ABSTRACT
Listeria monocytogenes infection leads to robust induction of an innate immune signaling pathway referred to as the cytosolic surveillance pathway (CSP), characterized by expression of beta interferon (IFN-β) and coregulated genes. We previously identified the IFN-β stimulatory ligand as secreted cyclic di-AMP. Synthesis of c-di-AMP in L. monocytogenes is catalyzed by the diadenylate cyclase DacA, and multidrug resistance transporters are necessary for secretion. To identify additional bacterial factors involved in L. monocytogenes detection by the CSP, we performed a forward genetic screen for mutants that induced altered levels of IFN-β. One mutant that stimulated elevated levels of IFN-β harbored a transposon insertion in the gene lmo0052. Lmo0052, renamed here PdeA, has homology to a cyclic di-AMP phosphodiesterase, GdpP (formerly YybT), of Bacillus subtilis and is able to degrade c-di-AMP to the linear dinucleotide pApA. Reduction of c-di-AMP levels by conditional depletion of the di-adenylate cyclase DacA or overexpression of PdeA led to marked decreases in growth rates, both in vitro and in macrophages. Additionally, mutants with altered levels of c-di-AMP had different susceptibilities to peptidoglycan-targeting antibiotics, suggesting that the molecule may be involved in regulating cell wall homeostasis. During intracellular infection, increases in c-di-AMP production led to hyperactivation of the CSP. Conditional depletion of dacA also led to increased IFN-β expression and a concomitant increase in host cell pyroptosis, a result of increased bacteriolysis and subsequent bacterial DNA release. These data suggest that c-di-AMP coordinates bacterial growth, cell wall stability, and responses to stress and plays a crucial role in the establishment of bacterial infection.
IMPORTANCE
Listeria monocytogenes is a Gram-positive intracellular pathogen and the causative agent of the food-borne illness listeriosis. Upon infection, L. monocytogenes stimulates expression of IFN-β and coregulated genes dependent upon host detection of a secreted bacterial signaling nucleotide, c-di-AMP. Using a forward genetic screen for mutants that induced high levels of host IFN-β expression, we identified a c-di-AMP phosphodiesterase, PdeA, that degrades c-di-AMP. Here we characterize L. monocytogenes mutants that express enhanced or diminished levels of c-di-AMP. Decreased c-di-AMP levels by conditional depletion of the diadenylate cyclase (DacA) or overexpression of PdeA attenuated bacterial growth and led to bacteriolysis, suggesting that its production is essential for viability and may regulate cell wall metabolism. Mutants lacking PdeA had a distinct transcriptional profile, which may provide insight into additional roles for the molecule. This work demonstrates that c-di-AMP is a critical signaling molecule required for bacterial replication, cell wall stability, and pathogenicity.
Introduction
Listeria monocytogenes is a Gram-positive, facultative intracellular pathogen that is the causative agent of the food-borne illness listeriosis. Both the infection cycle and a murine model of infection have been well characterized, making L. monocytogenes an attractive model organism for studying basic aspects of infection and immunity (1). During infection, L. monocytogenes rapidly escapes from a phagocytic vacuole into the host cytosol, where bacterial replication and spread to neighboring cells occur.
Several innate immune pathways are stimulated upon cytosolic entry of L. monocytogenes (2). One pathway is mediated by inflammasome activation, resulting in pyroptosis, an inflammatory host cell death. Levels of pyroptosis induced by L. monocytogenes are low compared to those induced during infection with other intracellular pathogens and are a result of detection of bacterial DNA released during infrequent bacteriolysis (3). Cytosolic L. monocytogenes also triggers the expression of interferon-beta (IFN-β) and coregulated genes (4) via a pathway termed the cytosolic surveillance pathway (CSP), which is dependent on the recently described cytosolic pattern recognition receptors STING and DDX41 (5–7). However, the role of c-di-AMP and CSP activation in L. monocytogenes pathogenesis is unclear, as STING-deficient mice are as susceptible to infection as wild-type mice (5). L. monocytogenes multidrug resistance transporters (MDRs) of the major facilitator superfamily (MFS) secrete c-di-AMP. (c-di-AMP) during infection, resulting in the induction of IFN-β (8, 9). Given the structural similarity to c-di-GMP, a well-characterized bacterial-specific second messenger nucleotide, we hypothesize that c-di-AMP serves an analogous but distinct signaling role in L. monocytogenes.
In this study, we identified an L. monocytogenes phosphodiesterase, PdeA, that mediates c-di-AMP degradation. By genetically modifying the expression of PdeA and the diadenylate cyclase DacA, we investigated the role of c-di-AMP in L. monocytogenes physiology and pathogenesis. These data support a role for c-di-AMP as a critical signaling molecule affecting a number of fundamental processes in L. monocytogenes, with significant consequences on bacterial growth and the ability to establish infection.
RESULTS
c-di-AMP metabolism in L. monocytogenes.
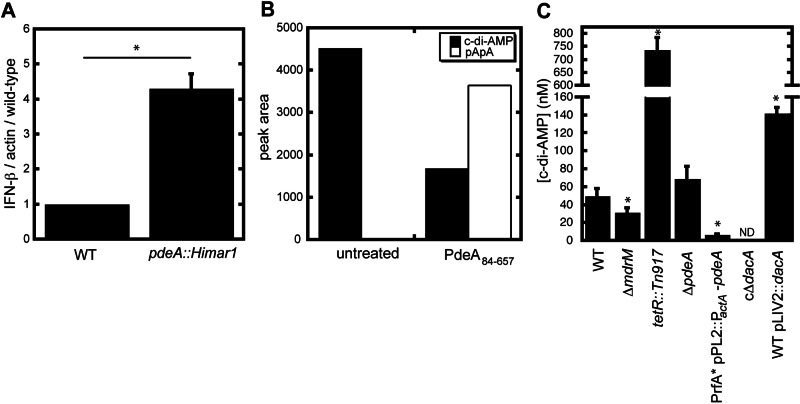
Previous studies from our laboratory identified L. monocytogenes DacA and MDRs as regulators of the CSP-stimulatory molecule c-di-AMP (8, 9). To identify novel determinants of CSP activation and hence c-di-AMP signaling, a forward genetic screen for mutants that affected host cell death and IFN-β production was conducted (3). Infection with a mutant that had a disruption of lmo0052 resulted in elevated levels of IFN-β compared to infection with wild-type L. monocytogenes (Fig. 1A), with no changes in the induction of host cell death (data not shown). Sequence analysis identified Lmo0052 as a homolog of Bacillus subtilis GdpP (previously YybT), which was shown to have c-di-AMP phosphodiesterase activity (10). Lmo0052, renamed here PdeA, shared 50% identity and 74% similarity with B. subtilis GdpP and the same multidomain structure of GdpP, with two N-terminal transmembrane spanning helices, a PAS domain, a GGDEF domain, and C-terminal DHH and DHH-associated domains. Recombinant protein lacking the transmembrane domains (PdeA84-657) catalyzed conversion of c-di-AMP to the linear dinucleotide pApA (Fig. 1B), as assessed by high-performance liquid chromatography (HPLC). Truncated constructs lacking the PAS and GGDEF domains localized this activity to the DHH/DHHA1 domains (data not shown), consistent with GdpP activity (10).
