Abstract
Mammals possess a remarkable ability to maintain and defend a constant internal milieu against diverse environmental threats. Unsurprisingly, the two systems tasked with these duties, metabolism and immunity, have evolved to share a common modular architecture that allows extensive bidirectional communication and coordination. Indeed, recent observations have highlighted numerous, functionally critical immune regulatory modules located within diverse metabolic circuits. In this Review, we discuss the architectural commonality between immunity and metabolism, and highlight how these two primordially disparate systems leverage shared regulatory axes to coordinate metabolic physiology under conditions of normality and chronic overnutrition. Such an integrated perspective both advances our understanding of basic physiology and highlights potential opportunities for therapeutic intervention in metabolic dysfunction.
Introduction
Life in the inconstant world is fraught with challenges. Reproduction, growth, and even simple homeostasis all demand reliable and uninterrupted maintenance, while the environment around us is in constant, unpredictable flux. Body temperature, for example, must be maintained within narrow limits, while ambient temperatures vary wildly. Nutrients must be maintained constant and in appropriate proportion, while food availability (to say nothing of composition) can unpredictably vary from feast to famine. Survival is predicated on the maintenance of a stable, Goldilocks-like internal milieu buffered from an inhospitable external environment. Mammals, however, are not unique in our preference for stable, nutrient-laden climes; the world around us teems with life—bacteria, viruses, fungi, and even other metazoans—all with similar preferences and many poised to invade and coopt our resources for their own priorities. Indeed, the disproportionate evolutionary success enjoyed by mammals is attributable largely to their ability to successfully maintain and defend this particular internal milieu (Wilson et al., 2012).
Shaped by millennia under these concurrent pressures, it is unsurprising that evolution has reached for common solutions when confronting these disparate necessities. Indeed, the systems tasked with maintaining and defending the internal environment, such as metabolism and immunity, organize responses along similar, modular lines, allowing our relatively limited genetic diversity to match the far greater diversity of unique environmental situations (Boehm, 2012). Moreover, these responses both match short-term environmental fluctuations as well as integrate them over time to detect and adapt to long-term patterns (e.g. changing seasons or recurring pathogens) (Humphries et al., 2003). Importantly, these commonalities of architecture (both employ the same modular architecture), challenges (both confront ever-varying threats), and biological goals (both strive to preserve the internal milieu) weld together these responses into the cooperative, collaborative, and coherent physiology necessary for complex life.
In this Review, we discuss the general architecture of canonical immune and metabolic responses with special emphasis on the modular organization of individual regulatory circuits. These same architectural principles apply to immune-mediated metabolic control, and allow us to understand how these circuits function and interact with more traditional regulatory modalities to preserve short-term stability and adapt to long-term environmental changes. Such a conceptual framework provides the context in which to systematically organize the existing knowledge of immune metabolic control, infer as yet undescribed regulatory components, and better target potential therapeutic interventions.
Architectural principles of immunity
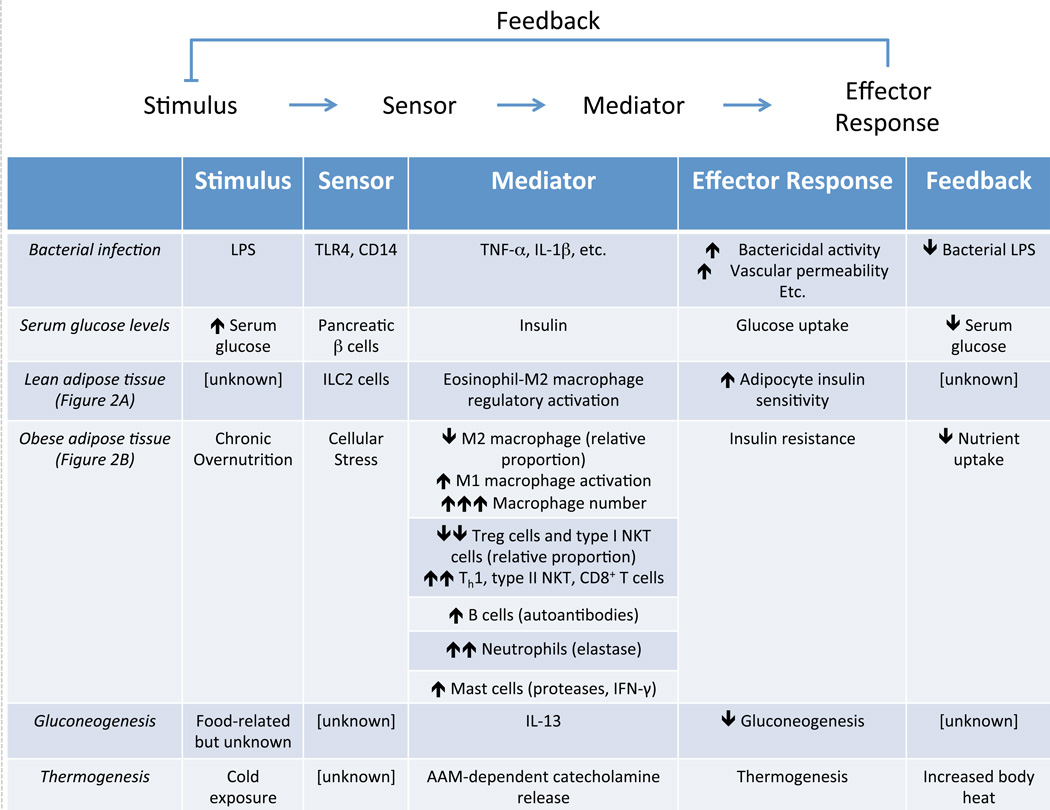
The mammalian immune system is remarkably complex; however, each individual function—and indeed the system as a whole—is patterned on the same, highly stereotyped modular architecture (Figure 1). In each, discrete component modules operate in directional series to transform specific inputs into reproducible outputs. While this organization is common to most biological systems, immune responses follow a further stereotyped organization, most often cued to primordial host defense functions and purposed to defend a static baseline of sterility (Medzhitov, 2008). As such, system inputs (stimuli) are generally indicators of a break in that sterility (such as pathogen-derived molecules) or at least the potential there for (self-derived danger signals such as necrotic cell debris, for example). Such stimuli activate specific sensor modules (e.g. Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, RIG-I-like receptors (RLRs), etc.), triggering a response (Elinav et al., 2011; Kawai and Akira, 2011; Lamkanfi and Dixit, 2012). The exact character of this initial response is varied (kinase activation, oligomerization, peptide release, etc.); however, all result in the transduction of the incident stimulus into a transmissible mediator (typified by cytokines and chemokines, but also including diffusible small molecules like prostaglandins and leukotrienes as well as intracellular mediators like calcium ions, kinases, or membrane depolarization) capable of downstream action. This mediator may act locally or distantly, on one target or many; however, irrespective of specific mechanism, it activates a downstream module to mount an effector response. In contrast to the mediator response initiated by the sensor, the direct purpose of the effector response is elimination of the incident stimulus and the return to the baseline condition of sterility.
