Abstract
As the science demonstrating strong evidence for voluntary medical male circumcision (VMMC) for HIV prevention has evolved, the President’s Emergency Plan for AIDS Relief (PEPFAR) has collaborated with international agencies, donors, and partner country governments supporting VMMC programming. Mathematical models forecast that quickly reaching a large number of uncircumcised men with VMMC in strategically chosen populations may dramatically reduce community-level HIV incidence and save billions of dollars in HIV care and treatment costs. Because VMMC is a 1-time procedure that confers life-long partial protection against HIV, programs for adult men are vital short-term investments with long-term benefits. VMMC also provides a unique opportunity to reach boys and men with HIV testing and counseling services and referrals for other HIV services, including treatment. After formal recommendations by WHO in 2007, priority countries have pursued expansion of VMMC. More than 1 million males have received VMMC thus far, with the most notable successes coming from Kenya’s Nyanza Province. However, a myriad of necessary cultural, political, and ethical considerations have moderated the pace of overall success. Because many millions more uncircumcised men would benefit from VMMC services now, US President Barack Obama committed PEPFAR to provide 4.7 million males with VMMC by 2014. Innovative circumcision methods—such as medical devices that remove the foreskin without injected anesthesia and/or sutures—are being rigorously evaluated. Incorporation of safe innovations into surgical VMMC programs may provide the opportunity to reach more men more quickly with services and dramatically reduce HIV incidence for all.
Keywords: male circumcision, HIV prevention, PEPFAR
THE SCIENTIFIC EVIDENCE FOR VOLUNTARY MEDICAL MALE CIRCUMCISION
Male circumcision, one of the world’s oldest surgical procedures, has been performed for cultural and religious reasons for millennia. The health benefits were documented as early as 1855, when Hutchinson’s observational studies of Jewish and Christian patients with venereal disease in London showed “the well-known greater exemption of the Jew to syphilitc infection, owing to the protecting influence of circumcision.1” In 1954, Wynder et al2 detailed the association of cervical cancer with a lack of circumcision in women’s sex partners. Decades later in 1986, the same year HIV was officially labeled the etiologic agent of AIDS, scientists hypothesized a link between the foreskin and AIDS in men.3 Shortly thereafter, Cameron et al4 prospective study of male clients of female sex workers in Nairobi, Kenya, found a greater than 8-fold increased risk of HIV acquisition among uncircumcised men. Scientists next looked to ecological data and found many populations where lack of circumcision was correlated with higher HIV prevalence.5-7 A meta-analysis published in 2000 of 27 observational studies from sub-Saharan Africa demonstrated a 58% protective effect of circumcision in general population males.8 That same year, a prospective study of discordant couples conducted in Rakai, Uganda, showed zero seroconversions among 50 circumcised male partners of HIV-positive women, compared with an incidence of 17 per 100 person-years among the 137 couples where the male partner was uncircumcised.9 Such correlations were further supported by findings published in 2001 from a study in 4 cities in sub-Saharan Africa that demonstrated male circumcision as the greatest predictor of HIV prevalence.10
Although these ecological and observational data indicated a causal relationship between circumcision and reduced HIV incidence, doubts remained due to potential confounding by unknown or inadequately measured factors including sexual behaviors, cultural practices, religion, and hygiene. In addition, observational data mainly referred to men circumcised many years previously, making determination of the timing of circumcision and a protective effect uncertain. These uncertainties were conclusively resolved by 3 randomized controlled trials (RCTs) among 10,000 HIV-negative African men randomized to immediate or delayed circumcision and followed for incident HIV infections. The 3 studies showed 60%,11 53%,12 and 51%13 reductions in HIV incidence in circumcised compared with uncircumcised male study participants from South Africa, Kenya, and Uganda, respectively. Men in the Kenya and Uganda studies who underwent extended follow-up at 66 months (Kenya) and 5 years (Uganda) exhibited sustained reductions in HIV incidence of 64%14 and 73%,15 respectively. In 2010, investigators in Orange Farm, South Africa, conducted a cross-sectional study of HIV incidence by laboratory assay methods and found that after adjusting for confounding, HIV incidence was reduced by 76% in circumcised versus uncircumcised men (adjusted incidence rate ratio = 0.24, 95% confidence interval [CI] =0.00 to 0.66].).16
Numerous studies have substantiated the biological plausibility for a causal link between male circumcision and lower susceptibility to HIV infection. Compared with the tissue of the outer foreskin, the inner foreskin may be more prone to micro tears during intercourse and has HIV target cells (Langerhan cell with CD4 receptors) closer to the epithelial surface.17-25 The warm moist microenvironment under the foreskin hosts a high density and diversity of anaerobic bacteria,26 providing conditions that may be conducive to greater risk of HIV acquisition in men with an intact foreskin. Circumcised men also have a lower susceptibility to other sexually transmitted infections, including Mycoplasma genitalium27 and herpes simplex virus type 2,28-cf31 and human papillomavirus (HPV), including high-risk strains of HPV.28,32,33 The link between the foreskin and cervical cancer in women, noted nearly 50 years prior, was further clarified when scientists demonstrated reduced incidence of HPV, including HPV genotypes that cause cervical cancer, (IRR = 0.77; 95% CI: 0.63 to 0.93)34 and reduced risk of cervical cancer (aOR = 0.42; 95% CI 0.23 to 0.79)35 in women with circumcised male sex partners.