FIG 1 .
PdeA is a c-di-AMP phosphodiesterase. (A) qRT-PCR analysis of IFN-β expression in bone marrow-derived macrophages infected with wild-type L. monocytogenes (WT) or pdeA::Himar1 mutants. (B) HPLC detection of c-di-AMP (black bars) and pApA (white bars) following incubation of c-di-AMP in the absence or presence of recombinant PdeA84-657 protein. HPLC data are representative of more than three independent experiments. (C) Detection of secreted c-di-AMP from L. monocytogenes mutants. Samples from the indicated strains were taken during stationary phase, and c-di-AMP secretion was evaluated by HPLC following chloroacetaldehyde derivatization of the samples. Values are averages of three independent measurements of samples from the indicated strains. Error bars represent standard deviations from the mean. *, P < 0.05 compared to the wild type, using Student’s t test. ND, none detected.
A series of L. monocytogenes strains were constructed to alter c-di-AMP levels. We constructed an in-frame deletion of pdeA and a strain that overexpressed the protein. To achieve overexpression, pdeA was placed downstream of the actA promoter (PactA) in the PrfA* G145S strain (11). Multiple attempts to delete dacA were unsuccessful. Therefore, we generated a conditional depletion (c∆dacA) strain with a single IPTG-inducible copy of the gene (12, 13).
To define the effects of altered expression of DacA, PdeA, and MDRs on c-di-AMP metabolism, secreted c-di-AMP was measured after bacterial growth in chemically defined minimal medium. Ectopic overexpression of the bacterial MDR, MdrT, by transposon disruption of the negative regulator tetR (tetR::Tn917) and overexpression of DacA both resulted in increased levels of c-di-AMP in the culture supernatant as previously reported (8, 9). In contrast, c∆dacA and overexpression of pdeA resulted in decreased levels of c-di-AMP in culture supernatants, indicating that both DacA and PdeA regulate c-di-AMP levels (Fig. 1C). Deletion of pdeA was predicted to result in increased levels of intracellular c-di-AMP, as previously reported for B. subtilis and Staphylococcus aureus mutants lacking GdpP (14, 15). However, PdeA deficiency did not significantly affect secreted levels under these culture conditions (Fig. 1C). This discrepancy suggests that although ΔpdeA mutants likely have high levels of intracellular c-di-AMP, there may be another factor that affects secretion in broth culture.
Deletion of pdeA affects bacterial response to acid stress.
The effects of c-di-GMP on gene expression are well documented (16–18). Therefore, we hypothesized that altered levels of c-di-AMP would have effects on RNA levels as well. To test this, we performed whole-genome microarray analysis comparing ∆pdeA mutants to wild-type bacteria (see Table S1 in the supplemental material). Two of the most highly (3- to 8-fold) upregulated genes in PdeA-deficient mutants were those encoding the glutamate decarboxylase, gadD2 (lmo2362), and a glutamate transporter, gadT2 (lmo2363). Together, GadD2 and GadT2 comprise one of two glutamate decarboxylase systems in L. monocytogenes and are associated with bacterial survival under severe acid stress (19, 20). We tested the effects of PdeA deficiency in acidified culture medium (pH 2.5). After 4 h, nearly 2 logs more L. monocytogenes ΔpdeA mutants than wild-type bacteria were recovered (Fig. 2A), consistent with a role for PdeA in acid resistance and previous reports in B. subtilis and L. lactis (10, 21).
FIG 2 .
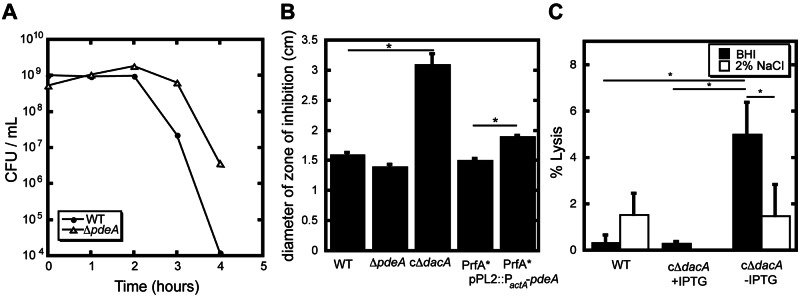
c-di-AMP affects acid resistance and cell wall stability. (A) Acid resistance of wild-type L. monocytogenes and ΔpdeA mutants was assessed by quantifying survival in BHI medium adjusted to pH 2.5. The plot is representative of three independent experiments. (B) Antibiotic susceptibility of indicated mutants as measured as zone of bacterial growth inhibition surrounding filter disks impregnated with cefuroxime (5 µg). Values are averages from more than three independent experiments, and error bars show the standard deviation from those means. (C) In vitro bacteriolysis was measured as the amount of β-galactosidase activity in broth supernatant during logarithmic growth in BHI medium or BHI supplemented with 2% NaCl from the indicated strains. *, P < 0.05, using Student’s t test. NM, not measured.
Deletion of pdeA leads to increased expression of resuscitation-promoting factors.
Microarray transcriptional analysis of ∆pdeA mutants also identified increased expression of lmo2522 (see Table S1 in the supplemental material). Although the increase was not significant by statistical analysis of microarrays (SAM), we also observed upregulation of lmo0186 that was confirmed by quantitative reverse transcription-PCR (qRT-PCR). Lmo2522 and Lmo0186 are homologous to the B. subtilis resuscitation-promoting factors/stationary-phase survival (Rpf/Sps) family members YocH and YabE (22, 23). Rpf, the first Rpf/Sps family member, was initially identified in Micrococcus luteus as an autolysin required for stimulating growth of dormant bacteria (24). We hypothesized that the absence of these two proteins may affect L. monocytogenes replication in broth culture or an in vivo mouse model of infection. However, deletion of lmo2522 and lmo0816, either independently or in combination, did not affect the L. monocytogenes growth rate or the time necessary to exit stationary phase (see Fig. S1A in the supplemental material). Similarly, no effect on the ability of L. monocytogenes to replicate in vivo was observed in the spleens and livers of mice infected with Δlmo2522, Δlmo0186, or Δlmo2522 Δlmo0186 mutants (see Fig. S1B in the supplemental material). Nevertheless, the effects of c-di-AMP levels on Rpf/Sps transcription established a potential link between PdeA, c-di-AMP levels, and bacterial cell wall metabolism and growth control.
Intracellular c-di-AMP levels affect bacterial growth rate.