Figure 1.
Immunity and metabolism share a common modular architecture. In most biological systems, sensor modules transduce stimuli into downstream mediators that, in turn, activate effector responses, which generally feed back to eliminate the incident stimulus and return the system to its baseline condition. Despite their disparate primordial functions, canonical host defense and metabolic circuits are both organized in this manner. Importantly, this shared architecture allows immunity to regulate metabolic processes, such as occurs in lean and obese adipose tissue. Such an architectural viewpoint allows unknown but putative functional modules to be predicted, such as in the regulation of hepatic gluconeogenesis and thermogenesis.
The simplest and most familiar examples of this organization are direct pathogen clearance responses, as initially proposed by Ruslan Medzhitov (Medzhitov, 2008). For example, recognition of bacteria-derived lipopolysaccharide (LPS, stimulus) by toll-like receptor (TLR)-4 (sensor) on a macrophage triggers activation of inhibitor of κB kinase (IKK), mitogen-activated protein kinase (MAPK), and interferon response factor 3 (IRF3) pathways (mediator) that results in increased bactericidal activity (effector response), thus enabling the cell to eliminate the LPS- producing bacteria (original stimulus, Figure 1). While this discussion treats component modules as singular entities—a single protein, molecule, or chemical reaction—they comprise several to many individual operations in most biological systems; for example, bacterial lysis, while a single function, is a complex, multistep process (Kawai and Akira, 2011). Similarly, feedback mechanisms often comprise entire regulatory circuits in and of themselves.
Such a modular architecture has profound functional implications. First, the use of discrete, independently functional modules allows a great diversity of processes to be constructed from a relatively small number of components through combinatorial variation. For example, a module that senses LPS may be used to initiate a bactericidal effector response, as above, or it may be used to drive production of cytokines, antigen presentation, or co-stimulation by coupling to different downstream mediator or effector response modules (Medzhitov et al., 1997). Furthermore, the same effector response may be placed downstream of other sensor modules to create systems that react in the same way to distinct stimuli (such as bactericidal responses to TLR ligands other than LPS) (Kawai and Akira, 2011). Importantly, by deploying the same modules in multiple distinct pathways (combinatorial diversity), modular organization allows responses to be coordinated across multiple different systems, enabling complex immunologic programs to be mounted and coordinated using a relatively limited number of functional components. As we saw above, TLR-4 activation by a single pro-inflammatory stimulus, LPS, activates a plethora of coordinated downstream responses to facilitate clearance of the offending bacterium (Medzhitov, 2008). Many of these are direct effector functions (such as increased bactericidal activity in phagocytes); however, others represent similarly organized but distinct circuits nested within the larger response (e.g. cytokine mediation of paracrine activation, chemokine recruitment of additional effector leukocytes, and increased vascular permeability to allow such an influx). As such, the presence of shared modules enables disparate processes to be coordinated into a coherent, effective whole.
Immunity and metabolism
Modular organization is familiar to any immunologist—as we saw above, the most well studied host defense responses are organized in this manner—however, these principles govern not only immunity but other biological processes as well. Indeed, evolution has shaped mammals’ myriad regulatory networks along strikingly similar lines, even to the extent of utilizing many of the same functional modules across systems with disparate primordial functions. Importantly, as modules shared within systems coordinate system-wide responses, modules shared between “unrelated” systems represent key inter-system communication nodes, cohering and coordinating all aspects of physiology into functioning organisms. This interconnectivity is perhaps best exemplified by the still-evolving intersection between immunity and metabolism. Over the past twenty years, common immune regulatory modules have been located in many metabolic pathways, mediating an immune axis of metabolic control operating in concert with the more canonical neuroendocrine, hormonal, and neural pathways (Hotamisligil, 2006; Odegaard and Chawla, 2013; Qatanani and Lazar, 2007; Samuel and Shulman, 2012). Moreover, common metabolic regulatory modules have been reported in immune pathways, similarly establishing metabolic influence over immune function (see the accompanying Review by Pearce et. al.). For example, diffusible and intracellular inflammatory mediators (typified by tumor necrosis factor (TNF)-α or IL-1β, and IKK or c-Jun BN-terminal kinases (JNKs), respectively) have now been shown to directly inhibit insulin signaling, while direct nutrient recognition by immune sensor modules (e.g. TLR-4 and peroxisome proliferator-activated receptor (PPAR)-δ ligation by saturated and poly-unsaturated fatty acids, respectively) influences immune activation (Odegaard et al., 2008; Pal et al., 2012). Indeed, despite the putative separation in their primordial functions, immunity and metabolism appear to be inextricably linked and coordinated by common regulatory axes. In the sections that follow, we present examples of how immunity participates in short term metabolic responses and integrates environmental inputs over time to effect long term metabolic adaptation in tissues.
