines elaborated by macrophages infected with the ppk2-deficient mutant and control strains.
RESULTS
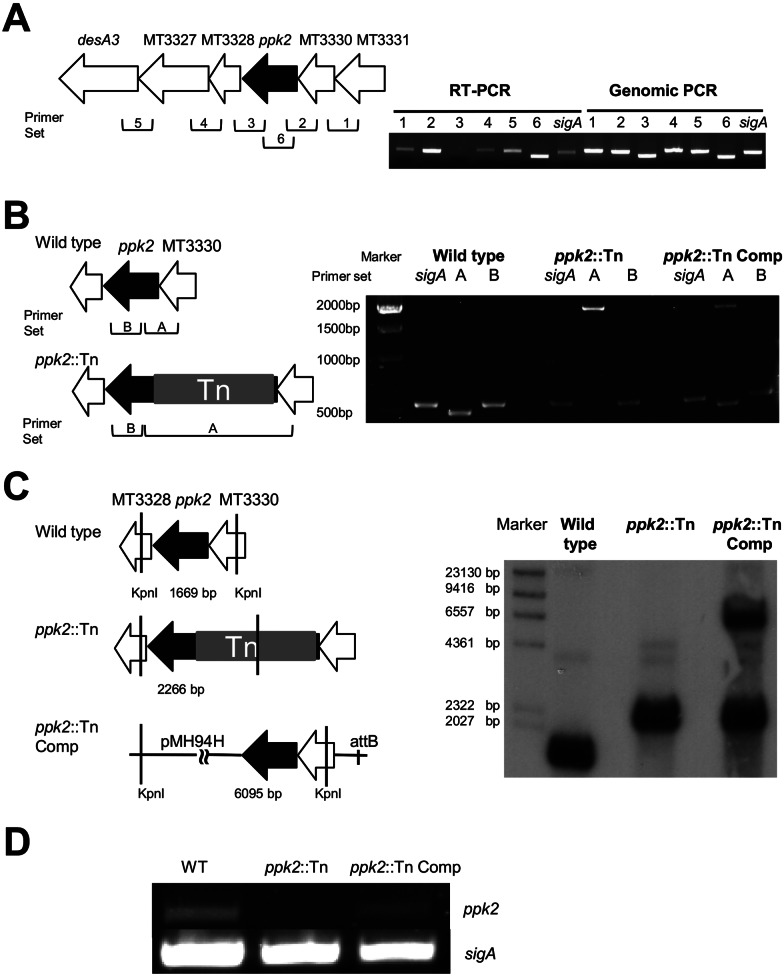
ppk2 is coexpressed as part of a three-gene operon.
In order to test the role of ppk2 (Rv3232c/MT3329) in M. tuberculosis virulence, a ppk2-deficient mutant (ppk2::Tn) was generated (14). To guide our complementation strategy, we studied potential cotranscription of genes flanking ppk2 using reverse transcription-PCR (RT-PCR). ppk2 is located within a putative operon comprising Rv3234c/MT3331, Rv3233c/MT3330, Rv3232c/MT3329, Rv3231c/MT3328, Rv3230c/MT3327, and Rv3229c/MT3326. As shown in Fig. 1A, the two genes upstream of ppk2 (Rv3234c/MT3331 and Rv3233c/MT3330) are cotranscribed with ppk2, but the three downstream genes are expressed independently in a single transcriptional unit.
FIG 1 .
Complementation of ppk2::Tn. (A) RT-PCR analysis of wild-type M. tuberculosis CDC 1551 expression of intergenic regions from MT3331 to desA3/MT3326 during late log phase. Chromosomal DNA was used as a control. Primer sets 1 to 5 (Table 1) target specific intergenic areas, as indicated in the figure; primer set 6 targets ppk2. (B) PCR analysis of genomic DNA from the wild-type, ppk2::Tn, and ppk2::Tn Comp strains. Primer set A targets the ppk2 gene (bp −50 to 430), yielding a 480-bp product in the wild type, a 2,547-bp product in the ppk2::Tn mutant, and both products in the ppk2::Tn Comp strain. Primer set B targets bp 108 to 661 of ppk2, yielding a 553-bp product in all strains. (C) Southern hybridization demonstrating binding of the ppk2-specific probe to KpnI-digested fragments of the expected size in the wild type (1,669 bp), ppk2::Tn mutant (2,266 bp), and ppk2::Tn Comp strain (2,266 and 6,095 bp). (D) RT-PCR analysis of ppk2 expression in the wild-type, ppk2::Tn, and ppk2::Tn Comp strains during early stationary phase of growth.
A DNA fragment containing the Rv3234c/MT3331, Rv3233c/MT3330, and ppk2 genes, as well as 269 bp of the 5′ flanking sequence of MT3331 was cloned into the pMH94H plasmid and then recombined into ppk2::Tn to generate a ppk2 complemented strain (ppk2::Tn Comp). PCR amplification with primers targeting ppk2 (Table 1) yielded products of 487 bp and 2,554 bp in the wild-type and ppk2::Tn strains, respectively, and both products were present, as expected, in the ppk2::Tn Comp strain (Fig. 1B). Southern hybridization, using a specific probe targeting ppk2 (Table 1) after restriction digestion of genomic DNA with KpnI confirmed the generation of a 1,669-bp fragment in the wild-type strain and a 2,266-bp fragment in the ppk2::Tn strain (Fig. 1C). The two expected products (2,266 bp and 6,095 bp) were observed in the ppk2::Tn Comp strain. As confirmation of functional ppk2 complementation in the ppk2::Tn Comp strain, partial restoration of ppk2 expression was observed during early stationary phase (Fig. 1D). RT-PCR demonstrated that expression of the two upstream genes (Rv3234c/MT3331 and Rv3233c/MT3330) and two downstream genes (Rv3231c/MT3328 and Rv3230c/MT3327) was not disrupted in the mutant or complemented strains (see Fig. S1 in the supplemental material). The ppk2::Tn mutant exhibited wild-type growth during the logarithmic phase in supplemented Middlebrook 7H9 broth but achieved a lower density relative to the wild type during stationary phase. The in vitro growth of the ppk2::Tn Comp strain was similar to that of the mutant (Fig. S2).
TABLE 1 .