Growth rates of L. monocytogenes mutants with altered DacA expression were measured. DacA overexpression did not affect in vitro bacterial replication, while conditional dacA depletion resulted in a doubling time of 84 min, compared to 44 min for wild-type L. monocytogenes (Table 1; also, see Fig. S2A in the supplemental material). In the presence of IPTG, the cΔdacA strain grew similarly to wild-type L. monocytogenes. To confirm that synthesis of c-di-AMP was critical for growth and not another feature of the DacA protein, two experiments were performed. First, we characterized growth of strains with altered PdeA expression. Deletion of pdeA did not affect in vitro bacterial replication (Table 1; also, see Fig. S2B). Overexpression of PdeA led to a doubling time of 56 min, compared to 41 min for the parent strain (Table 1; also, see Fig. S2C). Second, growth was characterized in a ∆dacA mutant containing an inducible copy of B. subtilis disA (cΔdacA pLIV2:disA) (see Fig. S1C), of which L. monocytogenes does not have a homologue. In addition to the DAC domain, B. subtilis DisA contains a DNA-binding domain that leads to chromosomal localization, in contrast to the membrane-anchored DacA. This orthogonal approach to generating c-di-AMP in L. monocytogenes rescued the growth defect of the dacA conditional depletion (Table 1; also, see Fig. S2D). To address the possibility that extracellular c-di-AMP regulates the growth of L. monocytogenes, we supplemented cΔdacA mutant cultures with 0 to 10 µM c-di-AMP, far exceeding the secreted levels observed in broth culture. No change in growth rate was observed, suggesting that intracellular c-di-AMP levels synthesized by DacA regulate bacterial replication (data not shown).
TABLE 1 .
c-di-AMP levels affect bacterial in vitro doubling time
| L. monocytogenes genotype or description | Doubling time (min)a |
|---|---|
| WT | |
| ΔpdeA | 44 |
| cΔdacA +IPTG | 44 |
| cΔdacA +IPTG | 84 |
| cΔdacA pLIV::disA +IPTG | 48 |
| cΔdacA pLIV::disA −IPTG | 113 |
| PrfA* G145S | 41 |
| PrfA* G145SpPL2::PactA-pdeA | 56 |
Bacterial doubling times were calculated during logarithmic growth of each strain. Values are representative of at least two independent experiments.
c-di-AMP affects bacterial cell wall stability.
To characterize cell wall stability in L. monocytogenes, susceptibility to peptidoglycan-targeting antibiotics was measured by antibiotic disk diffusion assays. L. monocytogenes ΔpdeA mutants were slightly more resistant to cefuroxime, whereas the DacA conditional depletion and the pdeA-overexpressing strains had increased susceptibility to cefuroxime (Fig. 2B) as well as penicillin and ampicillin (see Fig. S3A in the supplemental material). A similar trend was observed by measuring MICs of cefuroxime for each of the L. monocytogenes strains (see Fig. S3B in the supplemental material). These data indicated a role for c-di-AMP in regulating cell wall structure, consistent with similar findings reported for S. aureus and B. subtilis (15, 25).
We next assessed bacterial lysis during growth in broth by constitutively expressing β-galactosidase in wild-type L. monocytogenes and c∆dacA mutants and measuring β-galactosidase release into the culture medium by hydrolysis of ortho-nitrophenyl-β-galactoside (ONPG) (26). The L. monocytogenes cΔdacA mutant released nearly 10-fold more β-galactosidase than wild-type bacteria (Fig. 2C). A weakened cell wall may lead to osmotic stress, resulting in bacteriolysis. Therefore, c∆dacA and wild-type strains were grown in medium containing betaine (100 µM) or carnitine (100 µM), neither of which affected lysis or growth rate (data not shown). However, increased broth hypertonicity (2% NaCl) significantly reduced lysis of c∆dacA mutants (Fig. 2C), whereas wild-type bacteria exhibited a small but significant increase in bacteriolysis. Together these observations support a direct correlation between c-di-AMP production and cell wall stability in L. monocytogenes.
c-di-AMP is required for L. monocytogenes virulence.
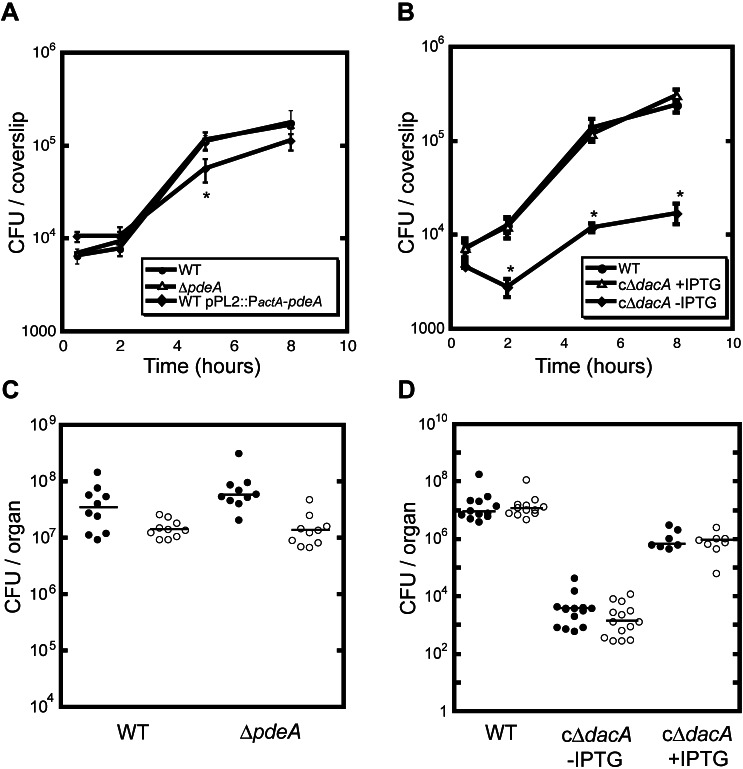
To investigate the role of c-di-AMP during L. monocytogenes infection, we examined bacterial intracellular replication in primary bone marrow-derived macrophages. The intracellular growth kinetics of the pdeA-deficient mutants were indistinguishable from those of wild-type bacteria, whereas strains that overexpressed pdeA upon cytosolic entry were unable to replicate to the same level (Fig. 3A). Conditional depletion of DacA resulted in attenuated bacterial replication (Fig. 3B) with comparable bacterial growth rates (70 min between 2 and 5 h postinfection) to those observed in broth culture (Table 1). While cΔdacA bacteria were able to reach the same final density as wild-type L. monocytogenes in broth culture, significantly lower levels of viable bacteria were recovered from infected macrophages at 5 and 8 h postinfection. To further characterize the role of c-di-AMP levels on L. monocytogenes virulence in a mouse model of infection, mice were infected intravenously with each of the mutants. The bacterial loads recovered from both livers and spleens were indistinguishable between ∆pdeA and wild-type-L. monocytogenes-infected mice (Fig. 3C). In contrast, nearly 4 logs fewer c∆dacA bacteria were recovered 48 h postinfection compared to recovery from mice infected with wild-type bacteria or to mice maintained on water containing IPTG throughout infection with c∆dacA mutants (Fig. 3D). Together, these results support the idea that c-di-AMP is required for in vitro replication in broth culture, intracellular growth in macrophages, and establishment of infection in vivo.