White adipose tissue
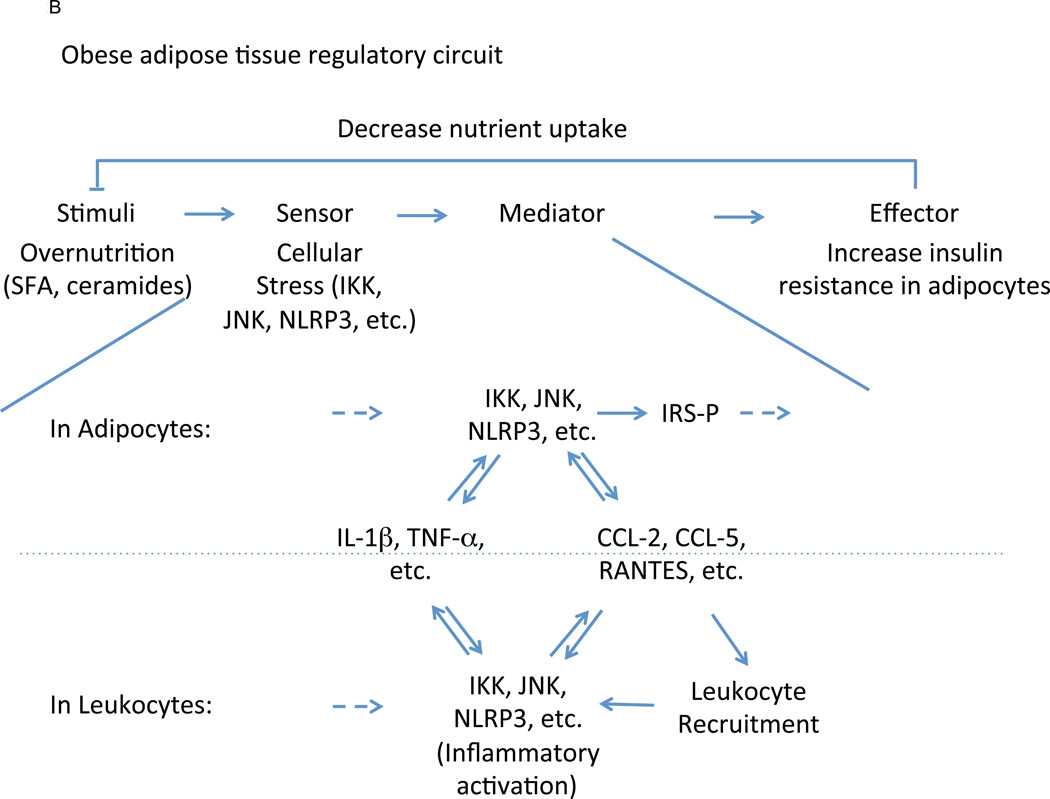
Nowhere is immunity’s metabolic influence better understood than in adipose tissue. As the primary site of mammalian energy storage, the core physiologic function of white adipose tissue is nutrient storage and release, both of which are orchestrated through the insulin signaling axis (Text Box and Figure 3) (Rosen and Spiegelman, 2006). In architectural terms, changes in serum glucose concentration (stimulus) are sensed by the pancreatic islet (sensor), where they are transduced to changes in insulin secretion (mediator) (Figure 1). In the adipose tissue, insulin drives uptake and storage of glucose and fatty acids, and inhibits lipolysis and fatty acid release (Rask-Madsen and Kahn, 2012). Overnutrition, however, delivers nutrients in quantities beyond the capacity of adipocytes to safely dispose, leading to varied manifestations of cellular stress and dysfunction, including dysregulated lipid balance (such as intracellular accumulation of diacylglycerols, saturated fatty acids, and ceramides), mitochondrial dysfunction, oxidative stress, and endoplasmic reticulum stress (Hotamisligil, 2010; Qatanani and Lazar, 2007). In turn, these diverse processes, acting as surrogates for nutrient excess, activate a host of intracellular signaling cascades that sense and communicate adipocyte nutrient overload through activation of JNK, IKK, and protein kinase R (PKR) signaling pathways (Hirosumi et al., 2002; Nakamura et al., 2010; Yuan et al., 2001). These signaling pathways, in turn, directly antagonize the insulin signaling pathway, primarily through inhibitory serine phosphorylation of insulin receptor substrate (IRS) proteins (Aguirre et al., 2002; Hotamisligil et al., 1996). While this effect acutely inhibits further nutrient uptake in an adipocyte-autonomous manner, JNK and IKK activation also initiate a paracrine inflammatory response involving cytokines such as TNF-α and IL-1β that broadcast intracellular conditions to other adipocytes and, importantly, resident leukocytes in the immediate microenvironment (Odegaard and Chawla, 2013). Chronic activation of this response results in striking remodeling of adipose tissue-associated leukocyte populations with concomitant effects on systemic metabolism and immune homeostasis.
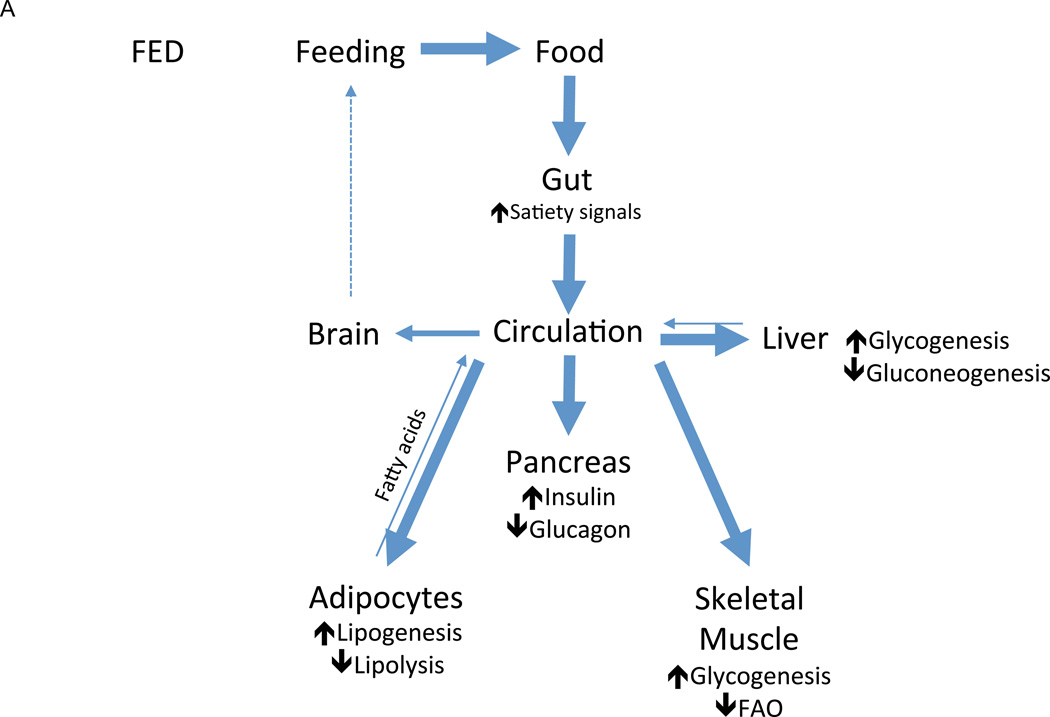
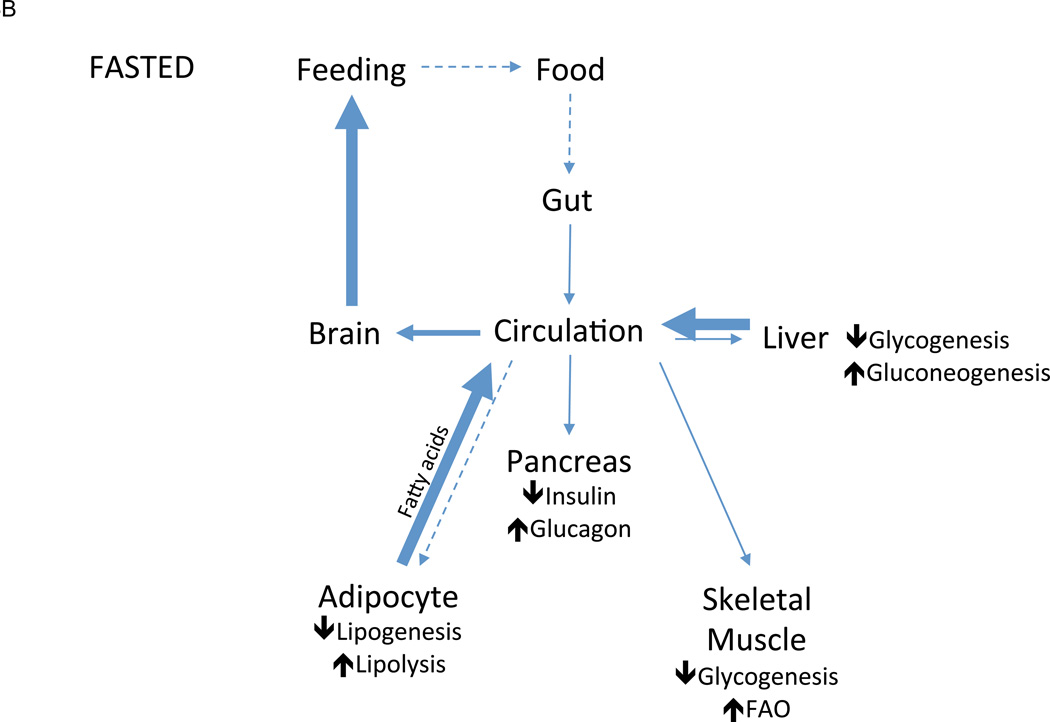
Figure 3.