Primers used in this study
| Primer | Directiona | Sequence |
|---|---|---|
| Primers used for coexpression studiesb | ||
| Primer set 1 | F | GTAACGAATTCCGGCCAACT |
| R | TACATCTGCGACTGGGTTCC | |
| Primer set 2 | F | CCAAGCTGCTGGAGACCTAC |
| R | CGGGAAGATGGGCAATATAA | |
| Primer set 3 | F | GTGGAAAAGCCAAAGGTCAA |
| R | ACCTGATCGATCGTGATTGG | |
| Primer set 4 | F | ACCAAGAGCTGCCATTTCTG |
| R | CACAATGGTCCCTGGCTTTA | |
| Primer set 5 | F | CGAAGTGGCAAGAGCGTAG |
| R | CCGATCTCCATGTTTTCGAT | |
| Primer set 6 (ppk2) | F | GTGGATATACCATCCGTTGATGTG |
| R | GCGTACAGAAACCCATGACC | |
| sigA primer set | F | ACCTCAAACAGATCGGCAAG |
| R | TGGATTTCCAGCACCTTCTC | |
| Primers used for complemented strain generation/confirmationc | ||
| 1st cloning | F | GCGGAGCTC GTACTCGACGACGGGTGTG |
| R | GCGTCTAGACAACGAGTCGCTGCAACA | |
| 2nd cloning | F | CGTCGCTCATGAGATGGTGCCCTTGGTGG |
| R | GCATCGAACGTTATGTTAAAACGACGGCCAGTGAAT | |
| Primer set A | F | GGTACTCGGTGATCCGTTTG |
| R | AAGCACTGGACGAGCTTCTG | |
| Primer set B | Same as primer set 6 | |
| ppk2 primer set (probe in Southern blot) | F | CGTCTACCAAGCCGAATTGT |
| R | GCACCATCATCTCGTCCTTT | |
| Gene-specific primers used for qRT-PCR studies | ||
| Rv0496 | F | AGAGGACCCTAACGGCAAAT |
| R | TTTCCACCGCTTCTATCGAC | |
| Rv1026 | F | AACTCGATTCGCTTGCTGAT |
| R | TGGTGAAACGTCAGCAGTTC | |
| ppk1 | F | CTCAAGACGCACTGCAAGAC |
| R | TGAACAAGTCGGTCAAGTCG | |
| sigA | F | TCGAGGTGATCAACAAGCTG |
| R | TGGATTTCCAGCACCTTCTC |
ppk2 is required for control of intrabacillary poly P levels and susceptibility to isoniazid.
Previous studies have shown that PPK2 hydrolyzes inorganic polyphosphate (poly P) to generate GTP from GDP, contributing to a decreased ATP/GTP ratio in Mycobacterium smegmatis (7). Consistent with a role for PPK2 in poly P hydrolysis during exponential growth of M. tuberculosis, we found that the ppk2::Tn mutant exhibited significantly higher poly P content relative to the isogenic wild-type (P = 0.0038) and ppk2::Tn Comp (P = 0.0028) strains (Fig. 2A). Quantitative RT-PCR (qRT-PCR) revealed that ppk1 expression was significantly reduced (P < 0.001) and expression of the putative exopolyphosphatase Rv1026 was increased (P = 0.004) in the ppk2::Tn mutant relative to the wild type (Fig. 2B), consistent with negative- and positive-feedback mechanisms, respectively. However, expression of the exopolyphosphatase gene Rv0496 was slightly decreased in the ppk2::Tn mutant than in the wild type (P = 0.028). Consistent with the hypothesis that poly P accumulation is associated with M. tuberculosis tolerance to the cell wall-active agent isoniazid (15), the MIC of isoniazid increased from 0.06 µg/ml for the wild-type and ppk2::Tn Comp strains to 0.24 µg/ml for the ppk2::Tn mutant strain. As in the case of M. smegmatis (7), M. tuberculosis ppk2 deficiency was associated with a higher ATP/GTP ratio relative to the wild-type (P = 0.07) and ppk2::Tn Comp (P = 0.02; Fig. 2C) strains. Each experiment was performed using three biological replicates.
FIG 2 .
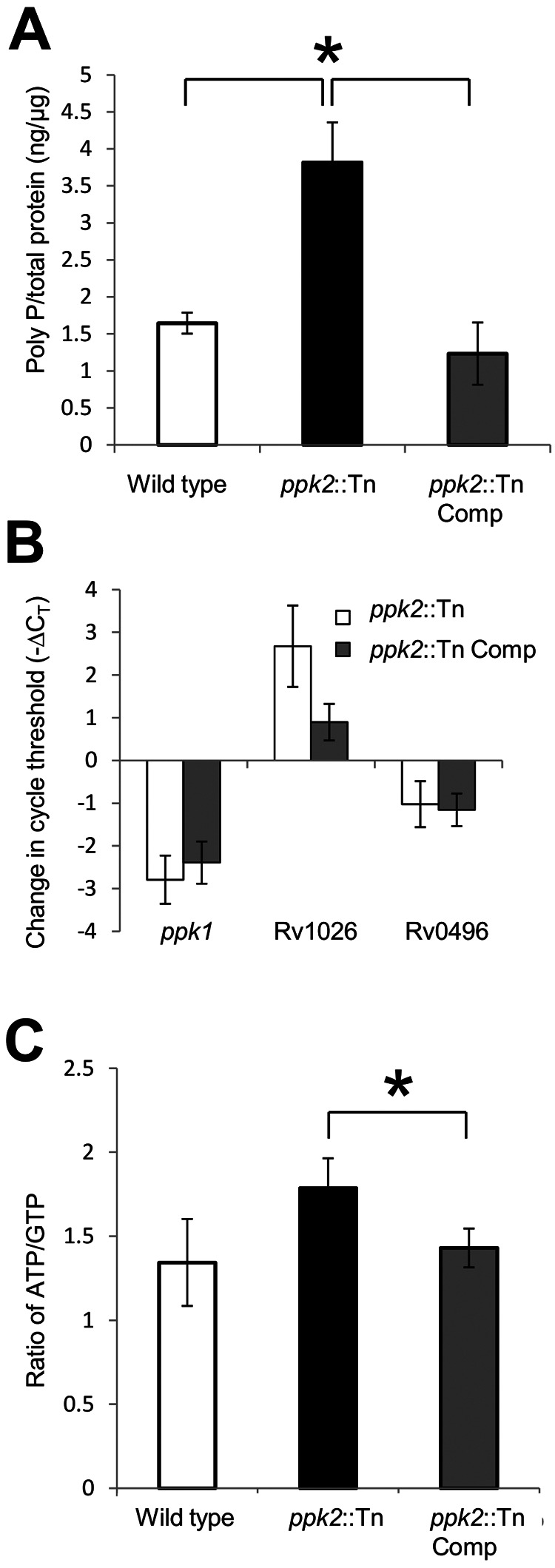
ppk2 is required for M. tuberculosis control of intracellular poly P levels and ATP/GTP ratio. (A) Intrabacillary poly P levels were measured in the wild-type, ppk2::Tn, and ppk2::Tn Comp strains during mid-log phase using a DAPI-based method and normalized to total protein content of extract lysate. Values that were significantly different (P < 0.05) are indicated by a bar and asterisk. Values are means ± standard deviations (error bars) for three biological replicate samples. (B) qRT-PCR analysis of gene expression of ppk2::Tn mutant and ppk2::Tn Comp strain compared to that of the wild type during late log phase of growth in supplemented Middlebrook 7H9 broth, expressed as a change in the normalized cycle threshold (−∆CT). (C) Intrabacillary ATP/GTP ratio of the wild-type, ppk2::Tn, and ppk2::Tn Comp strains during late log phase, as measured by HPLC. The values for ppk2::Tn and ppk2::Tn Comp strains were significantly different (P < 0.05) as indicated by the bar and asterisk. The values for the ppk2::Tn mutant and the wild-type strain were not significantly different (P = 0.07). Three biological replicate samples were used for each strain in each experiment.