FIG 3 .
C-di-AMP levels affect L. monocytogenes ability to establish infection. (A) Representative intracellular growth of wild-type L. monocytogenes, the ΔpdeA mutant, or pdeA-overexpressing mutants in bone marrow-derived macrophages. (B) Representative intracellular growth of wild-type L. monocytogenes or dacA-conditional mutants in the presence or absence of IPTG in bone marrow-derived macrophages. Error bars represent the standard deviation from the mean of triplicates within the representative experiment. (C) C57BL/6 mice were infected with 1 × 105 CFU of the wild type or ΔpdeA mutants. Organs were harvested at 48 hpi, and bacterial burden per spleen (closed circles) or liver (open circles) was enumerated. (D) C57BL/6 mice were infected with 1 × 105 CFU of the wild type or dacA-conditional mutants in the absence or presence of 10 mM IPTG in the drinking water. Organs were harvested at 48 hpi, and bacterial burden per spleen (closed circles) or liver (open circles) was enumerated. Median values are presented as horizontal lines. *, P < 0.05.
Altered c-di-AMP metabolism affects host innate immune activation.
To characterize the host response to bacterial mutants, we infected murine bone marrow-derived macrophages and quantified transcription of IFN-β as a measure of CSP activation. PdeA-deficient bacteria stimulated 5-fold more IFN-β than wild-type L. monocytogenes (Fig. 4A). Given that ∆pdeA mutants do not exhibit increased host cell death or DNA delivery, which would be indicative of increased lysis during infection, these observations suggest increased c-di-AMP secretion in the host cell cytosol. Surprisingly, infection with cΔdacA mutants also resulted in a significant increase in IFN-β (Fig. 4A), despite lower levels of bacterial growth in macrophages and decreased c-di-AMP secretion in vitro. Given that cΔdacA mutants lyse in broth culture, we hypothesized that released bacterial DNA may result in CSP stimulation. Cytosolic DNA also activates the AIM2 inflammasome, leading to caspase-1 cleavage and pyroptosis, an inflammatory host cell death (27–29). Infection with cΔdacA mutants led to a 2-fold increase in macrophage cell death relative to levels observed with wild-type L. monocytogenes or cΔdacA mutants in the presence of IPTG (Fig. 4B). Infection of macrophages deficient for the DNA inflammasome receptor, AIM2, resulted in reduced cell death following infection with c∆dacA mutants, indicating that bacterial DNA released by lysing bacteria stimulated pyroptosis (see Fig. S4 in the supplemental material). To quantify the extent of bacteriolysis during intracellular infection, we utilized a bacterial reporter plasmid encoding firefly luciferase under control of a cytomegalovirus promoter (3). A 4.5-fold increase and 0.5-fold decrease in luciferase expression were observed in macrophages infected with c∆dacA and ∆pdeA mutants, respectively (Fig. 4C), indicating that the c∆dacA mutants are more susceptible to bacteriolysis while ∆pdeA mutants may be more resistant. Together, these findings establish a correlation between c-di-AMP levels and cell wall stability during infection (Fig. 4C).
FIG 4 .
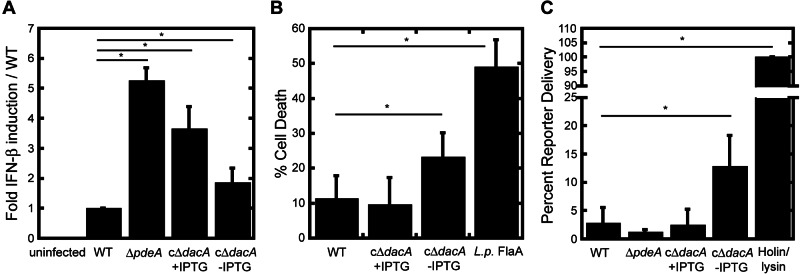
L. monocytogenes mutants with altered c-di-AMP have increased detection by host innate immune pathways. (A) IFN-β induction was measured by qRT-PCR following a 4-h infection of bone marrow-derived macrophages with the indicated strains. (B) Cell death was measured by lactate dehydrogenase release following a 6-h infection of bone marrow-derived macrophages with the indicated strains. “L. p. FlaA” indicates L. monocytogenes expressing the Nlrc4-stimulatory flagellin from Legionella pneumophila (4). Data are relative to a 100% Triton-induced lysis control and are the averages of at least three independent experiments. (C) Bacteriolysis was measured by delivery and expression of luciferase from the reporter plasmid pBHE573 following 6-h infection of IFNAR−/− bone marrow-derived macrophages. Bacteriolysis data are relative to infection with the holin/lysin-expressing strain (5) and are the averages from at least three independent experiments. Error bars represent the standard deviations from the mean. *, P < 0.05, using Student’s t test.
DISCUSSION
The results of this study extend the repertoire of proteins involved in c-di-AMP metabolism in L. monocytogenes by identifying a phosphodiesterase that regulates levels of the signaling nucleotide secreted during infection (Fig. 5). In addition, we provide evidence that c-di-AMP is required for optimal L. monocytogenes growth and contributes to bacterial cell wall stability. Our data show that c-di-AMP production is necessary for L. monocytogenes to establish and maintain growth within the host. Finally, we demonstrate that perturbation of c-di-AMP levels, both enhanced and diminished, directly affects detection by host innate immune pattern recognition receptors.
FIG 5 .
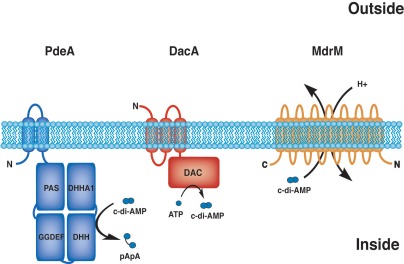
Model of c-di-AMP metabolism in L. monocytogenes, levels of which are regulated by the diadenylate cyclase DacA, which synthesizes the molecule, the phosphodiesterase PdeA, which is capable of degradation, and MDR transporters that secrete c-di-AMP from the cell.
Bacterial second messengers, such as cAMP, c-di-GMP, and ppGpp, function as signaling molecules whose levels are rapidly altered by generation and degradation of the signal in response to environmental cues. In L. monocytogenes, DacA mediates production and PdeA regulates degradation of the second messenger c-di-AMP. Although only a single report regarding regulation of a diadenylate cyclase exists (30), in vitro biochemical characterization showed that GdpP/PdeA proteins have multiple functions and regulatory inputs, including heme and nitric oxide binding (31), ATPase activity (10), and ppGpp binding (10). We speculate that GdpP/PdeA homologues function as a hub to control levels of c-di-AMP by integrating environmental and bacterium-derived signals.