INTERNATIONAL RESPONSE TO THE EVIDENCE
Upon publication of the Kenya and Uganda RCT results, WHO and UNAIDS announced a consultation to review scientific evidence and develop technical and policy guidance. The consultation, held in March 2007, led to 11 conclusions containing 43 recommendations for voluntary medical male circumcision (VMMC) for HIV prevention.36 The recommendations identified 13 priority VMMC countries with generalized HIV epidemics, high HIV prevalence, and low male circumcision prevalence. The recommendations were broad spanning issues from importance of conducting country-specific situation analysis to policy considerations, cultural sensitivities, gender issues, and human rights considerations. The need for a “minimum package” of HIV prevention services to complement circumcision surgery was deemed essential and identified the following services as requisite: removal of the foreskin; HIV testing and counseling (HTC) (voluntary); screening and treatment of sexually transmitted infections; pre-operative and postoperative education and risk-reduction counseling; and, promotion and provision of condoms. As program planning and implementation ensued, WHO and UNAIDS convened additional stakeholder consultations to develop normative guidance and tools. Many of the resources address complex cultural, religious, political, and ethical issues not specific to male circumcision, such as gender dimensions of programming, task shifting, sensitivity to cultural norms and religious practices, communications strategies, and informed consent. These aspects take considerably more effort to implement than the relatively simple and quick surgical removal of the foreskin.
PUBLIC HEALTH BENEFIT OF VMMC
In November 2011, Secretary of State Hillary Clinton included VMMC in her outline of 3 key evidence-based interventions essential to effective combination HIV prevention approaches: VMMC, prevention of mother to child transmission (PMTCT), and expanded HIV treatment. VMMC is a pivotal public health investment owing to a number of unique characteristics and is among the highest HIV prevention priorities for the PEPFAR program. VMMC is a 1-time, highly effective, relatively quick, and cost-savings intervention. The protection against HIV conferred is substantial, though partial, and repeated treatment is not required to maintain the benefits. No other HIV intervention currently available provides this permanence of effect. Microbicides, pre-exposure prophylaxis, and HIV treatment require considerable user adherence to realize the desired effectiveness. PMTCT programs are highly effective, but only protect the infant during pregnancy/birth/breastfeeding and shortly thereafter; other lifelong exposure risks remain. Once initiated, antiretroviral treatment and PMTCT programs must be sustained indefinitely.
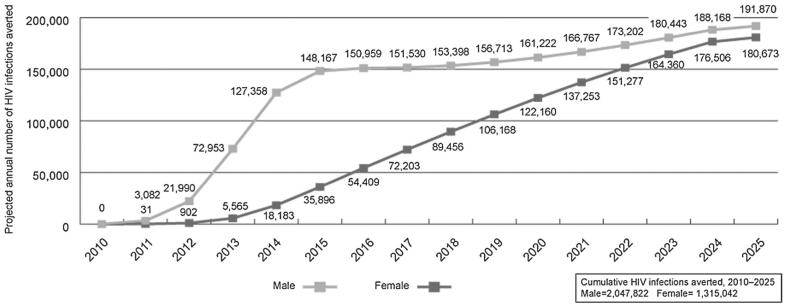
VMMC also reduces HIV risk for women, uncircumcised men, and eventually infants. With the risk of new HIV infection reduced in men as a direct result of becoming circumcised, the incidence and prevalence of HIV among men in the population decreases. Consequently, the likelihood that women will encounter HIV-infected male sex partners also decreases. Mathematical models suggest that the accruing indirect protection for women is substantial.37-39 As projected by the Decision Makers’ Programming Planning Toolkit model, increasing the prevalence of circumcision from country-specific baseline levels to 80% equally across males aged 15–49 in the priority countries within the next 5 years, for instance, can result in preventing nearly an equal number of new HIV infections annually in women as in men within 15 years38 (Fig. 1). Less HIV incidence and prevalence in women of reproductive age eventually means that fewer infants will be at risk of vertical transmission. In time, even uncircumcised men will face a lower risk of acquiring HIV due to reduced HIV prevalence in the community.