Nutrient handling in the fed and fasted states. In fed mammals (A), the presence of nutrients in the gut lumen (stimulus) triggers the production of satiety signals (mediator) that exert anorectic influences on the brain to decrease feeding (effector response), thus limiting further nutrient intake (feedback). As circulating nutrient concentrations rise (stimulus), anabolic mediators such as insulin are released, which simultaneously drives nutrient uptake and storage in peripheral tissues such as skeletal muscle, adipose tissue, and liver and inhibits their release of stored nutrients. The net result of this effector response is the decrease in circulating nutrient concentrations, which diminishes the incident stimulus for nutrient storage (feedback). In fasted mammals (B), nutrient levels fall below baseline (stimulus), triggering catabolic mediators such as glucagon that drive mobilization of stored nutrients into circulation while inhibiting their uptake (effector response), replenishing circulating nutrient concentrations and decreasing the incident stimulus (feedback). These catabolic mediators also drive a parallel effector response of increased hunger and, thus, feeding behavior, which eventually similarly increases the circulating concentration of nutrients (feedback).
Leukocyte influence over short term metabolic changes in the adipose tissue is largely unclear—adipocyte-autonomous mechanisms seem dominant in this context—however, as discussed below, the collective data suggest that over longer time frames, the immune microenvironment integrates environmental inputs and accordingly adapts the metabolic baseline to which more conventional metabolic responses are calibrated by varying the number and phenotype of adipose tissue leukocytes (Medzhitov, 2008; Odegaard and Chawla, 2012). Under long-term conditions of healthy nutrient provision, for example, the immune microenvironment adopts a regulatory phenotype that actively maintains high adipocyte insulin sensitivity. Chronic overnutrition, however, results in persistent cellular stress to which the immune system responds by increasing the number and inflammatory timbre of adipose tissue leukocytes to create a new metabolic set point characterized by smoldering inflammation and insulin resistance (Medzhitov, 2008; Odegaard and Chawla, 2012). Importantly, once established, this new set point represents the new baseline metabolic condition both within the adipose tissue and systemically, and is defended by short-term metabolic regulatory circuits even in the absence of continued overnutrition, providing an immunological basis for obesity’s tenacious resistance to weight loss.
Macrophages
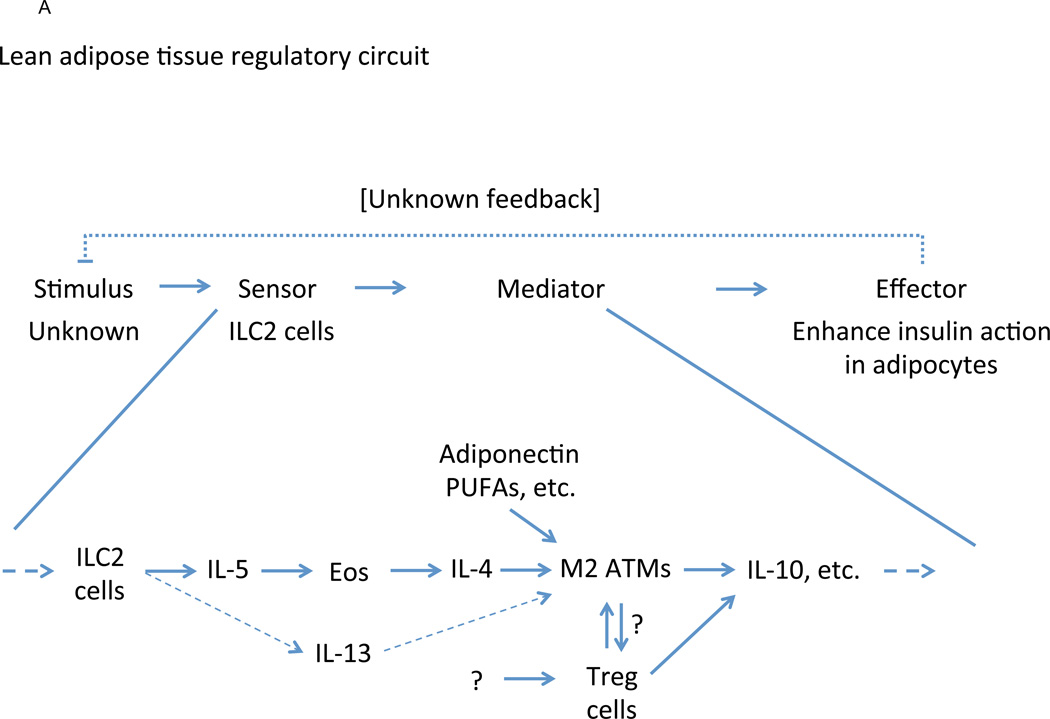
As the most populous resident leukocyte in adipose tissue, the macrophage is a central lineage through which overnutrition-associated inflammatory signals act (Figure 1). In lean adipose tissue, macrophages comprise ~10% of total cellularity and demonstrate an immunoregulatory phenotype (CD206+ CD301+ Arg1+ IL10+) in response to tonic production of IL-4 by resident eosinophils and, to a lesser degree, IL-13 by resident type 2 innate lymphoid cells (ILC2) (Lumeng et al., 2007; Molofsky et al., 2013; Odegaard et al., 2007; Weisberg et al., 2003; Wu et al., 2011) (Figure 2A). While the incident stimulus and sensor for this circuit is unknown, these macrophages actively promote adipocyte insulin sensitivity (effector response), most likely through the production of IL-10 and other mediators (Kang et al., 2008; Lumeng et al., 2007; Odegaard et al., 2007). Chronic overnutrition, however, stimulates intracellular stress programs (sensor) within the adipocyte, as well as activating similar pathways within macrophages (Figure 2B). In these cells, high concentrations of saturated fatty acids are sensed directly by TLR-4 using the bridging molecule fetuin-A (Pal et al., 2012) and, via conversion to ceramides, indirectly through activation of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (Nlrp3) inflammasome, a central nexus for IL-1β-mediated inflammation (Stienstra et al., 2010; Vandanmagsar et al., 2011; Wen et al., 2011). These phlogistic effector responses activate JNK and IKK pathways within adipocytes to inhibit the insulin-sensitizing activity of alternatively activated macrophages and skew the regulatory timbre of the macrophage population within the adipose tissue. Importantly, these mediators include potent chemotactants, such as chemokine C-C motif ligand (CCL)-2, CCL-5, and CCL-8, that recruit large numbers of Ly6cHi monocytes, which differentiate into tissue macrophages with a moderately inflammatory phenotype (CD11c+ NOS2+ TNF-α+) (Inouye et al., 2007; Kamei et al., 2006; Kanda et al., 2006; Weisberg et al., 2006). While the inflammatory activity of these newly-recruited cells is substantially less than that observed in canonical macrophage host defense responses (Hotamisligil, 2006; Medzhitov, 2008), their large number allows even their somewhat tepid inflammatory influence to overwhelm the pre-existing regulatory macrophage population, skewing the immune microenvironment and driving macrophage representation to as high as 50% of all non-adipocytes in obese adipose tissue (Weisberg et al., 2003; Xu et al., 2003). Importantly, abrogation of either the recruitment or inflammatory activation of macrophages blunts overnutrition-associated dysfunction both in the adipose tissue and systemically, whereas contrapositive interventions (such as impairment of alternative macrophage activation or eosinophil depletion) have the opposite effect (Odegaard and Chawla, 2013).