Regulation of PdeA activity within the context of infection of macrophages or mouse models remains largely unexplored. Our data show that L. monocytogenes ∆pdeA mutants did not exhibit increased c-di-AMP secretion during growth in broth despite increased IFN-β production by infected host cells. These observations are consistent with in vivo regulation of c-di-AMP levels by PdeA during intracellular infection, perhaps in response to host-derived signals. Given the increased resistance of ∆pdeA mutants to acid stress, phagosomal acidification may be a signal for the bacteria to alter c-di-AMP levels during infection. Indeed, pretreatment of BMDM with bafilomycin A, which prevents vacuolar acidification, led to lower levels of IFN-β induced by both ΔpdeA mutants and wild-type bacteria (C. E. Witte and D. A. Portnoy, unpublished data). These observations do not address why c-di-AMP levels remain unchanged during broth growth. We hypothesize that feedback inhibition or the presence of another phosphodiesterase regulates nucleotide levels during growth in broth. Nonsporulating members of the Actinobacteria, including Mycobacteria, Gordonia, and Rhodococcus, have predicted DAC domain-containing proteins but do not have GdpP/PdeA homologs, suggesting the existence of alternative mechanisms to regulate c-di-AMP levels.
A variety of stresses and cellular cues likely modulate DacA and PdeA activity, controlling c-di-AMP levels and altering phenotypic outputs. Our results establish two fundamental processes regulated by c-di-AMP: peptidoglycan homeostasis and bacterial growth. Bacteria with a reduced capacity to generate c-di-AMP lysed spontaneously during growth in rich medium and in macrophages, while the sensitivity of L. monocytogenes mutants to cell wall-targeting antibiotics was inversely correlated with c-di-AMP levels. We propose that lysis under conditions of low levels of c-di-AMP is due to a weakened cell wall that cannot withstand the high internal pressure, estimated to be between 15 and 25 atm in Gram-positive bacteria (32). Similar to c∆dacA mutants, spheroplasts and various peptidoglycan-defective mutants are susceptible to lysis and are osmotically stabilized by the addition of sucrose or NaCl (33). A similar role for c-di-AMP in maintaining cell wall homeostasis was reported in B. subtilis, where inactivation of B. subtilis GdpP rescued bacterial resistance to cefuroxime and overexpression increased antibiotic susceptibility (25). Additionally, mutation of the S. aureus GdpP resulted in increased peptidoglycan cross-linking and rescued the severe growth defects of mutants lacking lipoteichoic acids (15). Although transcriptional analysis identified altered expression of cell wall-metabolizing proteins (see Table S1 in the supplemental material), the mechanism by which cell wall stability is affected is currently unresolved. Also of note, glmM, which encodes glucosamine mutase, is adjacent to dacA on the L. monocytogenes chromosome. GlmM generates the precursor for all peptidoglycan biosynthesis, glucosamine-1-phosphate. This genetic organization is highly conserved among bacteria that contain DacA homologs and is consistent with a link between c-di-AMP and amino sugar metabolism.
The second major phenotype we report is a direct correlation between c-di-AMP production and L. monocytogenes growth rate. Because the c∆dacA strains may still contain low levels of DacA, we cannot definitively define DacA as essential to bacterial replication. However, a recent study with B. subtilis as well as previous high-throughput screens defined DacA homologs in Mycoplasma and Streptococcus spp. as equally important for bacterial growth (30, 34–36). How and why c-di-AMP is crucial for bacterial growth remains an intense area of research. PdeA is encoded within an operon containing a ribosomal protein and replicative DNA helicase, both predicted to be essential. This genomic architecture is conserved among many microbes with PdeA homologs and suggests a link between c-di-AMP metabolism and bacterial translation and replication. Furthermore, we identified altered expression of two peptidoglycan-metabolizing proteins referred to as resuscitation-promoting factors or stationary-phase survival factors (Rpf/Sps). In Micrococcus luteus these proteins promote growth of dormant bacteria and in B. subtilis stimulate emergence from stationary-phase growth (24). Therefore, the correlation between c-di-AMP and Rpf/Sps expression levels is consistent with a progrowth effect of c-di-AMP, although deletion of lmo2522 and/or lmo0186 did not result in a growth defect under the conditions tested here.
c-di-AMP, c-di-GMP, and the newly discovered cyclic GMP-AMP (cGAMP) (37) represent small bacterial secondary messengers that fulfill the criteria of pathogen-associated molecular patterns (PAMPs) in that they are essential and/or conserved microbial molecules recognized by host innate immune receptors. L. monocytogenes mutants that lack c-di-AMP still trigger the host IFN-β response likely due to DNA released during bacteriolysis. The host response to cyclic dinucleotides and DNA requires the host protein STING. Interestingly, it was recently discovered that the STING-dependent response to DNA involves a host-derived cyclic dinucleotide, cGAMP, as a secondary messenger (38). Perhaps it is more than coincidental that host innate immune recognition of L. monocytogenes involves both bacterial and host-derived cyclic dinucleotide secondary messengers.
MATERIALS AND METHODS
Bacterial strains, growth medium, and cell culture.
L. monocytogenes strains generated for this study are listed in Table S2 in the supplemental material. For in vitro growth, acid stress susceptibility, and gene expression experiments, wild-type 10403 S L. monocytogenes and isogenic strains were grown in brain heart infusion (BHI) medium at either 30°C or 37°C overnight to stationary phase, except for c-di-AMP secretion assays, in which L. monocytogenes strains were grown in a defined minimal medium (39). All Escherichia coli strains used for in-frame gene construction, complementation, and protein expression were grown on Luria-Bertani (LB) medium. Antibiotics were used at the following final concentrations: streptomycin, 200 µg/ml; chloramphenicol, 7.5 to 20 µg/ml; kanamycin, 30 µg/ml.
Bone marrow-derived macrophages were prepared as previously described (40). IFNAR−/− mice were previously described (41). Immortalized C57BL/6 macrophages were a gift from Russell Vance. AIM2 short hairpin RNA knockdown vectors were a gift from Katherine Fitzgerald. Lentivirus-mediated knockdowns were performed using the pLKO.1 system as previously described (42).
Deletions of pdeA (lmo0052), lmo2522, and lmo0186 were performed by allelic exchange (43). Overexpression of pdeA was achieved by placing the gene downstream of the PactA. Briefly, PactA, and pdeA were amplified and then combined by SOE PCR. The product was digested and ligated into a digested pPL2-derivative vector (44). The plasmid was transformed into E. coli SM10. Following confirmation by sequencing at the UC Berkeley, Sequencing Facility, it was conjugated into wild-type L. monocytogenes or PrfA* G145S mutants.
Construction of DacA conditional depletion strain.
The IPTG inducible pLIV2:dacA plasmid (8) was modified to contain a tetracycline resistance cassette (Tetr). Tetr was amplified from the Tn917 transposon, digested with NdeI, and ligated into the pLIV2:dacA plasmid to replace the chloramphenicol resistance cassette. The resulting plasmid, pLIV2-Tc:dacA, was subsequently integrated into the L. monocytogenes genome as described previously. Deletion of the chromosomal copy of dacA was accomplished by allelic exchange (43).