FIGURE 1.
Mathematical model-based estimates of annual HIV infections averted, by gender, as a result of increasing male circumcision prevalence from country-specific baseline levels to 80% equally across males aged 15–49 years in 13 priority countries.
VMMC provides a unique opportunity to reach men and adolescent boys with an array of behavioral and clinical health programs that might not otherwise be possible. Most notably, HIV testing rates among men have been historically low—less than 25 %—in almost half of the priority VMMC countries.40-45 Given that VMMC clients are routinely recommended to receive HIV testing pre-operatively, circumcision programs may increase awareness of HIV status among tens of millions of boys/men who might otherwise forego HIV testing. HIV-positive men are identified and linked with HIV care and treatment services, and antiretroviral treatment-induced viral suppression among HIV-infected men adhering to therapy will further reduce HIV incidence in women.46
In support of WHO/UNAIDS recommendations and country strategies to scale-up VMMC, PEPFAR endeavors to utilize US Government resources to assist partner country governments and communities to maximally and quickly reduce HIV incidence. This is in collaboration with other international donors, particularly the Bill and Melinda Gates Foundation (BMGF). PEPFAR’s resources—financial and technical—are being leveraged through support in 2 phases. The first phase (“catch-up” VMMC) is geared to scale-up services to the large number of uncircumcised men who already are or soon will be sexually active and at risk of HIV infection in the short term. Phase-1 support will continue until most or all men who want to become circumcised have been reached with services. Because the objective is to achieve high VMMC coverage quickly among such men—to maximally reduce HIV incidence—this first phase should be short, ideally less than 5 years, and would not be sustained thereafter. There are in excess of 20 million uncircumcised men who may benefit from VMMC for HIV prevention through “catch-up” services in the 13 priority countries.38 Once “catch-up” is completed, the need for VMMC services for such men will no longer exist or be comparatively small. Thus, support in this first phase is intended to achieve immediate results.
The second phase of implementation must ensure availability of medical male circumcision for younger males who were not old enough to receive services during the adult “catch-up” phase. Because male babies are born continuously, adding to the uncircumcised male population, phase-2 services must be indefinitely available once initiated. Though the second phase of support is ongoing, the annual number of male births is relatively small (compared with the number of adults in “catch-up”). Some countries may wish to maintain high male circumcision coverage after “catch-up” services by routinely offering circumcision to all parents of newborn males, all boys entering adolescence, or some combination of both. Once phase-2 services are implemented, they must be sustained, meaning that ministries of health will need to integrate these ongoing VMMC services into the existing health system.
Countries do not need to wait for all men to be circumcised before starting medical male circumcision activities for newborns and adolescents. As phase 1 support results in larger numbers of men receiving VMMC, then resources could logically be transitioned to phase-2 services. PEPFAR supports the needs of both VMMC program phases, though current funding is prioritized toward the first phase of adult “catch-up”, so that men at risk now receive VMMC as quickly as possible. Other donors may prioritize circumcision of male newborns ahead of adults, and some countries may wish to do the same with their own or other financial assistance.
TECHNICAL SUPPORT
VMMC is one of the newest biomedical HIV prevention interventions recommended for scale-up. In addition to the surgical procedure, service delivery programs must address a variety of technical issues, in accordance with the WHO-defined “minimum package” of services. Recent evidence demonstrates the prevention benefits of HIV treatment,46 and VMMC programs are incorporating active referral systems to link HIV-positive men from VMMC sites to HIV care and treatment. In consultation with ministries of health, WHO, UNAIDS, BMGF, and implementing partners, the PEPFAR Male Circumcision Technical Working Group provides technical leadership for planning and executing quality safe services throughout the region. In addition, and in collaboration with ministries of health and implementers, the Technical Working Group conducts external quality assurance exercises to assist with the assessment of program safety, quality, and requisite service efficiency and volume to achieve the intended epidemic impact.