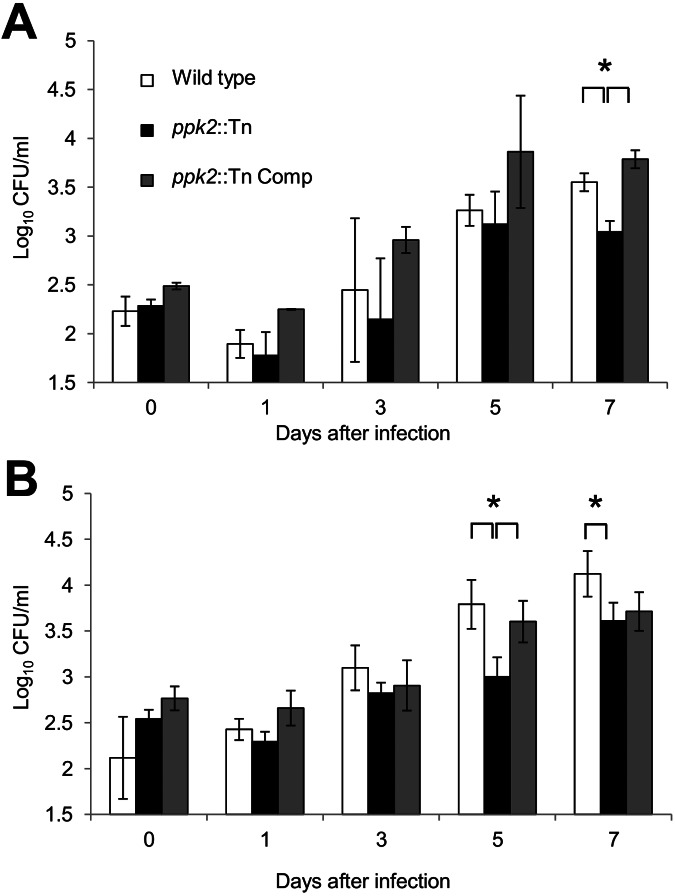
ppk2 is required for M. tuberculosis survival during infection of naive macrophages and suppression of proinflammatory cytokine release.
On the basis of previous reports, ppk2 appears to play an important role in M. tuberculosis intracellular survival (7). To further investigate the mechanism of defective growth of the ppk2::Tn mutant, we infected naive and activated murine J774 macrophages with each of the three strains. The numbers of intracellular CFU were lower in gamma interferon (IFN-γ)-activated macrophages 7 days after infection with the ppk2::Tn mutant relative to the corresponding values for the wild-type (P = 0.004) and ppk2::Tn Comp strains (Fig. 3A). On day 1 after infection, activated macrophages infected with the ppk2::Tn mutant produced significantly higher levels of granulocyte colony-stimulating factor (G-CSF) relative to macrophages infected with the wild-type strain (data not shown; P = 0.03). The levels of all other cytokines and chemokines elaborated by activated macrophages on day 1 or day 3 were either below the limit of detection of the assay or showed no differences between groups. The ppk2::Tn mutant showed defective growth relative to the wild-type and ppk2::Tn Comp strains on day 5 (P = 0.016) and day 7 (P = 0.049) after infection of naive macrophages (Fig. 3B). At day 1 after infection of naive macrophages, cytokine and chemokine levels were either below the limit of detection of the assay or showed no differences between groups. However, on day 3 after infection, the levels of interleukin 2 (IL-2) (P = 0.044), IL-9 (P = 0.04), IL-10 (P = 0.012), IL-12(p70) (P < 0.001), IL-13 (P = 0.046), eotaxin (P = 0.005), IFN-γ (P = 0.035), monocyte chemotactic peptide 1 (MCP-1) (P = 0.02), macrophage inflammatory protein 1b (MIP-1b) (P = 0.011), and RANTES (regulated upon activation, normal T cell expressed and secreted) (P = 0.004) were more highly induced by the ppk2::Tn mutant than by the wild-type and ppk2::Tn Comp strains, despite similar intracellular CFU values (Fig. 4). Each macrophage CFU and cytokine experiment was performed using three biological replicates.
FIG 3 .
ppk2 deficiency impairs M. tuberculosis growth and survival in J774 macrophages. Growth and survival of the wild type, ppk2::Tn mutant, and ppk2::Tn Comp strain after infection of activated (A) and naive (B) J774 murine macrophages. Values that were significantly different (P < 0.05) are indicated by a bar and asterisk. Three biological replicate samples were used for each strain in each experiment.
FIG 4 .