The c∆dacA::pLIV2-disA strain DP-L5937 was constructed by replacing the IPTG-inducible dacA of the cΔdacA strain with an IPTG-inducible B. subtilis disA gene. Briefly, disA was amplified from an asporogenic derivative of B. subtilis strain ZB307 (45) and then digested and ligated into an IPTG-inducible vector, pLIV2 (12). The resulting plasmid, pLIV2-disA, was integrated into the 10403s chromosome and transduced using the listeriophage U153 (46) into the cΔdacA strain, replacing the IPTG-inducible dacA element (Tetr) with an IPTG-inducible disA (Cmr), and was confirmed by PCR and antibiotic resistance.
The c∆dacA strain was maintained in solid and liquid media containing 500 µM IPTG. Prior to infection or broth growth analysis, bacteria were passaged overnight in BHI liquid culture in the absence of IPTG to deplete DacA levels. Infection and growth in nutritive medium were subsequently analyzed in the presence or absence of 500 µM IPTG.
PdeA protein expression and purification.
Using L. monocytogenes genomic DNA as a template, pdeA fragments encoding the PAS-GGDEF-DHH/DHHA1 (PdeA84-657) truncation were amplified. This fragment was ligated into the T7 expression vector pET-28(b), introducing a C-terminal 6×His tag. The plasmids were transformed into Rosetta E. coli BL21, and protein expression was analyzed as previously described (10). The bacteria were pelleted by centrifugation, resuspended in 20 ml lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 5% glycerol, 0.1% β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride [PMSF]), and lysed with a cell disruptor (Branson). Cell debris was pelleted by centrifugation for 30 min followed by filtration. The supernatant was incubated with 1 ml nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen) for 1 h at 4°C and then washed with 50 ml wash 1 buffer (lysis buffer with 20 mM imidazole) followed by 20 ml wash 2 buffer (lysis buffer with 50 mM imidazole). The bound protein was eluted using a step gradient method with elution buffers containing 50 mM Tris (pH 8.0), 150 mM NaCl, 5% glycerol, and 200 mM, 300 mM, or 500 mM imidazole. The fractions were assessed for purity by SDS-PAGE, and fractions with estimated >95% purity were dialyzed overnight at 4°C in Tris (50 mM, pH 8.0) and NaCl (150 mM). For storage, 5% glycerol was added and aliquots were flash frozen.
Enzymatic synthesis of c-di-AMP.
Recombinant DisA was expressed and purified as described previously (47). Purified DisA (0.6 µM final) was added to a solution of ATP (1 mM), Tris (40 mM, pH 7.5), NaCl (100 mM), β-mercaptoethanol, and MgCl2 (20 mM). Reaction mixtures were incubated for 24 h at 30°C. Protein was precipitated by boiling the sample for 10 min and removed by filtration. Following 5-fold dilution with deionized water, nucleotide was applied to anion exchange resin (Q-Sepharose, 10 ml; Pharmacia). The resin was washed with 5 bed volumes of deionized water. Elution of adsorbed nucleotide was accomplished with ammonium acetate (NH4Ac, 2 M) until the A260 in the eluate returned to baseline levels. Dissolved c-di-AMP was speed vacuumed to dryness and then resuspended in water. This process was repeated two more times to ensure complete removal of NH4Ac. Sample purity was assessed by HPLC analysis and confirmed to be >98% based upon peak absorbance (data not shown).
Derivatization and detection of secreted c-di-AMP.
To assess secretion of c-di-AMP, bacterial mutants were grown in defined minimal medium (39) for 24 h at 37°C with shaking. Bacteria were removed from the supernatant by centrifugation. Samples (50 µl) of culture supernatants were combined with 50 µl sodium acetate (1 M, pH 4.5) and 5 µl chloroacetaldehyde (4 M) and incubated at 80°C for 20 min to derivatize all adenyl purine molecules, as previously described (48). Samples were analyzed using an HPLC system (Agilent 1100 series) fitted with a Nova-Pak C18 column (150 by 3.9 mm, 4 m; Waters) equipped with an Alltima C18-LL guard column (5 m; Alltech). Solvent A consisted of sodium phosphate (30 mM), tetrabutylammonium bisulfate (5 mM, pH 6.0), and solvent B contained 100% acetonitrile. The samples were eluted using an isocratic flow of 14% solvent B over 14 min followed by a gradient from 14 to 40% B over 0.5 min, 40% B for 5 min, a return to 14% B over 0.5 min, and 5 min at 14% B to re-equilibrate the column. The injection volume was 100 µl, and the flow rate was 0.7 ml/min. Excitation of N6-etheno-derivatized c-di-AMP was done at 278 nm, and emission was monitored at 418 nm. Nucleotides were quantified by comparing the fluorescent peak area to similarly derivatized standards of purified c-di-AMP. Concentrations of standards were determined spectrophotometrically based upon the absorbance at 259 nm (ε = 30,000 M−1 cm−1).
PdeA enzyme activity.
Reaction mixtures containing 40 µM c-di-AMP were incubated with 1.5 to 2.5 µM enzyme in assay buffer consisting of Tris (100 mM, pH 8.0), potassium chloride (20 mM), manganese sulfate (0.5 mM), and reactions were allowed to proceed for 30 min at 37°C prior to analysis using an HPLC system (Agilent 1100 series) Nova-Pak C18 column (150 by 3.9 mm, 4 m; Waters) equipped with an Alltima C18-LL guard column (5 m; Alltech). Solvent A consisted of sodium phosphate (30 mM), tetrabutylammonium bisulfate (5 mM, pH 6.0), and solvent B contained 100% acetonitrile. The samples were eluted using a linear gradient from 5 to 12% solvent B over 20 min followed by a gradient from 12 to 40% over 3 min. The injection volume was 100 µl, and the flow rate was 0.7 ml/min; c-di-AMP and pApA (Biolog) standards were run in each experiment.
Bacterial transcriptional analysis.
Microarray analysis of L. monocytogenes ∆pdeA was compared to analysis of the WT as previously described (49). Selected genes that exhibited >2-fold increases in transcription were confirmed by qRT-PCR.
Susceptibility to acid stress.
Bacterial cultures were grown to stationary phase in BHI broth (OD ≈ 2.5) at 37°C with shaking, and then the pH was adjusted to 2.5 with HCl. Viable-cell counts were performed at various intervals by plating on LB plates.
Bacteriolysis in broth and in macrophages.
A U153 transducing lysate was generated with donor strain DP-L967, which contains a Tn917-LTV3 insertion that leads to constitutive expression of the contained lacZ gene. Transduction of the cΔdacA and wild-type L. monocytogenes strains was performed as described previously (46). Broth lysis was assessed as the amount of β-galactosidase activity secreted into the broth supernatant during exponential growth. Overnight cultures of each strain were grown at 37°C with shaking. Cultures were diluted 1:100 in fresh BHI medium and grown to mid-log phase (OD at 600 nm [OD600] = 0.5). Bacteria were removed by centrifugation, and culture supernatants were sterilized with syringe filters (Millex GP, 0.22 µm; Millipore). The bacterial pellet was resuspended in fresh BHI and lysed by vortexing for 10 min at 4°C with zirconia/silica beads (~200 µl; Biospec Products Inc.). The lysed bacteria were then centrifuged to remove cellular debris and the beads. Cellular lysates and sterile filtered culture supernatants of each strain were diluted (1- to 16-fold) and separately mixed with Z buffer (100 µl, 0.1 M phosphate, 0.01 M KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol, pH 7.0) and ONPG (4 mg/ml in 0.1 M phosphate, pH 7.0). Samples were placed in a 96-well plate, and A420 was monitored at 37°C. β-Galactosidase activity was calculated based on the rate of change in the A420. Dilutions of bacterial lysates were used to generate a standard curve of lysis. Broth lysis was calculated based upon the activity observed in the culture supernatant relative to this standard curve.