FINANCIAL SUPPORT
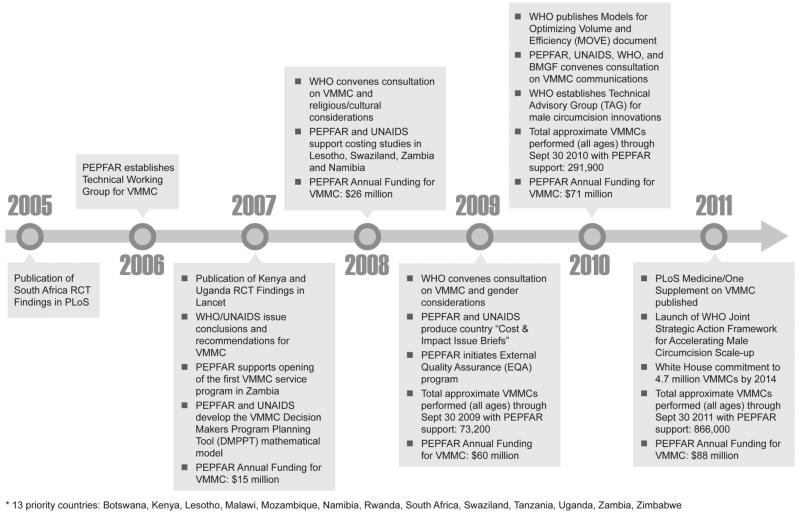
To date, PEPFAR has been the primary funder of VMMC programs Africa. In anticipation of favorable findings from the RCTs, PEPFAR offices in sub-Saharan Africa countries were encouraged in 2006 to request funding for feasibility, acceptability, and policy development activities related to VMMC. Funding levels for VMMC have progressively increased, with a total of approximately $250 million dollars allocated to VMMC activities through 2011. In addition to the 13 countries identified by WHO/UNAIDS, PEPFAR has provided limited support to Burundi, Ethiopia, and Nigeria. See Figure 2 for annual PEPFAR funding levels, relative to key achievements/milestones. The PEPFAR support has been accompanied by more than $140 million in support from the BMGF dating back to 2001, which has been particularly collaborative and instrumental to programs in Kenya, Botswana, Swaziland, Zambia, Zimbabwe, and Uganda. More recently, the Global Fund has also approved petitions for funding of VMMC programs in the region.
FIGURE 2.
Timeline of key VMMC milestones and PEPFAR funding for VMMC in 13 countries.
EXPERIENCE TO DATE-SUCCESSES AND CHALLENGES
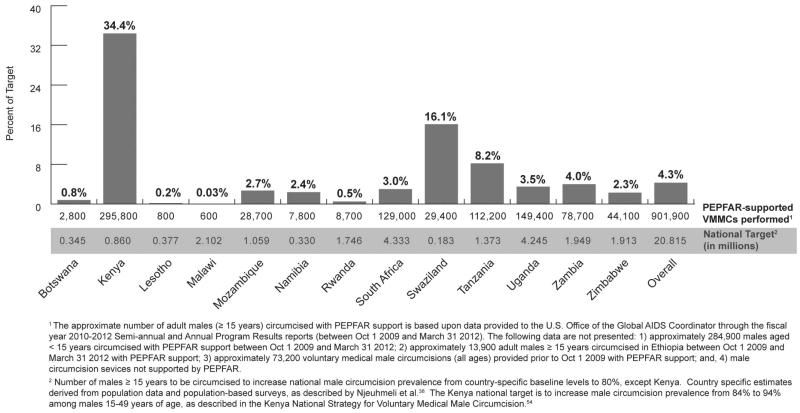
Five years after WHO/UNAIDS recommended VMMC to reduce HIV incidence in sub-Saharan Africa, over 1.2 million boys and men have recedived VMMC services with support from PEPFAR, with the greatest progress achieved to date in Kenya (Fig. 3). Kenya’s successes are described in the Case Study below. All priority countries have incorporated VMMC into their HIV prevention portfolios and are developing policies and operational plans to enable expansion of the program. There has been wide adoption of the minimum packages of HIV prevention services, and thousands of doctors, clinical officers, nurses, counselors, health educators, and hygiene officers have been trained to provide comprehensive VMMC services. This progress represents a translation of scientific evidence into health policy and program implementation in a relatively short period.
FIGURE 3.
Progress in scale-up of VMMC through March 2012, assuming national-level male circumcision prevalence targets of 80% within 5 years of program start among males 15–49 years of age.
CASE STUDY: KENYA
Nyanza Province, Kenya, was home to one of the 3 RCTs of male circumcision for HIV prevention described previously. Kenya’s national and provincial government agencies were thus poised to move quickly after the WHO recommendations in 2007. Before rapidly implementing routine VMMC services throughout noncircumcising communities, however, Kenyan health officials first engaged the elders in these communities to sensitize them on the RCT results, lobby for their support, and obtain their guidance. This process was neither certain nor particularly quick, as influential elders initially opposed the idea of circumcision. Thoughtful and continued discussions clarified the “voluntary” and “medical” nature of male circumcision performed for HIV prevention purposes. Eventually, consensus was achieved. With visible support for VMMC from within communities, government leaders then championed the HIV prevention benefits of the program, providing consistent and responsive leadership in all aspects of programming.