Figure 2.
Immune regulation of adipose tissue function. In lean adipose tissue (A), ILC2-derived IL-5 supports the accumulation and maintenance of eosinophils, which alternatively activate resident macrophages via IL-4 (also supported, in part, by ILC2-derived IL-13 and dietary factors such as PUFA). In conjunction with regulatory T cells, alternatively activated macrophages produce IL-10 and other mediators that directly promote adipocyte insulin sensitivity. While ILC2 cells likely occupy the sensor role in this context, the stimulus and how it is sensed is unclear as are the factors controlling regulatory T cell accumulation. Under conditions of chronic overnutrition (B), however, supra-physiologic nutrient concentrations activate intracellular stress signaling pathways with two primary effects. First, these pathways directly inhibit insulin signaling in adipocytes, thus reducing uptake of additional nutrients into already-stressed cells. Second, these pathways trigger moderate inflammatory activation and accumulation of tissue-associated leukocytes that reinforce the inflammatory microenvironment, indirectly further blunting insulin action. Both mediator paths lead to an end effector response of decreased adipocyte nutrient uptake, thus reducing intracellular nutrient concentrations and decreasing the incident stimulus of nutrient overload. Abbreviations: Eos – eosinophils, ATM – adipose tissue macrophages, SFA – saturated fatty acids, IRS – insulin receptor substrates.
Mast cells
Similar to macrophages, mast cells are also found in lean adipose tissue and support the inflammatory transition associated with chronic overnutrition (Figure 1), though the relative significance of their contribution is debated. While studies using KitW-sh/W-sh and KitWv/W animals are complicated by systemic multilineage immune derangements, mast cell repletion (“add-back”) models implicate mast cell-derived IL-6 and interferon-γ (IFN-γ) in obesity-related metabolic dysfunction (Liu et al., 2009). Congruently, mast cell-stabilizing therapeutics already in clinical use (e.g. chromolyn and ketotifen) improve metabolic function and promote weight loss in rodent models of diet-induced obesity (Liu et al., 2009).
T cells
While macrophages form the numeric and functional bulk of adipose tissue leukocytes, T lymphocytes play crucial roles in regulating macrophage and adipocyte phenotypes alike. T helper-2 (Th2), type I (or invariant) natural killer (iNKT) (Ji et al., 2012; Lynch et al., 2012; Schipper et al., 2012), and regulatory T cells are found in substantial numbers in lean adipose tissue and participate in maintaining the tolerogenic immune microenvironment through production of type 2 cytokines, such as IL-4 and IL-10 (Feuerer et al., 2009) (Figure 1 and Figure 2A). Indeed, RAG1-null mice (which lack T cells) demonstrate impaired insulin signaling in adipocytes and increased susceptibility to weight gain relative to wild-type controls, a phenotype that is rescued by either Th2 or regulatory T cell repletion (though not by CD8+ or CD4+ Th1 T cells) (Winer et al., 2009), whereas depletion of regulatory T cells impairs adipocyte insulin signaling in otherwise normal lean animals (Feuerer et al., 2009). While the mechanism is not currently known, this effect is possibly mediated by participation in the ILC2-eosinophil-macrophage regulatory immune axis described above (Figure 2A). With chronic overnutrition, however, Th2–iNKT–regulatory T cell dominance is diluted by large numbers of CD8+, type II NKT (Nishimura et al., 2009; Satoh et al., 2012), and Th1 T cells recruited by adipocyte- and macrophage-derived chemokines (most notably CCL20, RANTES, and CXCL12), skewing the overall T cell activation bias towards inflammatory responses that synergize with those of the newly-recruited macrophages (Winer et al., 2009) (Figure 1). Aside from their influence on the inflammatory timbre of the microenvironment, T cells may also influence adipocytes directly, possibly through the CD40-CD40L pathways, for example (Poggi et al., 2011). Additionally, a recent report suggests that adipocytes themselves are important antigen-presenting cells that influence T cell activation in obesity; however, the specific contribution of this pathway will require additional verification using adipocyte-specific disruption of antigen presentation (Deng et al., 2013). Importantly, most adipose tissue T cells, regardless of their specific lineage, demonstrate distinct and limited T cell receptor repertoires relative to their circulating counterparts, suggesting antigen-specific selection (though the restriction mechanisms and cognate antigens are unknown).