To assess intracellular bacteriolysis, L. monocytogenes strains were engineered to carry the luciferase reporter plasmid pBHE573 and used to infect IFNAR−/− macrophages, as previously described (3). L. monocytogenes engineered to express bacteriophage holin and lysin was used as a 100% lysis control, to which other values were normalized (3).
Antibiotic susceptibility.
Approximately 2 × 107 CFU of bacteria were spread on BHI plates and allowed to dry. Sterile 7-mm-diameter paper disks (Whatman 3 MM) containing 5 µg cefuroxime, 5 µg ampicillin, or 5 µg penicillin were placed on the plates. Following 24 h of incubation at 37°C, the diameter of the zone of growth inhibition surrounding the disks was measured. Antibiotic susceptibility was also assessed by determining the MIC. Briefly, stationary-phase cultures were diluted 1:100 into fresh BHI medium containing 2-fold serial dilutions of cefuroxime. Cultures were growth for 12 h at 37°C, and OD600 readings were taken. The MIC was determined as the antibiotic concentration required to inhibit bacterial growth by 75%.
In vivo mouse infections.
This study was performed in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (50). Protocols were approved by the Animal Care and Use Committee of the University of California, Berkeley.
Prior to infection, bacterial strains are grown to stationary phase (OD600 = 1.2) at 30°C, then diluted into fresh BHI, and grown at 37°C with shaking until the OD600 was 0.3 to 0.5. Cultures were diluted in 1× PBS and used to intravenously infect female C57BL/6 mice between 6 and 8 weeks of age with a final inoculum of 105 bacteria. At 48 h postinfection, mice were sacrificed and organs collected. Bacterial burdens were enumerated by plating organ homogenates on LB plates and incubating overnight.
Host response to infection.
Macrophage cell death was assessed by lactate dehydrogenase release and qRT-PCR of IFN-β was performed as previously described (3). L. monocytogenes mutants expressing Legionella pneumophila flagellin were used as positive controls for robust inflammasome activation (51).
SUPPLEMENTAL MATERIAL
Resuscitation-promoting factors/stationary phase-survival factors are upregulated in L. monocytogenes ΔpdeA mutants. (A) Broth growth curves of indicated L. monocytogenes strains grown in BHI medium at 37°C with shaking. The plot is representative of three independent experiments. (B) C57BL/6 mice were infected with 1 × 105 CFU of wild-type L. monocytogenes or the Δlmo2522, Δlmo0186, or Δlmo2522 Δlmo0186 mutant. Organs were harvested at 48 hpi, and bacterial burden per spleen (closed circles) or liver (open circles) was enumerated. Median values are presented as horizontal lines. Download
c-di-AMP levels affect bacterial in vitro growth. Broth growth curves of L. monocytogenes strains grown in BHI medium at 37°C with shaking. (A) Wild-type L. monocytogenes (circles) and cΔdacA mutants with IPTG (triangles) and without IPTG (diamonds). (B) Wild-type L. monocytogenes (circles) and ΔpdeA mutants (triangles). (C) PrfA* L. monocytogenes (circles) and the PdeA overexpression strain, PrfA* pPL2::PactA-pdeA (triangles). (D) Wild-type L. monocytogenes (circles), cΔdacA pLIV2::disA mutants with IPTG (triangles) and without IPTG (diamonds). Each plot is representative of three independent experiments. Download
c-di-AMP affects bacterial cell wall stability. (A) Antibiotic susceptibility of indicated mutants as measured as zone of bacterial growth inhibition surrounding filter disk impregnated with ampicillin (5 µg) or penicillin (5 µg). Values are averages from more than three independent experiments, and error bars show standard deviations from those means. (B) Cefuroxime susceptibility, represented by the MIC. Download
Pyroptosis induced by cΔdacA mutants is dependent on the DNA receptor AIM2. Cell death was measured by lactate dehydrogenase release following 6-h infection of wild-type immortalized macrophages (black bars) or AIM2 knockdown macrophages (gray bars). Data are presented relative to a 100% Triton-induced lysis control and are the averages from at least three independent experiments. Error bars represent standard deviations from the means. Download
Microarray analysis of transcriptional changes in mid-log-phase ΔpdeA mutants grown in BHI medium.
Oligonucleotide primers and L. monocytogenes strains used in this study.
ACKNOWLEDGMENTS
We thank John Helmann for helpful discussions and sharing of unpublished data.
This work was supported by National Science Foundation GRFP DGE 1106400 (A.T.W.), American Cancer Society postdoctoral fellowship PF-07-066-01-LIB (J.D.S.), National Institutes of Health grant 1PO1 AI63302, National Institutes of Health grant 1RO1 AI27655 to D.A.P. and National Institutes of Health F32 A184372 to J.J.W.
Daniel A. Portnoy has a consulting relationship with and a financial interest in Aduro Biotech, and both he and the company stand to benefit from the commercialization of the results of this research.
Footnotes
Citation Witte CE, Whiteley AT, Burke TP, Sauer J-D, Portnoy DA, Woodward JJ. 2013. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4(3):e00282-13. doi:10.1128/mBio.00282-13.