A willingness to innovate has defined the Kenya VMMC program and may be the source of its many successes. Efficient partnerships were created at the national and regional level in Nyanza Province to co-ordinate VMMC services. When conventional surgical approaches in public sector facilities were not able to meet program targets, leadership tested mobile service models, including VMMC in tents, to avail services to a greater number of people in a wider array of settings. When the pool of clinical officers was insufficient to meet service needs, Kenya broadened national task-shifting policies and approved nurses to provide VMMC. The Kenyans also marshaled health care teams comprised of private, public, and volunteer providers to offer campaign-style services in diverse outreach settings during periods of peak demand. At each and every challenge, the Kenyans determined a solution—often policy and regulatory—instead of remaining entrenched in bureaucratic barriers.
Kenya continues to translate financial and technical support into a volume of VMMCs sufficient to impact their HIV epidemic. Among the PEPFAR-supported countries, Kenya has received the highest level of US Government financial support for VMMC thus far. A shared appreciation of the importance of reaching high VMMC coverage levels quickly to reduce HIV incidence is paramount, and the Government of Kenya, WHO/UNAIDS, the US Government, and the BMGF remain committed to ensuring success. Although the VMMC program undoubtedly offers collateral benefits through the health system, the primary goal of the Kenya Ministry of Health is to quickly reach as many men possible with safe VMMC services. Focus, flexibility, and an ability to deliver are hallmarks of the most successful VMMC for HIV prevention program to date.
Despite the progress in VMMC programming, and given that one new HIV infection may be prevented for every 10 male circumcisions in the South Africa region,37 stakeholder are understandably impatient to more rapidly reach greater numbers of men with VMMC. Similar to HTC, PMTCT, and antiretroviral therapy (ART) programs—which all experienced similar protracted timelines between the issuance of normative guidance and substantial achievements toward scale-up—VMMC scale-up has not happened overnight. The factors impeding scale-up vary by country, but several experiences have been common.
With the exception of a few countries, political prioritization for planning and implementing MC programs has been challenging. Though mathematical models project that millions of new HIV infections may be prevented by male circumcision and billions of dollars may be saved in HIV care and treatment costs, these benefits accrue over a generation’s time.38 Other health care needs are more immediate and tangible, and dedicating scarce resources—human and material—to an elective medical procedure may be unpopular. The contribution of financial resources by PEPFAR and others do not often quickly or completely remedy the shortage of clinicians and suitable clinical space. Also, shifting service burdens to nonphysicians may be contentious for medical officers, clinical officers, and nurses alike; and some ministries of health have been reluctant to pursue task shifting policies. Implementation of a large public health intervention like VMMC is preceded by a lengthy list of formative work, sociocultural considerations, ethical and human rights evaluations, awareness and demand creation activities (but not too soon or too great), trainings, logistics and supply chain management work, and assurances for safety/quality. Such activities, although essential to effective VMMC programs, are labor and time-intensive endeavors. Too, the timeline for completing such preparatory work is dependent upon the capacity of partner governments to commit to completion. National elections, civil unrest, worsening domestic and international economic crises, Global Fund Round 11 cancellation, for instance, have all transpired in the past 5 years, impacting program planning and implementation.
Because medical removal of the foreskin in adolescents/adults is achieved by surgical techniques, implementation has generally benefited the overall health system, particularly the clinical/surgical capacity of public sector facilities. Innovative approaches to service delivery have also demonstrated benefits in productivity through novel implementation models (eg, VMMC in tents, supply kits, flexible staffing plans with task shifting). Although such models may have fewer benefits to public sector facilities, their ability to extend services more widely achieves the primary goal of the program: VMMC to reduce HIV incidence.
When VMMC was introduced after the 2007 WHO/UNAID recommendations, there were boys and men in many countries who presented almost immediately for services (“early adopters”). During this period, the delay of communications activities was logical, to avoid creating more demand than could be met with service supply. Service capacities eventually increased, and simultaneously, the “early adopters” were no longer in need of VMMC. At the nexus of this shifting dynamic, some countries are committed to expanding VMMC services in the face of unsustained demand. Communications strategies to match service supply with demand, in these countries, have lagged, particularly among older, sexually active men.