B cells
While B-cells (especially B2-cells) are found in lean adipose tissue (Winer et al., 2011), no role for this lineage in normal tissue homeostasis has yet been identified. In obese adipose tissue, however, B-cells increase in number, adopt an activated phenotype, and demonstrate immunoglobulin repertoire restriction similar to that seen in the associated T cell receptor pool, though here at least some of their cognate antigens are clearly defined by the presence of pathogenic autoantibodies to intracellular antigens such as Brutons kinase (BTK), glial fibrillary acidic protein (GFAP), and golgi SNAP receptor complex member 1 (GSOR1) (Winer et al., 2011). Aside from their role in autoantibody production, B-cells in obese adipose tissue promote CD8+ T cell responses, possibly through antigen presentation, exacerbating metabolic dysfunction in obesity (Figure 1). [If I understand correctly, while macrophages are beneficial to resetting the metabolic checkpoint in adipocyte overnutrition, B cells actually exacerbate the problem?]
Neutrophils
The same chemoattractant storm that recruits additional macrophages and lymphocytes also attracts other leukocytes that normally eschew adipose tissue. Neutrophils, for example, are largely absent from lean adipose tissue; however, they rapidly infiltrate adipose tissue under conditions of nutrient excess and have been reported to persist for months (Figure 1). Importantly, these neutrophils secrete elastase, a protease capable of both blunting insulin signaling through IRS-1 degradation and augmenting the inflammatory response through TLR4 signaling pathways (Talukdar et al., 2012). Basophils, NK cells, and other innate leukocytes have similarly been reported to contribute to adipose tissue dysfunction in obesity; however, the data for these lineages is scant and often controversial.
Liver
While white adipose tissue serves as the primary long-term energy repository in the body, the liver serves as the primary short-term energy handling depot. As in the adipose tissue, these functions are largely coordinated by the stimulus, sensor, and mediator modules of the insulin signaling axis; however, the effector functions of the liver comprise the synthesis of both lipids and glucose (gluconeogenesis), their short term storage (glycogen), and release (Figure 3A and B) (Lin and Accili, 2011). While a great diversity of leukocytes are found within the liver, relatively little is known of their contributions to these routine hepatic functions. Despite this lack of knowledge, multiple observations suggest a critical role for immune regulation of routine hepatic function. The most convincing example of such observations involves the liver’s resident macrophage population (Kupffer cells). These cells comprise ~10% of the liver’s cellularity and demonstrate an immunoregulatory phenotype similar to that observed in the adipose tissue. While these cells are critical in host defense and xenobiotic metabolism, depletion of this lineage in healthy animals also disrupts hepatic function in the absence of any toxic, infectious, or dietary challenge, with specific impairments in hepatocyte insulin sensitivity and lipid metabolism (Huang et al., 2010; Papackova et al., 2012). Importantly, this phenotype can be partially recapitulated through the selective ablation of Kupffer cell alternative activation via macrophage-specific PPARδ deficiency (Kang et al., 2008; Odegaard et al., 2008). Taken together, these data suggest that alternatively activated Kupffer cells actively promote insulin sensitivity in the hepatocyte; however, as in the adipose tissue, the mechanisms by which this effect is exerted are unclear (though IL-10 is an attractive candidate). Moreover, unlike the adipose tissue, the upstream factors regulating tonic macrophage activation have not been defined.
Whereas these data implicate a specific lineage (alternatively activated macrophages) with unclear mediators, other data implicate specific mediators but remain unclear regarding specific lineages, sensors, stimuli, or other components of the metabolic circuit (Figure 1). For example, a recent report has implicated IL-13 in the hepatic response to feeding (Stanya et al., 2013). In the healthy liver, food intake is accompanied by decreases in hepatic gluconeogenesis as systemic metabolism shifts from catabolism to anabolism (Lin and Accili, 2011). This inhibition had been thought to rely entirely on insulin (Figure 3A, B); however, Stanya et al. describe an immune-mediated regulatory loop in which IL-13 (thought to be derived from NKT cells) suppresses the hepatocyte gluconeogenic program in response to food intake. Interestingly, IL-13 is reported to mediate this effect by signaling through STAT3 in a non-canonical signal transduction pathway (Stanya et al., 2013). Despite this intriguing mechanism, key components of this metabolic circuit—including the exact stimulus, sensor, and cell of origin—are yet unknown, highlighting the spotty nature of current knowledge of immune contributions to healthy liver function.
White adipose tissue’s striking hypertrophy and profound functional changes have justifiably made it the most well studied manifestation of obesity-associated metabolic dysfunction; however, the liver, as the central glucose and lipid handling tissue, is acutely susceptible to nutrient toxicity (i.e. metabolic substrate overload) as well. As in the adipose tissue, nutrient excess in the liver leads to hepatocyte stress and activation of similar cell autonomous signaling modules shared between metabolic and immune pathways (primarily IKK and JNK), which in the short term directly antagonize insulin signaling to prevent acute nutrient toxicity (Arkan et al., 2005; Cai et al., 2005; Hirosumi et al., 2002). Unlike adipose tissue, however, long-term activation of these responses in the liver results in increased glucose production rather than decreased glucose uptake (though insulin resistance at both sites increases fatty acid release). This effect, in turn, augments the accompanying peripheral insulin resistance to raise serum concentrations of glucose.
While the cell autonomous responses to nutrient excess are similar in both liver and fat, the liver-specific effects on resident leukocyte populations are much less well described. In both tissues, there is a general shift in the immune microenvironment from a regulatory bias to an inflammatory one; however, the dramatic changes in population size typical in the adipose tissue are not generally seen, perhaps because of the liver’s more rigid tissue architecture. The tissue macrophage population, for example, swaps an alternatively activated phenotype for a more inflammatory bent (CD11c+ NOS2+ TNFα+); however, unlike in the fat, this occurs without a significant increase in cell number (specifically, the most detailed data suggest a small decrease in resident macrophages that is offset by newly recruited cells) (Obstfeld et al., 2010). While the result is similar—inflammation antagonizes parenchymal insulin signaling both directly and via inhibition of macrophage alternative activation programs—the mechanism appears to involve either the phenotypic conversion of individual cells or their replacement, neither of which has been described in the adipose tissue or liver. Importantly, this inflammatory switch in macrophage phenotype is mechanistically crucial despite the absence of marked numerical changes as either genetic abrogation of macrophage inflammatory signaling (Arkan et al., 2005) or wholesale lineage depletion dramatically reduces the overnutrition-associated phenotype (Huang et al., 2010; Papackova et al., 2012). Other liver resident leukocyte populations undergo similar, isometric inflammatory activation in obesity that, to varying degrees, supports metabolic dysfunction; however, whether this is due to primary, liver-specific processes or to systemic inflammation is unclear. As in the adipose tissue, neutrophils are not known to contribute to the resident leukocyte population of healthy liver; however, they are recruited to chronically overfed livers where they contribute to metabolic dysfunction through TLR4 signaling augmentation and direct degradation of IRS-1 within hepatocytes (Talukdar et al., 2012).