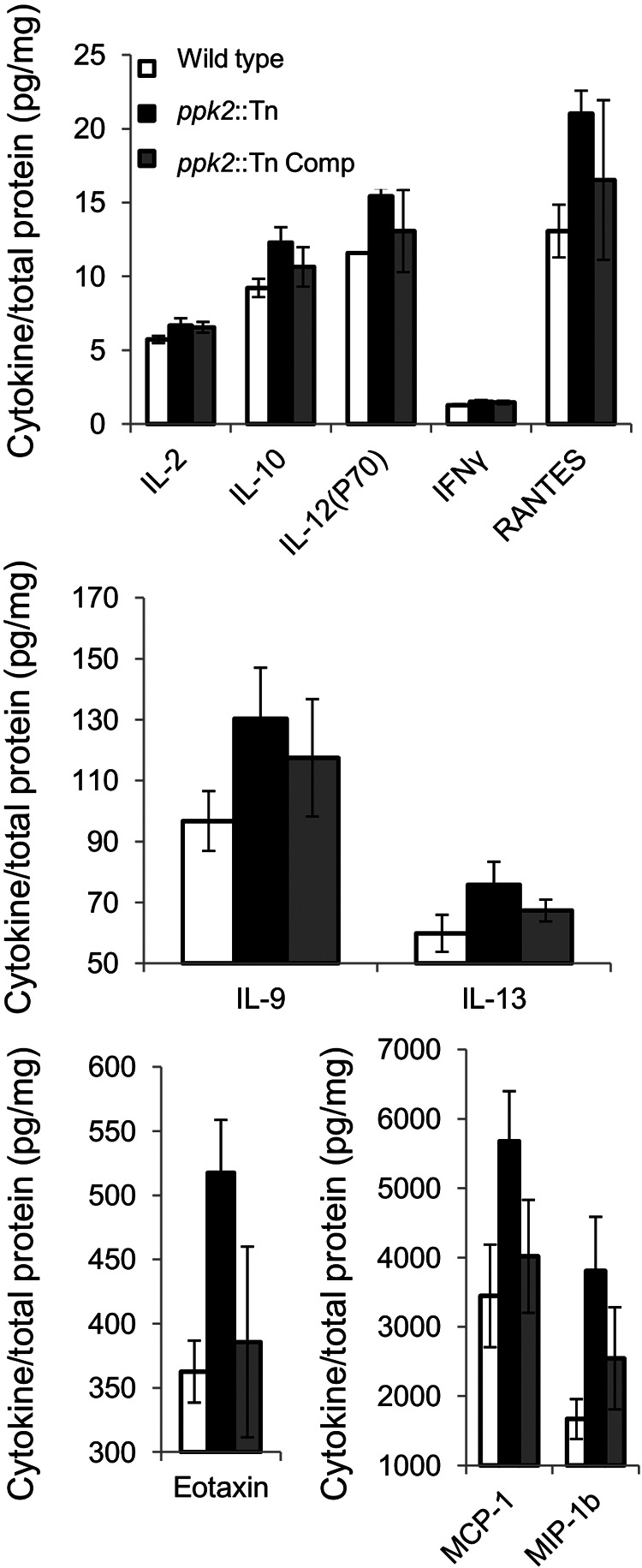
M. tuberculosis ppk2 deficiency alters cytokine and chemokine release by naive macrophages by immunobead cytokine assays. Cytokines and chemokines released by naive macrophages 72 h after infection with the wild-type, ppk2::Tn, and ppk2::Tn Comp strains. Cytokine levels were normalized to total protein content of the culture supernatant. The values for all cytokines were significantly different (P < 0.05) comparing the value for the ppk2::Tn mutant with the value of the wild type. Three biological replicate samples were used for each strain in each experiment.
ppk2 enhanced M. tuberculosis growth and survival in mouse lungs.
In order to test the role of ppk2 in M. tuberculosis virulence in the mammalian host, separate groups of BALB/c mice were aerosol infected with the wild type (1.82 ± 0.1), ppk2::Tn mutant (2.24 ± 0.06), and ppk2::Tn Comp strain (1.9 ± 0.1) (all values log10 number of CFU/lung). During the first 14 days after infection, wild-type and ppk2::Tn Comp strains showed typical exponential growth, increasing by 4.34 log10 units and 4.15 log10 units, respectively. On the other hand, ppk2::Tn showed a severe growth defect during the acute phase of the infection relative to wild type, increasing by only 2.29 log10 units (P < 0.001) (Fig. 5A). After the onset of adaptive immunity (day 28), the wild-type bacillary burden declined to 5.85 ± 0.15 1og10 units, after which a relatively stable lung census was maintained. Interestingly, the lung bacillary burden of the ppk2::Tn mutant continued to increase until day 56, when it was equivalent to that of animals infected with the wild type but then declined during the chronic phase of infection, such that by day 119, it was 0.63 log10 unit lower than that of wild type (P = 0.098). The ppk2::Tn mutant also showed defective growth in mouse spleens, as the numbers of CFU in ppk2::Tn-infected mouse spleens were significantly lower than the numbers in spleens in mice infected with the wild-type and ppk2::Tn Comp strains at days 28, 84, and 119 (P < 0.001 for all time points) (Fig. 5B).
FIG 5 .
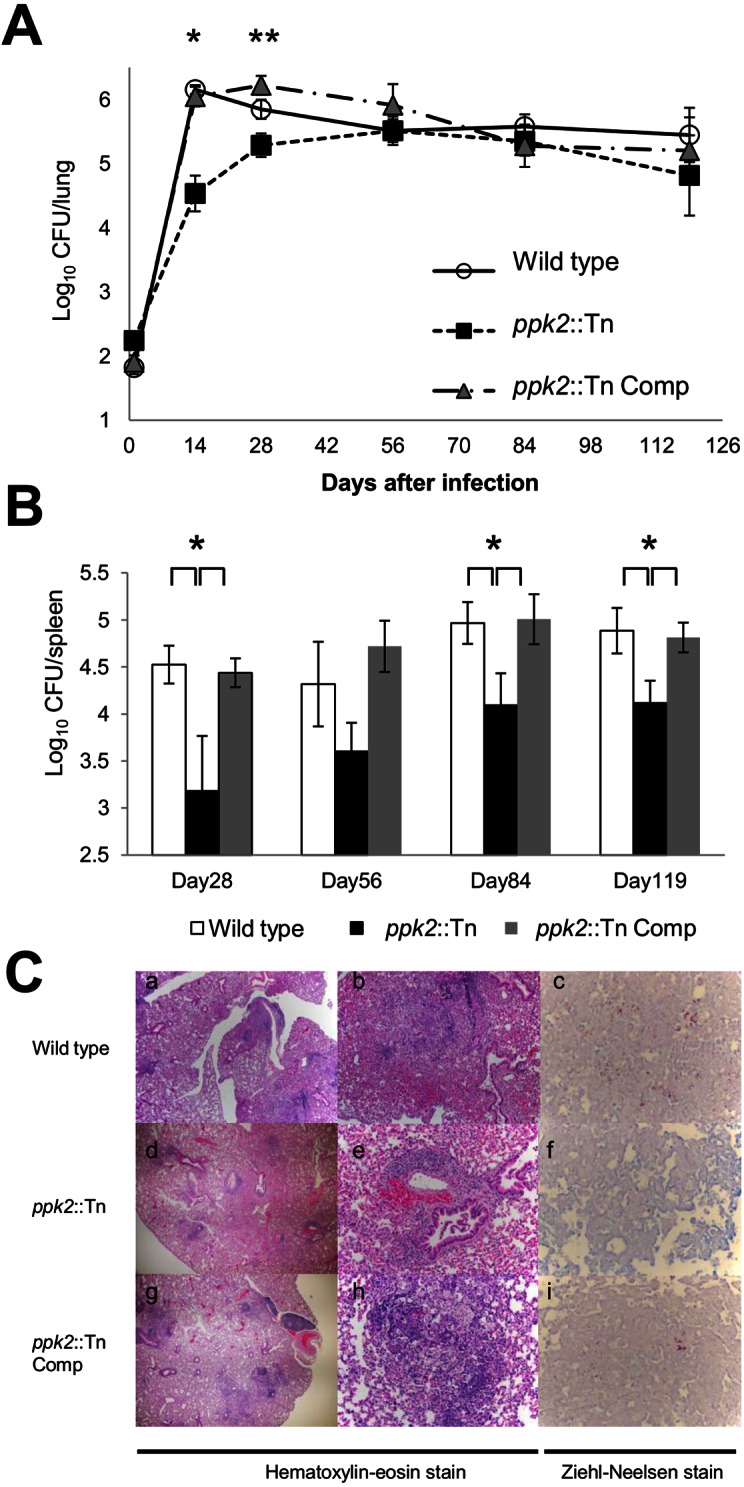
ppk2 is required for M. tuberculosis virulence in the murine model. (A) Separate groups of mice were aerosol infected with the wild-type, ppk2::Tn, and ppk2::Tn Comp strains, and the number of CFU in the lungs of mice were determined at various time points postinfection. Values that were significantly different are indicated as follows: *, P < 0.001 comparing the value for the ppk2::Tn mutant with the value for the wild type or ppk2::Tn Comp strain; **, P < 0.001 comparing the value for the ppk2::Tn mutant with the value for the wild type or ppk2::Tn Comp strain. The values for the ppk2::Tn Comp strain and the wild type were significantly different (P = 0.004). Five biological replicate samples were used for each strain in each experiment. (B) Spleen CFU of the wild-type, ppk2::Tn, and ppk2::Tn Comp strains at various time points postinfection. Values that were significantly different (P < 0.05) are indicated by a bar and asterisk. Five biological replicate samples were used for each strain in each experiment. (C) Histological analysis of mouse lungs infected with wild-type, ppk2::Tn, and ppk2::Tn Comp strains at day 28 postinfection. Magnifications, ×40 (a, d, and g), ×200 (b, e, and h), and ×400 (c, f, and i).
Mouse body weights were not significantly different between groups at any time point (see Fig. S3A in the supplemental material). However, by day 28 after infection, the mean normalized lung (see Fig. S3B in the supplemental material) and spleen (see Fig. S3C) weights of ppk2::Tn-infected mice were significantly lower than the corresponding values of mice infected with the wild type (P < 0.001 and P = 0.023, respectively). Gross pathology revealed no major differences in the number or size of lung tubercle lesions between groups at any time point (Fig. S4).