Finally, stakeholders in the priority VMMC countries and beyond have expressed concerns about whether men receiving VMMC (and their sex partners) might increase sexual risk behaviors due to a perceived protection that is greater than actual (called risk compensation or disinhibition). However, among men in the RCTs, there were no meaningful differences noted in almost all sexual risk behaviors between those in circumcised versus uncircumcised study arms.47,48 Among men in communities where VMMC services have been dramatically expanded and sexual risk behaviors assessed, there were no differences in risk behaviors between circumcised and uncircumcised men.16 Modeling studies suggest that the favorable epidemiologic impact and cost-effectiveness would be maintained, even if risk behaviors increased by 30% after circumcision, in all countries except Rwanda.38
WAY FORWARD
Of the countries presently receiving donor support for VMMC, the majority have adopted scale-up strategies that call for circumcision service expansion to raise male circumcision prevalence to 80% from baseline levels among adult men, nationally or regionally, within 5 years of the start of the program. In support of these goals, the US Government made additional commitments. On World AIDS Days 2011, the White House pledged support for 4.7 million circumcisions by 2014.
Achieving 4.7 million VMMCs by 2014 and many millions more thereafter will require the most efficient use of resources, financial and otherwise, and continual innovation. Just as rapid diagnostic testing technology transformed the landscape of HTC—taking HIV testing out of the clinic and into communities—medical devices capable of removing the foreskin without the need for injected anesthesia, sutures, or an aseptic clinical space may facilitate a similarly rapid advancement for VMMC. Alongside WHO/UNAIDS and the BMGF, PEPFAR encourages technological innovations for VMMC to ensure that safe and acceptable services are as widely available as possible. It is hoped that increasingly efficient innovative methods, such as device-based VMMC, may be used by nonphysicians, especially outside of hospitals and clinics, and thus support rather than compete with existing health care priorities. Two medical devices—PrePex and Shang Ring—are currently being evaluated in clinical trials, in accordance with WHO guidance, and preliminary results around safety, efficacy, acceptability, task shifting, and time savings are promising.49-52 Medical device-based alternatives to conventional surgical circumcision may soon be a reality.
VMMC has affirmatively answered the following crucial questions for a public health intervention: does it work, is it safe, is it acceptable, is it feasible, and is it affordable? The challenges faced in scaling-up VMMC are greatly outweighed by the benefits of its impact on the HIV epidemic. Despite tremendous progress in HIV prevention, for every 1 person started on ART today in sub-Saharan Africa, there are still 2–3 more who become newly infected with HIV.53 Although solutions are sought to overcome the logistical, behavioral, and financial difficulties of achieving universal viral suppression through ART in Africa, the cost-savings from a highly effective and relatively straightforward intervention such as VMMC cannot be overlooked.
As Nelson Mandela reminds us, “It always seems impossible until it’s done.” Let us remember that vision as we continue the long, but winnable fight against HIV. We must accelerate the scale-up of the important public health tool of VMMC if we are to achieve an HIV-free generation.
ACKNOWLEDGMENTS
The authors wish to thank Jonathan Grund, Alpa Patel-Larson, Jordana DeLeon, Funmi Adesanya, Claudia Day, and Denise Duran for their thoughtful review of the information included in this paper.
Various authors receive financial support or have professional relationships from PEPFAR (either as employees of PEPFAR-supported US Government agencies or as grantees/contractors) as outlined in the Copyright Transfer Agreement Forms.
Footnotes
The authors have no other funding or conflicts of interest to disclose.
Prior iterations of data on scale-up progress were most recently presented at ICASA 2012.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the US Government, or the World Health Organization.