As in the adipose tissue, liver-specific immune responses integrate long-term environmental conditions and accordingly adjust the metabolic baseline state, promoting insulin sensitivity under conditions of metabolic normalcy. The immune response to chronic overnutrition, in contrast, alters the leukocyte landscape to promote insulin resistance, stemming the nutrient influx into already-stressed hepatocytes. This new metabolic set point is furthermore stable, protected by short-term metabolic regulatory circuits as the new homeostatic baseline.
Brown adipose tissue
While the majority of the work on immunity-metabolism interactions has focused on glucose homeostasis (and thus on white adipose tissue, liver, and skeletal muscle), temperature regulation is another important area in which immune metabolic regulation has been shown to play a key role. In mammals, thermoregulation is comprised of both heat loss-reducing (such as piloerection and cold aversion behavior) and heat-generating strategies of which non-shivering thermogenesis is the most important (Cannon and Nedergaard, 2011; Lowell and Spiegelman, 2000; Tseng et al., 2010). In this response, cold exposure (stimulus) triggers an increase in sympathetic nervous system tone (mediator), which instructs brown adipocytes to dramatically increase uncoupled fatty acid oxidation, liberating large amounts of heat (effector response). Until recently, this response was thought to be mediated entirely by direct sympathetic innervation of brown adipocytes; however, recent work demonstrated an unexpected requirement for alternatively activated adipose tissue macrophages. Interestingly, these cells not only form a critical component of the sympathetic efferent limb, accounting for ~50% of secreted catecholamines, but also comprise a second, integrated metabolic circuit in which the fatty acids necessary to fuel thermogenesis are mobilized from the adjacent white adipose tissue (Nguyen et al., 2011). Congruently, the acute thermogenic response is markedly blunted in both amplitude and duration in animals with impaired macrophage IL-4 and IL-13 signaling. Despite their common requirement for macrophage alternative activation, however, cold exposure and the basal nutrient handling cues discussed above activate distinct effector modules within these cells. IL-10, for example, has not been implicated in the thermogenic response, while catecholamine production is similarly uninvolved in the maintenance of adipocyte insulin sensitivity.
In parallel with its role in short-term regulation of body temperature, the macrophage-adrenergic axis may also participate in metabolic adaptation to long-term temperature changes. Long-standing observations have demonstrated that chronic exposure to cold or β3-activating adrenergic agents results in increased brown adipose tissue mass both through hyperplasia of existing brown adipocytes and acquisition of thermogenic capacity (“browning”) by existing white adipocytes (Frontini and Cinti, 2010; Lee et al., 2012). From an architectural perspective, mediators derived from short-term response activation are ideally positioned to further mediate these long-term adaptations as well. For example, macrophage-dependent adrenergic signaling might activate long-term adaptations in white adipose tissue in addition to short-term thermogenic responses, possibly via fibroblast growth factor (FGF)-21 signaling, which was recently implicated in cold-induced browning of white adipocytes (Fisher et al., 2012).
While parkas and central heating may have decreased thermogenesis’s primeval evolutionary imperative, the structure and regulation of thermogenic responses have never been more hotly pursued due to their therapeutic potential in the current obesity epidemic (Tseng et al., 2010). In this context, brown adipose tissue, with its remarkable capacity to burn fat, is seen by many as an ideal counter to overnutrition—turn it on, and the excess calories will simply burn away. While at this point only speculative, a great deal of evidence supports this approach, including the therapeutic success of transplantation, pharmacologic activation, and genetic augmentation of brown adipose tissue in mice (Wu et al., 2013). Importantly, however, the dependence of thermogenesis on IL-4 signaling and alternative macrophage activation raises important questions about how it is affected by obesity’s inflammatory influence, especially in the context of the anatomic and functional overlap between brown and white adipose tissues.
Central metabolic regulation in the hypothalamus
Metabolic regulation, as seen in the examples above, is often as much the adaptation to behavioral variation as to environmental. Bridging this divide, central metabolic regulation is an intriguing aspect of metabolic study in which researchers have begun to describe immune networks with more long-lasting implications for central metabolism, most notably in the context of energy balance and feeding behavior. Indeed, despite strikingly divergent phenotypes, cachexia (a wasting syndrome associated with chronic inflammatory conditions such as cancer) and obesity-related metabolic disease have now both been causally linked to activation of the same inflammatory signaling pathways in the hypothalamus. In cachexia, chronic production of proinflammatory mediators, such as TNF-α and IL-1β, activate JNK and IKK signaling in pro-opiomelanocortin (POMC)-expressing neurons in the medial basal hypothalamus, triggering the classic pairing of anorexia and increased energy expenditure as an effector response (Cai and Liu, 2011). While the long term consequence of this new metabolic baseline (wasting) is undoubtedly maladaptive, decreased appetite may function adaptively in the short term to decrease further exposure to ingested pathogens, whereas increased basal metabolic rate may augment host defense responses (Medzhitov, 2008). Interestingly, sustained moderate elevation of the same inflammatory mediators, as occurs during overnutrition, activates the same signaling pathways in both agouti-related peptide- and POMC-expressing arcuate neurons with opposite effects—food intake is increased, while whole-body energy expenditure is decreased (Belgardt et al., 2010; Purkayastha et al., 2011; Sabio et al., 2010; Zhang et al., 2008). As with cachectic response, long-term activation establishes a new, apparently detrimental baseline condition that promotes persistence and exacerbation of metabolic dysfunction. The putative adaptive functions served by short-term activation are unclear, but may include activation of downstream effectors to mitigate cellular stress.
While these processes regulate metabolic responses (basal metabolic rate and appetite) through immune mediators (inflammatory cytokines and signaling pathways), little is known about the contributions of individual leukocyte lineages to this process. Certainly their aggregate effects in the periphery are central; however, the hypothalamus comprises resident and transient leukocyte populations (such as microglia and lymphocytes, respectively) similar to other tissues. The complete relevance of each of these cells is not yet clear; however, IKK-mediated microglial activation, for example, has been implicated in overnutrition-associated neuron and neural stem cell damage (Li et al., 2012).
Conclusion
Immunity and metabolism have co-evolved through millennia with a common architectural organization and common, vital biological goals (Afacan et al., 2012). Given these commonalities and their extended cohabitation, it is unsurprising that immunity and metabolism have become entangled, instructing and coordinating their activities through common regulatory axes such that host defense and metabolic provision are coordinated and mutually maintained. Recognizing this, functional models that integrate immunity and metabolism into a common architectural organization have fundamentally changed our understanding of both disciplines. Similarly, our grasp of immune phenomena as basic as macrophage activation has been reshaped by the inclusion of metabolism (Cramer et al., 2003; Vats et al., 2006). Moreover, the intrinsic regularity of the architectural context itself highlights the gaps remaining in our understanding. While examples of this may be found in the original hypotheses behind many of today’s facts, current models still expose vacancies. For example, functional models in brown adipose tissue explain short-term thermogenic responses and describe similarly directed long-term adaptations; however, the mechanism linking the two remains unclear. Applying architectural observations from other metabolic systems suggests that short-term thermoregulatory responses should connect in some way to long-term mechanisms; however, none have yet been described. Similarly, IL-13, an immune mediator, has been assigned an important role in regulating gluconeogenesis; however, the exact stimulus and sensor modules are unknown.