After 28 days of infection, histological evaluation revealed moderate inflammation with a predominance of macrophages and bronchiolar obliteration in the lungs of mice infected with the wild-type and ppk2::Tn Comp strains (Fig. 5C). In lung samples from mice infected with the ppk2::Tn mutant, mild inflammation predominantly comprising lymphocytes was noted. Lymphocytic involvement was observed in 15/41 (36.6%) bronchioles in mice infected with the wild type, 8/50 (16%) in mice infected with the ppk2::Tn mutant, and 12/21 (57.1%) in mice infected with the ppk2::Tn Comp strain. Acid-fast staining revealed more bacilli in lungs in mice infected with the wild-type and ppk2::Tn Comp strains than in mice infected with the ppk2::Tn mutant. After 56 days of infection, the lungs of each group exhibited similar degrees of bronchiolitis, bronchiolar obliteration, intra-alveolar inflammation, and perivascular lymphocytic inflammation, with equal numbers of histiocytes and lymphocytes. Each data point from mouse experiments represents data obtained from 5 animals.
DISCUSSION
M. tuberculosis is a highly adapted pathogen, with the ability to evade host immunity and persist for prolonged periods within infected tissues. The regulatory molecule poly P has been implicated in the bacterial stringent response (16). The presence of poly P in M. tuberculosis was reported several decades ago (17), and recent studies have used scanning transmission electron microscopy together with energy-dispersive X-ray spectroscopy to visualize poly P granules in individual M. tuberculosis bacilli during nutrient starvation (18). In addition to its role in inducing the stringent response (11, 19), poly P levels have been shown to influence susceptibility of M. tuberculosis to certain TB drugs and M. tuberculosis virulence in guinea pigs (20). The M. tuberculosis polyphosphate kinase PPK2 catalyzes the synthesis of GTP from GDP using poly P as a phosphate donor (7). In this study, we confirm the role of ppk2 in M. tuberculosis poly P hydrolysis in vivo by demonstrating that ppk2 deficiency leads to increased intrabacillary poly P content. We also found that poly P accumulation was accompanied by tolerance to the bactericidal drug isoniazid, which targets actively multiplying organisms by inhibiting the mycolic acid synthesis pathway (21). These phenotypes are consistent with those observed for another poly P-accumulating M. tuberculosis strain deficient in the exopolyphosphatase Rv0496/PPX1 (exopolyphosphatase 1) (15). In addition, we demonstrate that ppk2 is an important virulence gene required for exponential growth of M. tuberculosis during the acute phase of infection in mouse lungs and for bacillary persistence during the chronic phase of infection.
Previous work has shown that PPK2 plays an important role in maintaining intracellular GTP levels in M. smegmatis by directly synthesizing GTP from GDP and poly P and by interacting with the nucleoside diphosphate kinase Ndk, thereby promoting production of GTP over CTP or UTP (7). Our study corroborates the importance of PPK2 in nucleotide pool maintenance in M. tuberculosis, as we found that the ATP/GTP ratio was increased in the ppk2-deficient mutant. The significance of such changes in the ratios of nucleotides and their relationship to growth arrest and antibiotic tolerance remain to be determined. Similar to our findings, ppk2 deficiency in Campylobacter jejuni is associated with significant survival defects under osmotic, nutrient, aerobic, and antimicrobial stresses and within human intestinal epithelial cells (9).