REFERENCES
- 1. Hamon M, Bierne H, Cossart P. 2006. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4:423–434 [DOI] [PubMed] [Google Scholar]
- 2. Witte CE, Archer KA, Rae CS, Sauer JD, Woodward JJ, Portnoy DA. 2012. Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Adv. Immunol. 113:135–156 [DOI] [PubMed] [Google Scholar]
- 3. Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. 2010. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. U. S. A. 99:13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. 2011. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of STING in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79:688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. 2012. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 13:1155–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crimmins GT, Herskovits AA, Rehder K, Sivick KE, Lauer P, Dubensky TW, Jr, Portnoy DA. 2008. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc. Natl. Acad. Sci. U. S. A. 105:10191–10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. 2010. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J. Biol. Chem. 285:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lauer P, Hanson B, Lemmens EE, Liu W, Luckett WS, Leong ML, Allen HE, Skoble J, Bahjat KS, Freitag NE, Brockstedt DG, Dubensky TW., Jr. 2008. Constitutive activation of the PrfA regulon enhances the potency of vaccines based on live-attenuated and killed but metabolically active Listeria monocytogenes strains. Infect. Immun. 76:3742–3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischetti VA. 2006. Gram-positive pathogens. ASM Press, Washington, DC [Google Scholar]
- 13. Li Z, Zhao X, Higgins DE, Frankel FR. 2005. Conditional lethality yields a new vaccine strain of Listeria monocytogenes for the induction of cell-mediated immunity. Infect. Immun. 73:5065–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 12:594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 7:e1002217 http://dx.doi.org/:10.1371/journal.ppat.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69:376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beyhan S, Tischler AD, Camilli A, Yildiz FH. 2006. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 188:3600–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Méndez-Ortiz MM, Hyodo M, Hayakawa Y, Membrillo-Hernández J. 2006. Genome-wide transcriptional profile of Escherichia coli in response to high levels of the second messenger 3′,5′-cyclic diguanylic acid. J. Biol. Chem. 281:8090–8099 [DOI] [PubMed] [Google Scholar]
- 19. Cotter PD, Hill C. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cotter PD, Ryan S, Gahan CG, Hill C. 2005. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl. Environ. Microbiol. 71:2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rallu F, Gruss A, Ehrlich SD, Maguin E. 2000. Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol. Microbiol. 35:517–528 [DOI] [PubMed] [Google Scholar]
- 22. Ravagnani A, Finan CL, Young M. 2005. A novel firmicute protein family related to the actinobacterial resuscitation-promoting factors by non-orthologous domain displacement. BMC Genomics 6:39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah IM, Laaberki MH, Popham DL, Dworkin J. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB. 1998. A bacterial cytokine. Proc. Natl. Acad. Sci. U. S. A. 95:8916–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo Y, Helmann JD. 2012. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol. Microbiol. 83:623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camilli A, Portnoy A, Youngman P. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10:266–272 [DOI] [PubMed] [Google Scholar]
- 28. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J. 2013. Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J. Biol. Chem. 288:2004–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rao F, Ji Q, Soehano I, Liang ZX. 2011. Unusual heme-binding PAS domain from YybT family proteins. J. Bacteriol. 193:1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whatmore AM, Reed RH. 1990. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J. Gen. Microbiol. 136:2521–2526 [DOI] [PubMed] [Google Scholar]
- 33. Gnarpe H, Edebo L. 1970. Conditions affecting the viability of spheroplasts in urine. Infect. Immun. 1:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song JH, Ko KS, Lee JY, Baek JY, Oh WS, Yoon HS, Jeong JY, Chun J. 2005. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol. Cells 19:365–374 [PubMed] [Google Scholar]
- 35. Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA, III, Smith HO, Venter JC. 2006. Essential genes of a minimal bacterium. Proc. Natl. Acad. Sci. U. S. A. 103:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. French CT, Lao P, Loraine AE, Matthews BT, Yu H, Dybvig K. 2008. Large-scale transposon mutagenesis of Mycoplasma pulmonis. Mol. Microbiol. 69:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davies BW, Bogard RW, Young TS, Mekalanos JJ. 2012. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. 2013. Cyclic GMP-AM is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phan-Thanh L, Gormon T. 1997. A chemically defined minimal medium for the optimal culture of Listeria. Int. J. Food Microbiol. 35:91–95 [DOI] [PubMed] [Google Scholar]
- 40. Jones S, Portnoy DA. 1994. Intracellular growth of bacteria. Methods Enzymol. 236:463–467 [DOI] [PubMed] [Google Scholar]
- 41. Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. 2008. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 4:e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD. 2003. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camilli A, Tilney LG, Portnoy DA. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lauer P, Chow MY, Loessner MJ, Portnoy DA, Calendar R. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zuber P, Losick R. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodgson DA. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312–323 [DOI] [PubMed] [Google Scholar]
- 47. Witte G, Hartung S, Buttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30:167–178 [DOI] [PubMed] [Google Scholar]
- 48. Katayama M, Matsuda Y, Shimokawa K, Tanabe S, Kaneko S, Hara I, Sato H. 2001. Simultaneous determination of six adenyl purines in human plasma by high-performance liquid chromatography with fluorescence derivatization. J. Chromatogr. B Biomed Sci. Appl. 760:159–163 [DOI] [PubMed] [Google Scholar]
- 49. Crimmins GT, Schelle MW, Herskovits AA, Ni PP, Kline BC, Meyer-Morse N, Iavarone AT, Portnoy DA. 2009. Listeria monocytogenes 6-phosphogluconolactonase mutants induce increased activation of a host cytosolic surveillance pathway. Infect. Immun. 77:3014–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Research Council National. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 51. Sauer JD, Pereyre S, Archer KA, Burke TP, Hanson B, Lauer P, Portnoy DA. 2011. Listeria monocytogenes engineered to activate the Nlrc4 inflammasome are severely attenuated and are poor inducers of protective immunity. Proc. Natl. Acad. Sci. U. S. A. 108:12419–12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Resuscitation-promoting factors/stationary phase-survival factors are upregulated in L. monocytogenes ΔpdeA mutants. (A) Broth growth curves of indicated L. monocytogenes strains grown in BHI medium at 37°C with shaking. The plot is representative of three independent experiments. (B) C57BL/6 mice were infected with 1 × 105 CFU of wild-type L. monocytogenes or the Δlmo2522, Δlmo0186, or Δlmo2522 Δlmo0186 mutant. Organs were harvested at 48 hpi, and bacterial burden per spleen (closed circles) or liver (open circles) was enumerated. Median values are presented as horizontal lines. Download
c-di-AMP levels affect bacterial in vitro growth. Broth growth curves of L. monocytogenes strains grown in BHI medium at 37°C with shaking. (A) Wild-type L. monocytogenes (circles) and cΔdacA mutants with IPTG (triangles) and without IPTG (diamonds). (B) Wild-type L. monocytogenes (circles) and ΔpdeA mutants (triangles). (C) PrfA* L. monocytogenes (circles) and the PdeA overexpression strain, PrfA* pPL2::PactA-pdeA (triangles). (D) Wild-type L. monocytogenes (circles), cΔdacA pLIV2::disA mutants with IPTG (triangles) and without IPTG (diamonds). Each plot is representative of three independent experiments. Download
c-di-AMP affects bacterial cell wall stability. (A) Antibiotic susceptibility of indicated mutants as measured as zone of bacterial growth inhibition surrounding filter disk impregnated with ampicillin (5 µg) or penicillin (5 µg). Values are averages from more than three independent experiments, and error bars show standard deviations from those means. (B) Cefuroxime susceptibility, represented by the MIC. Download
Pyroptosis induced by cΔdacA mutants is dependent on the DNA receptor AIM2. Cell death was measured by lactate dehydrogenase release following 6-h infection of wild-type immortalized macrophages (black bars) or AIM2 knockdown macrophages (gray bars). Data are presented relative to a 100% Triton-induced lysis control and are the averages from at least three independent experiments. Error bars represent standard deviations from the means. Download
Microarray analysis of transcriptional changes in mid-log-phase ΔpdeA mutants grown in BHI medium.
Oligonucleotide primers and L. monocytogenes strains used in this study.