Despite its importance, the identification of individual regulatory circuit components is only the prelude to applying those functional models to practical, real-world gain. Most poignantly, overnutrition-associated metabolic dysfunction is sweeping both the developed and developing worlds with epidemics of diabetes, cardiovascular disease, cancer, and other end-pathology confederates (Adams et al., 2006; Finucane et al., 2011). Though in their infancy, efforts to understand this pathology in a way that integrates metabolism and immunity have already led to the identification of salicylates (e.g. salsalate), direct cytokine antagonists (e.g. canakinumab, infliximab, entanercept), leukocyte depleting agents (e.g. CD3- and CD20-targetting antibodies), and other immunomodulators (e.g. amlexanox, resolvins) as potential treatments for metabolic disease (Brooks-Worrell et al., 2012; Reilly et al., 2013). Moreover, metabolic agents (e.g. polyunsaturated fatty acids, AMP-activated protein kinase agonists, PPAR agonists, DPP-4 inhibitors) also show promise as novel immunomodulatory interventions (O'Neill and Hardie, 2013).
The recent progress reviewed above establishes immunity as a bona fide metabolic regulatory axis and opens exciting new therapeutic avenues; however, immune influence over many metabolic processes and tissues remains unclear: How does immunity participate in metabolic homeostasis in the pancreatic islet? In the gut? In the muscle? How does it participate in fasting responses, in weight loss, or in times of stress? How are these effects modulated by interaction with traditional and other non-traditional metabolic axes? In each of these cases, evidence exists that suggests immune participation; however, important questions remain outstanding, the answers to which promise to increase our understanding of immunity, metabolism, and their joint dysfunction and bolster our ability to therapeutically intervene there in.
Text Box: Energy homeostasis.
While exceedingly complex in its specifics, whole-body energy homeostasis functions itself as metabolic circuit in which nutrient need (stimulus), sensed as hunger, drives an effector response of feeding behavior to replenish metabolic stores (Figure 3A). The presence of nutrients in the gut lumen triggers short term regulatory feedback (satiety signals) that inhibit acute feeding, while long term patterns in feeding are governed, in part, by leptin, a regulatory mediator produced in proportion to available nutrient stores (adipose tissue). Once ingested, nutrients are apportioned via another metabolic circuit in which rising glucose concentrations (stimulus) trigger the β-cells of the pancreas (sensor) to secrete insulin (mediator), which, in turn, drives glucose uptake and storage in skeletal muscle, liver, and fat (effector response), thus returning glucose concentrations to baseline. With fasting (Figure 3B), glucose concentrations fall below baseline, stimulating glucagon secretion, which mediates a variety of catabolic responses. Skeletal muscle subsists on intracellular stores of glycogen, while liver mobilizes its own stores via glycogenolysis—supplemented by newly synthesized glucose derived from gluconeogenesis—into circulation. Adipose tissue similarly mobilizes its energy stores by releasing fatty acids. These actions collectively raise circulating nutrient concentrations and restore nutrient balance. The simplicity of this schema is belied, of course, by the intricacy apparent on closer inspection of any given component, each of which comprise entire regulatory circuits themselves. Furthermore, recurring modules occupy key positions throughout these circuits (for example, the insulin receptor is expressed on many different metabolic tissues), forming the structural basis for the familiar neuroendocrine, hormonal, and neural axes of metabolic regulation that coordinate whole body metabolism down to the level of single cells.
Acknowledgments
We thank L. Mukundan for constructive comments on this manuscript. A.C. was supported by grants from: NIH (HL076746, DK094641), Larry L. Hillblom Foundation Network Grant, Diabetes Family Fund (UCSF), AHA Innovative Award (12PILT11840038) and an NIH Director’s Pioneer Award (DP1AR064158). We regret that we are unable to cite all relevant citations from our colleagues due to space limitations. The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- Afacan NJ, Fjell CD, Hancock RE. A systems biology approach to nutritional immunology - focus on innate immunity. Molecular aspects of medicine. 2012;33:14–25. doi: 10.1016/j.mam.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Bronneke HS, Brodesser S, Hampel B, Schauss AC, Bruning JC. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci U S A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T. Evolution of vertebrate immunity. Current biology CB. 2012;22:R72–R2732. doi: 10.1016/j.cub.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Brooks-Worrell B, Narla R, Palmer JP. Biomarkers and immune-modulating therapies for type 2 diabetes. Trends Immunol. 2012;33:546–553. doi: 10.1016/j.it.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Annals of the New York Academy of Sciences. 2011;1243:E1–E39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, Ren Y, Yin Z, Hamilton DJ, Reardon PR, et al. Class II Major Histocompatibility Complex Plays an Essential Role in Obesity-Induced Adipose Inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MM, Thomas DW, Kramer DL. The role of energy availability in Mammalian hibernation: a cost-benefit approach. Physiological and biochemical zoology : PBZ. 2003;76:165–179. doi: 10.1086/367950. [DOI] [PubMed] [Google Scholar]
- Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, Flier JS. Absence of CC Chemokine Ligand 2 Does Not Limit Obesity-Associated Infiltration of Macrophages into Adipose Tissue. Diabetes. 2007 doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annual review of cell and developmental biology. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Jr., Ables EV, Ferrante AW. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Leukocyte set points in metabolic disease. F1000 Biol Rep. 2012;4:13. doi: 10.3410/B4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012 doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Papackova Z, Palenickova E, Dankova H, Zdychova J, Skop V, Kazdova L, Cahova M. Kupffer cells ameliorate hepatic insulin resistance induced by high-fat diet rich in monounsaturated fatty acids: the evidence for the involvement of alternatively activated macrophages. Nutrition & metabolism. 2012;9:22. doi: 10.1186/1743-7075-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi M, Engel D, Christ A, Beckers L, Wijnands E, Boon L, Driessen A, Cleutjens J, Weber C, Gerdes N, Lutgens E. CD40L Deficiency Ameliorates Adipose Tissue Inflammation Metabolic Manifestations of Obesity in Mice. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.231357. [DOI] [PubMed] [Google Scholar]
- Purkayastha s, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes & development. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, Davis RJ. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes & development. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HS, Rakhshandehroo M, van de Graaf SF, Venken K, Koppen A, Stienstra R, Prop S, Meerding J, Hamers N, Besra G, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature. 2012;483:457–460. doi: 10.1038/nature10880. [DOI] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]