Although the ppk2::Tn mutant was implanted at a higher dose in mouse lungs relative to the control strains, the number of lung CFU in mutant-infected animals was ~1.5 log10 units lower than those infected with the control strains after 14 days of infection. Our macrophage data shed some light on the impaired growth of the ppk2::Tn mutant during the acute phase of infection in mouse lungs. Although the number of intracellular CFU of the ppk2::Tn mutant was similar or even slightly lower than the numbers of intracellular CFU of the control strains following a multiplicity of infection (MOI) of 1:1, naive macrophages infected with the mutant strain showed significantly greater induction of the proinflammatory cytokines IL-12(p70) and IFN-γ, which serve to control M. tuberculosis infection in the lungs (22, 23). Furthermore, the ppk2::Tn mutant induced significantly greater expression of the chemotactic factors MIP-1b, MCP-1, eotaxin, and RANTES by naive macrophages (24, 25). Finally, M. tuberculosis deficiency of ppk2 was also associated with increased macrophage expression of IL-2, which can control inflammation by interfering with IL-6-dependent Th17 differentiation, and IL-10, which limits immune system-mediated damage to the host following infection (26). The net effect of these changes on macrophage function may have contributed to improved control of the ppk2::Tn mutant in mouse lungs, as well as more limited lung histopathology in mutant-infected animals 28 days after infection, as manifested by milder, predominantly lymphocytic infiltration with relative preservation of bronchiolar architecture. Interestingly, the ppk2::Tn mutant continued to grow in mouse lungs to wild-type levels by day 56 after infection. Whether continued growth after the onset of adaptive immunity is due to compensatory metabolic changes in the mutant (e.g., poly P hydrolysis through increased expression of exopolyphosphatase [PPX]) and/or defective host immunity remains to be determined.
We observed incomplete restoration of all wild-type phenotypes following complementation of ppk2. Despite our efforts to preserve the native promoter of ppk2 in the ppk2::Tn Comp strain, it is possible that regulation of the gene may be altered at the recombination site, which is supported by the RT-PCR data showing mildly decreased expression of ppk2 in the ppk2::Tn Comp strain relative to the wild type during early stationary phase (Fig. 1D). Another difference between the wild-type and complemented strains is the presence of two copies of the upstream genes Rv3233c/MT3330 and Rv3234c/MT3331 in the latter. However, the expression levels of these genes did not differ significantly between these two strains by RT-PCR (see Fig. S1 in the supplemental material). Incomplete complementation may be attributed to polar effects of the transposon insertion in ppk2; however, expression of the two upstream and two downstream genes was not disrupted, as revealed by RT-PCR (Fig. S1).
In summary, our data show that ppk2 (Rv3232c/MT3329) is required to maintain M. tuberculosis homeostasis of poly P and nucleotide pools. In particular, dysregulation of poly P balance in the ppk2::Tn mutant may have contributed to phenotypic tolerance to isoniazid. Finally, we show for the first time that PPK2 plays an important role in M. tuberculosis-host interactions, perhaps by suppressing macrophage production of proinflammatory cytokines and chemokines, thereby promoting bacillary growth during the acute phase of infection in mouse lungs.
MATERIALS AND METHODS
Bacterial strains.
An M. tuberculosis strain deficient in ppk2/Rv3232c/MT3329 (ppk2::Tn) was generated by transposon mutagenesis of the wild-type M. tuberculosis strain CDC 1551 (14). The point of insertion of the himar1 transposon, which contains a kanamycin resistance cassette, was determined to be at nucleotide position 8 in the ppk2 gene (27). A DNA fragment containing MT3329 and the coregulated genes MT3330 and MT3331, along with 269 bp of upstream and downstream flanking sequences, was cloned into the mycobacterial integrating vector pMH94H using the BspHI and AclI sites to yield a single-copy plasmid conferring hygromycin resistance (28, 29). The complementation plasmid was introduced into the ppk2::Tn mutant by electroporation, and transformants were selected on hygromycin-containing 7H10 plates. The complemented strain (ppk2::Tn Comp) was confirmed by PCR and Southern blotting. Briefly, genomic DNA from the wild-type, ppk2::Tn, and ppk2::Tn Comp strains was isolated, digested with KpnI (NEB), separated by 1% agarose gel electrophoresis, and blotted onto positive-charge nylon membranes by standard capillary transfer. The DNA templates were hybridized with PCR-generated, digoxigenin-11-dUTP-labeled gene probes recognizing the region of ppk2 from 108 to 601 bp. After cross-linking, probe binding was detected with an antidigoxigenin alkaline phosphatase conjugate antibody and developed with disodium 3-(4-methoxyspiro {1,2-dioixetane-3,2′-(5′-chloro)tricycle[3.3.1.13,7]decan}-4-yl) phenyl phosphate (CSPD) substrate (Roche Diagnostics).
Determination of intrabacillary inorganic polyphosphate and nucleotide content.
A 4′,6-diamidino-2-phenylindole (DAPI)-based method was used to determine intracellular polyphosphate (poly P) content in each strain (15, 30). Briefly, the bacteria were lysed by bead beating in 5 M guanidinium thiocyanate (GITC; Sigma)—50 mM Tris-HCl (pH 7.0) buffer. The total protein levels of the lysates were determined by colorimetric protein assay (Bio-Rad) and used to normalize poly P content. Poly P was harvested by glass milk from Geneclean III kit (MP Biomedicals LLC) and then treated with DNase (Ambion) and RNase (NEB) before elution with distilled water (95°C, pH 8.0). Each elution was further diluted 1:10 in distilled water and mixed with DAPI (Roche Diagnostics) at a final concentration of 100 µg/ml. The poly P concentration was determined by fluorescence of the DAPI-poly P complex following excitation at 415 nm and emission at 525 nm by FLUOstar OPTIMA (BMG Labtech). The fluorescence intensity of DAPI-containing solution was used as a background signal for blanks. Increasing concentrations of poly P (type 65; Sigma-Aldrich) ranging from 2.5 µg/ml to 312.5 ng/ml were used to generate a standard curve. For nucleotide measurements, bacilli were pelleted by centrifugation at 8,000 rpm at 4°C for 10 min. The pellets were resuspended in ice-cold buffer (acetonitrile-methanol-water [40:40:20]) and incubated on ice for 15 min. The cell suspension was heated at 95°C for 10 min and cooled on ice for 5 min. The cell debris was removed by centrifugation, and the supernatant was concentrated by evaporation and reconstituted in distilled water. ATP and GTP (Sigma) were used to generate the reference peak. Cell lysate (50 µl) was injected into a reverse-phase high-performance liquid chromatograph (HPLC) (Waters 2690) fitted with a Sunfire C18 column and UV detector (Waters 2487) to study ATP and GTP contents at the same time. The resulting peaks were analyzed with Waters Empower software, and the area of ATP and GTP peaks was used to determine the ratio for further comparison.