REFERENCES
- 1.Hutchinson J. On the influence of circumcision in preventing syphilis. Medical Times and Gazette, NS. 1855;2:542–543. [Google Scholar]
- 2.Wynder EL, Cornfield J, Schroff PD, et al. A study of environmental factors in carcinoma of the cervix. Am J Obstet Gynecol. 1954;68:1016–1047. doi: 10.1016/s0002-9378(16)38400-9. [DOI] [PubMed] [Google Scholar]
- 3.Fink AJ. A possible explanation for heterosexual male infection with AIDS. N Engl J Med. 1986;315:1167. [PubMed] [Google Scholar]
- 4.Cameron DW, Simonsen JN, D’Costa LJ, et al. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;2:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 5.Bongaarts J, Reining P, Way P, et al. The relationship between male circumcision and HIV infection in African populations. AIDS. 1989;3:373–377. doi: 10.1097/00002030-198906000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Moses S, Bradley JE, Nagelkerke NJ, et al. Geographical patterns of male circumcision practices in Africa: association with HIV seroprevalence. Int J Epidemiol. 1990;19:693–697. doi: 10.1093/ije/19.3.693. [DOI] [PubMed] [Google Scholar]
- 7.Halperin DT, Bailey RC. Male circumcision and HIV infection: 10 years and counting. Lancet. 1999;354:1813–1815. doi: 10.1016/S0140-6736(99)03421-2. [DOI] [PubMed] [Google Scholar]
- 8.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2000;14:2361–2370. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, et al. Rakai Project Study Group Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Eng J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 10.Auvert B, Buvé A, Lagarde E, et al. Male circumcision and HIV infection in four cities in sub-Saharan Africa. AIDS. 2001;15(suppl 4):S31–S40. doi: 10.1097/00002030-200108004-00004. [DOI] [PubMed] [Google Scholar]
- 11.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 13.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 14.Bailey RC, Moses S, Parker CB, et al. The protective effect of male circumcision is sustained for at least 42 months: results from the Kisumu, Kenya Trial [THAC0501]; Presented at: XVII International AIDS Conference; Mexico City, Mexico. August 3–8, 2008. [Google Scholar]
- 15.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a post trial follow-up study. AIDS. 2012;26:609–615. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auvert B, Taljaard D, Rech D, et al. Effect of the Orange Farm (South Africa) male circumcision roll-out (ANRS-12126) on the spread of HIV [WELBC02]; Presented at: 6th IAS Conference on HIV Pathogensis, Treatment and Prevention; Rome, Italy. July 17-20, 2011. [Google Scholar]
- 17.Serour F, Samra Z, Kushel Z, et al. Comparative periurethral bacteriology of uncircumcised and circumcised males. Genitourin Med. 1997;73:288–290. doi: 10.1136/sti.73.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo R, Short RV. How does male circumcision protect against HIV infection? BMJ. 2000;320:1592–1594. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson BK, Landay A, Siegel JN, et al. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alanis MC, Lucidi RS. Neonatal circumcision: a review of the world’s oldest and most controversial operation. Obstet Gynecol Surv. 2004;59:379–395. doi: 10.1097/00006254-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 21.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 22.Donoval BA, Landay AL, Moses S, et al. HIV-1 target cells in foreskins of African men with varying histories of sexually transmitted infections. Am J Clin Pathol. 2006;125:386–391. [PubMed] [Google Scholar]
- 23.Kigozi G, Wawer M, Ssettuba A, et al. Foreskin surface area and HIV acquisition in Rakai, Uganda (size matters) AIDS. 2009;23:2209–2213. doi: 10.1097/QAD.0b013e328330eda8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KE, Sherman ME, Ssempiija V, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS. 2009;23:1807–1815. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinh MH, Fahrbach KM, Hope TJ. The role of the foreskin in male circumcision: an evidence-based review. Am J Reprod Immunol. 2011;65(3):279–283. doi: 10.1111/j.1600-0897.2010.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price LB, Liu CM, Johnson KE, et al. The effects of circumcision on the penis microbiome. PLoS One. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta SD, Gaydos C, Maclean I, et al. The Effect of medical male circumcision on urogenital Mycoplasma genitalium among men in Kisumu, Kenya. Sex Transm Dis. 2012;39:276–280. doi: 10.1097/OLQ.0b013e318240189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–19. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study of Orange Farm, South Africa. J Infect Dis. 2009;199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Eng J Med. 2009;360:1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta SD, Moses S, Parker CB, et al. Circumcision status and incident HSV-2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–1149. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobian AA, Kigozi G, Redd AD, et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J Infect Dis. 2012;205:486–490. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rositch AF, Poole C, Hudgens MG, et al. Multiple human papillomavirus infections and type competition in men. J Infect Dis. 2012;205(1):72–81. doi: 10.1093/infdis/jir709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wawer MJ, Tobian AA, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. 2011;377:209–218. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellsagué X, Bosch FX, Muñoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization, Joint United Nations Programme on HIV/AIDS [Accessed February 29, 2012];New data on male circumcision and HIV prevention: policy and program implications. 2007. Available at: http://libdoc.who.int/publications/2007/9789241595988_eng.pdf.
- 37.UNAIDS/WHO/SACEMA Expert Group on Modelling the Impact and Cost of Male Circumcision for HIV Prevention Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modeling contribute to informed decision making? PLoS Med. 2009;6:e1000109. doi: 10.1371/journal.pmed.1000109. doi:10.1371/journal.pmed.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Njeuhmeli E, Forsythe S, Reed J, et al. VMMC: modeling the impact and cost of expanding male circumcision for HIV prevention in eastern and southern Africa. PLoS Med. 2011;8:e1001132. doi: 10.1371/journal.pmed.1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. [Accessed February 29, 2012];Mozambique AIDS Indicator Survey 2009. Available at: http://www.measuredhs.com/What-We-Do/Survey-Types/AIS.cfm.