MIC determination.
Logarithmically growing wild-type, ppk2::Tn, and Comp cultures (5 × 104/ml) were inoculated in 15-ml conical tubes containing 2 ml of Middlebrook 7H9 broth without Tween 80 and with increasing concentrations of isoniazid, as follows: 0, 0.03, 0.06, 0.12, 0.24, 0.48, and 0.96 µg/ml. Bacterial growth was determined by the presence of visible pellets after 10 days of standing culture at 37°C. The MIC was recorded as the lowest concentration of antibiotic for which there was no visible pellet.
Quantitative polymerase chain reaction and RT-PCR.
Total RNA was extracted from 50-ml M. tuberculosis cultures and treated with DNase, and cDNA was generated using Superscript III (Invitrogen) (31). cDNA corresponding to each transcript was subjected to 40 cycles of PCR for quantification using gene-specific primers (Table 1) and an iCycler 5.0 (Bio-Rad). The cycle threshold (CT) value obtained for each gene was normalized with that of the housekeeping gene sigA (32), and the normalized change in CT (ΔCT) was calculated (15). Reverse transcription-PCR (RT-PCR) was used to determine genes coexpressed with ppk2/Rv3232c/MT3329 using the intergenic primers listed in Table 1. RT-PCR was also used to determine the expression of genes upstream and downstream of the ppk2 transposon insertion in the mutant and complemented strains (see Fig. S1 in the supplemental material). Primers used for the expression of upstream and downstream genes include the following: for Rv3234c/MT3331, forward (F) primer CAATGTATGTCGGGTTGCTG and reverse (R) primer TGCAGTTGCTCGTCACTACC; for Rv3233c/MT3330, F primer CTGGTCGATGCCAGGACTAT and R primer AGGTCTCCAGCAGCTTGGTA; for Rv3231c/MT3328, F primer TCACGATCGATCAGGTTGTC and R primer CAGAAATGGCAGCTCTTGGT; for Rv3230c/MT3327, F primer GCTTCCTGTCCACCCACTT and R primer CGTTCGAAGCATCGACATTA; and for sigA, F primer TCGAGGTGATCAACAAGCTG and R primer TGGATTTCCAGCACCTTCTC.
Murine infections and virulence endpoints.
BALB/c mice (4 to 6 weeks old, female; Charles River Laboratories, Wilmington, MA) were housed in a biosafety level 3, specific-pathogen-free facility and fed water and chow ad libitum. All procedures followed protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins University. Separate groups of mice were infected with wild-type, ppk2::Tn, or ppk2::Tn Comp strains (~100 bacilli implanted per animal lung) via the aerosol route using a Glas-Col inhalation exposure system. Five mice in each group were sacrificed at days 1, 14, 28, 56, 84, and 119. Organs were homogenized and plated for CFU determination, and randomly selected sections were fixed with formalin for histological analysis, which was performed by a pulmonary pathologist blind or unaware of the specimen identity (D. A. Belchis). Lung and spleen samples harvested from ppk2::Tn-infected mice at the last time point were plated on Middlebrook 7H11 agar plates with or without kanamycin. No difference in CFU was observed, indicating stability of the transposon insertion throughout the entire experiment.
Macrophage infections and cytokine assays.
The mouse macrophage-like cell line J774.1 was maintained and infected with each M. tuberculosis strain (33). Macrophages were activated by treatment with mouse gamma interferon (IFN-γ) (Invitrogen) overnight and lipopolysaccharide from Escherichia coli O26:B6 (Sigma) for 3 h before infection. Naive macrophages were divided 1 day before infection without any treatment. At day 0, 104 macrophages were infected with an equal number of logarithmically growing bacilli of the wild-type, ppk2::Tn, and ppk2::Tn Comp strains. Intracellular M. tuberculosis was recovered and plated on days 0, 1, 3, 5, and 7. At days 1 and 3, the supernatant of each culture was collected and frozen at −80°C until analysis. Macrophage-secreted cytokines were analyzed by immunobead cytokine assays (mouse cytokine 23-plex assay; Bio-Rad).
Statistical analysis.
Data from at least three biological replicates were used to calculate the means and standard deviations (SDs) for graphing purposes. Statistical analysis employed the unpaired Student’s t test, and a P value of <0.05 was considered significant.
SUPPLEMENTAL MATERIAL
Gene expression analysis of the wild type, ppk2::Tn mutant, and ppk2::Tn Comp strain by RT-PCR during late log phase of growth in supplemented Middlebrook 7H9 broth. Download
Growth curves of wild-type, ppk2::Tn, and ppk2::Tn Comp strains during axenic growth in supplemented Middlebrook 7H9 broth. Values that were significantly different (P < 0.005) for the wild type and the ppk2::Tn mutant or for the wild type and the ppk2::Tn Comp strain are indicated by an asterisk. Download
Body (A), lung (B), and spleen (C) weights of mice infected with wild-type, ppk2::Tn, and ppk2::Tn Comp strains at various time points postinfection.Values that were significantly different (P < 0.05) are indicated by a bar and asterisk. Download
Gross lung pathology of mice infected with the wild-type, ppk2::Tn, and ppk2::Tn Comp strains after 28, 56, 84, and 119 days of infection. Download
ACKNOWLEDGMENTS
This work was supported by NIH grants AI083125 and HL106786.
Footnotes
Citation Chuang YM, Belchis DA, Karakousis PC. 2013. The polyphosphate kinase gene ppk2 is required for Mycobacterium tuberculosis inorganic polyphosphate regulation and virulence. mBio 4(3):e00039-13. doi:10.1128/mBio.00039-13.
REFERENCES
- 1. Lawn SD, Zumla AI. 2011. Tuberculosis. Lancet 378:57–72 [DOI] [PubMed] [Google Scholar]
- 2. Fauci AS, NIAID 2008. Multidrug-resistant and extensively drug-resistant tuberculosis: the National Institute of Allergy and Infectious Diseases Research agenda and recommendations for priority research. J. Infect. Dis. 197:1493–1498 [DOI] [PubMed] [Google Scholar]
- 3. Rao NN, Gómez-García MR, Kornberg A. 2009. Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78:605–647 [DOI] [PubMed] [Google Scholar]
- 4. Kulaev I, Kulakovskaya T. 2000. Polyphosphate and phosphate pump. Annu. Rev. Microbiol. 54:709–734 [DOI] [PubMed] [Google Scholar]
- 5. Kornberg A, Rao NN, Ault-Riché D. 1999. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68:89–125 [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Ishige K, Kornberg A. 2002. A polyphosphate kinase (PPK2) widely conserved in bacteria. Proc. Natl. Acad. Sci. U. S. A. 99:16678–16683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sureka K, Sanyal S, Basu J, Kundu M. 2009. Polyphosphate kinase 2: a modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol. Microbiol. 74:1187–1197 [DOI] [PubMed] [Google Scholar]
- 8. Ishige K, Zhang H, Kornberg A. 2002. Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proc. Natl. Acad. Sci. U. S. A. 99:16684–16688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gangaiah D, Liu Z, Arcos J, Kassem II, Sanad Y, Torrelles JB, Rajashekara G. 2010. Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni. PLoS One 5:e12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindner SN, Vidaurre D, Willbold S, Schoberth SM, Wendisch VF. 2007. NCgl2620 encodes a class II polyphosphate kinase in Corynebacterium glutamicum. Appl. Environ. Microbiol. 73:5026–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sureka K, Dey S, Datta P, Singh AK, Dasgupta A, Rodrigue S, Basu J, Kundu M. 2007. Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol. Microbiol. 65:261–276 [DOI] [PubMed] [Google Scholar]
- 12. Jagannathan V, Kaur P, Datta S. 2010. Polyphosphate kinase from M. tuberculosis: an interconnect between the genetic and biochemical role. PLoS One 5:e14336 http://dx.doi.org/10.1371/journal.pone.0014336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shum KT, Lui EL, Wong SC, Yeung P, Sam L, Wang Y, Watt RM, Tanner JA. 2011. Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry 50:3261–3271 [DOI] [PubMed] [Google Scholar]
- 14. Lamichhane G, Zignol M, Blades NJ, Geiman DE, Dougherty A, Grosset J, Broman KW, Bishai WR. 2003. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 100:7213–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thayil SM, Morrison N, Schechter N, Rubin H, Karakousis PC. 2011. The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence. PLoS One 6:e28076 http://dx.doi.org/10.1371/journal.pone.0028076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hengge-Aronis R. 2002. Signal transduction and regulatory mechanisms involved in control of the sigma(s) (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winder FG, Denneny JM. 1957. The metabolism of inorganic polyphosphate in mycobacteria. J. Gen. Microbiol. 17:573–585 [DOI] [PubMed] [Google Scholar]
- 18. Ward SK, Heintz JA, Albrecht RM, Talaat AM. 2012. Single-cell elemental analysis of bacteria: quantitative analysis of polyphosphates in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sureka K, Ghosh B, Dasgupta A, Basu J, Kundu M, Bose I. 2008. Positive feedback and noise activate the stringent response regulator rel in mycobacteria. PLoS One 3:e1771 http://dx.doi.org/10.1371/journal.pone.0001771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh R, Singh M, Arora G, Kumar S, Tiwari P, Kidwai S. 12 April 2013. Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J. Bacteriol. http://dx.doi.org/10.1128/JB.00038-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vilchèze C, Jacobs WR., Jr. 2007. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu. Rev. Microbiol. 61:35–50 [DOI] [PubMed] [Google Scholar]
- 22. Tomioka H, Tatano Y, Sano C, Shimizu T. 2011. Development of new antituberculous drugs based on bacterial virulence factors interfering with host cytokine networks. J. Infect. Chemother. 17:302–317 [DOI] [PubMed] [Google Scholar]
- 23. Sundareshan V, Modi J, Khardori NM. 2011. Mycobacteria and biological response modifiers: two sides of the relationship. Infect. Dis. Clin. North Am. 25:865–893 [DOI] [PubMed] [Google Scholar]
- 24. Lee SJ, Song OR, Lee YC, Choi YL. 2003. Molecular characterization of polyphosphate kinase (ppk) gene from Serratia marcescens. Biotechnol. Lett. 25:191–197 [DOI] [PubMed] [Google Scholar]
- 25. Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. 1994. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 179:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banchereau J, Pascual V, O’Garra A. 2012. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat. Immunol. 13:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamichhane G, Tyagi S, Bishai WR. 2005. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect. Immun. 73:2533–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee MH, Pascopella L, Jacobs WR, Jr, Hatfull GF. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and Bacille Calmette-Guerin. Proc. Natl. Acad. Sci. U. S. A. 88:3111–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen P, Ruiz RE, Li Q, Silver RF, Bishai WR. 2000. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigF. Infect. Immun. 68:5575–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aschar-Sobbi R, Abramov AY, Diao C, Kargacin ME, Kargacin GJ, French RJ, Pavlov E. 2008. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 18:859–866 [DOI] [PubMed] [Google Scholar]
- 31. Karakousis PC, Yoshimatsu T, Lamichhane G, Woolwine SC, Nuermberger EL, Grosset J, Bishai WR. 2004. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J. Exp. Med. 200:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manganelli R, Dubnau E, Tyagi S, Kramer FR, Smith I. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31:715–724 [DOI] [PubMed] [Google Scholar]
- 33. Bisson GP, Mehaffy C, Broeckling C, Prenni J, Rifat D, Lun DS, Burgos M, Weissman D, Karakousis PC, Dobos K. 2012. Upregulation of the phthiocerol dimycocerosate biosynthetic pathway by rifampin-resistant, rpoB mutant Mycobacterium tuberculosis. J. Bacteriol. 194:6441–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene expression analysis of the wild type, ppk2::Tn mutant, and ppk2::Tn Comp strain by RT-PCR during late log phase of growth in supplemented Middlebrook 7H9 broth. Download
Growth curves of wild-type, ppk2::Tn, and ppk2::Tn Comp strains during axenic growth in supplemented Middlebrook 7H9 broth. Values that were significantly different (P < 0.005) for the wild type and the ppk2::Tn mutant or for the wild type and the ppk2::Tn Comp strain are indicated by an asterisk. Download
Body (A), lung (B), and spleen (C) weights of mice infected with wild-type, ppk2::Tn, and ppk2::Tn Comp strains at various time points postinfection.Values that were significantly different (P < 0.05) are indicated by a bar and asterisk. Download
Gross lung pathology of mice infected with the wild-type, ppk2::Tn, and ppk2::Tn Comp strains after 28, 56, 84, and 119 days of infection. Download