- 40.Ministry of Health (Mozambique), National Statistical Institute (INE) (Mozambique), and ICF Macro . Mozambique AIDS Indicator Survey 2009. ICF Macro; Calverton, USA: [Accessed February 29, 2012]. 2010. Available at: http://www.measuredhs.com/pubs/pdf/SR179/SR179.pdf. [Google Scholar]
- 41.Institut National de la Statistique du Rwanda (INSR) and ORC Macro . Rwanda Demographic and Health Survey 2005. INSR and ORC Macro; Calverton, Maryland, U.S.A: [Accessed February 29, 2012]. 2006. Available at: http://www.measuredhs.com/pubs/pdf/FR183/00FrontMatter00.pdf. [Google Scholar]
- 42.Central Statistical Office (CSO) [Swaziland], and Macro International Inc . Swaziland Demographic and Health Survey 2006–07. Central Statistical Office and Macro International Inc.; Mbabane, Swaziland: [Accessed February 29, 2012]. 2008. Available at: http://www.measuredhs.com/pubs/pdf/FR202/FR202.pdf. [Google Scholar]
- 43.Ministry of Health (MOH) [Uganda] and Macro International Inc . Uganda Atlas of HIV/AIDS Indicators 2004–05. Ministry of Health and Macro International Inc; Calverton, Maryland, USA: [Accessed February 29, 2012]. 2007. 2007. Available at: http://www.measuredhs.com/pubs/pdf/ATR5/GS6.pdf. [Google Scholar]
- 44.Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia, and Macro International Inc . Zambia Demographic and Health Survey 2007. CSO and Macro International Inc; Calverton, Maryland, USA: [Accessed February 29, 2012]. 2009. Available at: http://www.measuredhs.com/pubs/pdf/FR211/FR211[revised-05-12-2009].pdf. [Google Scholar]
- 45.Central Statistical Office (CSO) [Zimbabwe] and Macro International Inc . Zimbabwe Demographic and Health Survey 2005–06. CSO and Macro International Inc; Calverton, Maryland: [Accessed February 29, 2012]. 2007. Available at: http://www.measuredhs.com/pubs/pdf/FR186/FR186.pdf. [Google Scholar]
- 46.Cohen M, Chen Y, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattson C, Campbell R, Bailey R, et al. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS one. 2008;3:e2443. doi: 10.1371/journal.pone.0002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegfriend N, Muller M, Deeks JJ, et al. Male circumcision for the prevention of the heterosexual acquisition of HIV in men (review) Cochrane Database Syst Rev. 2009;(2):CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barone M, Ndede N, Li P, et al. The Shang Ring device for adult male circumcision: a proof of concept study in Kenya. J Acquir Immune Defic Syndr. 2011;57:E7–E12. doi: 10.1097/QAI.0b013e3182158967. [DOI] [PubMed] [Google Scholar]
- 50.Awori Q. Randomized controlled trial of the Shang Ring versus conventional surgical techniques for adult male circumcision in Kenya and Zambia; Presented at: 16th International Conference on AIDS and STDs in Africa; Addis Ababa, Ethiopia. December 2011. [Google Scholar]
- 51.Bitega JP, Ngeruka M, Hategekimana T, et al. Safety and efficacy of the PrePex device for rapid scale-up of male circumcision for HIV prevention in resource-limited settings. J Acquir Immune Defic Syndr. 2011;58:127–134. doi: 10.1097/QAI.0b013e3182354e65. [DOI] [PubMed] [Google Scholar]
- 52.Mutabazi V. One arm, open label, prospective, cohort field study to assess the safety and efficacy of the PrePex device for scale up of nonsurgical circumcision when performed by nurses in resource limited settings for HIV prevention; Presented at: 16th International Conference on AIDS and STDs in Africa; Ethiopia. December 2011; [DOI] [PubMed] [Google Scholar]
- 53.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed April 5, 2012];AIDS at 30: Nations at the crossroads. 2011 Available at: http://www.unaids.org/unaids_resources/aidsat30/aids-at-30.pdf.
- 54.Kenya Ministry of Public Health and Sanitation . Kenya National Strategy for Voluntary Medical Male Circumcision. Kenya Ministry of Public Health and Sanitation; Nairobi, Kenya: 2009. [Google Scholar]